Determination of carotene, tocopherols and tocotrienols in residue oil
from palm pressed fiber using pressurized liquid extraction-normal
phase liquid chromatography
M. Marsin Sanagi
∗, H.H. See, Wan Aini Wan Ibrahim, Ahmedy Abu Naim
Department of Chemistry, Faculty of Science, Universiti Teknologi Malaysia, 81310 Skudai, Johor, Malaysia
Received 28 September 2004; received in revised form 29 January 2005; accepted 11 February 2005 Available online 10 March 2005
Abstract
Pressurized liquid extraction (PLE) along with normal phase liquid chromatography (NPLC) is evaluated as a new approach for the determination of-carotene and Vitamin E isomers in residue oil obtained from palm pressed fiber (PPF). The relative extraction recoveries obtained by optimized PLE were found to be equivalent (∼100%) to conventional Soxhlet extraction. Optimized PLE was obtained (5 g of sample, 80◦C, 1500 psi, 2×10 min static extractions with flush volume 50%) combined with NPLC system. Typical extractions give 3.7–4.0 mg ml−1of Vitamin E isomers and-carotene of 3.3–3.5 mg ml−1, which is similar to or greater than classical Soxhlet extraction with
n-hexane or chloroform. The new developed method demonstrated an acceptable performance with good efficiency in terms of total extraction time, total solvent usage, total-carotene and Vitamin E isomers contents as well as exceptional method repeatability.
© 2005 Published by Elsevier B.V.
Keywords: Pressurized liquid extraction; Liquid chromatography; Tocopherol; Tocotrienol; Palm oil
1. Introduction
Tocopherol was first reported in 1922 by a biologist, Evans when they studied the reproduction of the rat[1]. In 1937, Emerson et al.[2]described the existence of various Vitamin E homologues having different abilities to prevent Vitamin E deficiency. The naturally occurring tocopherols and tocotrienols constitute the majority of the Vitamin E group of compounds and they are composed of eight vitamers: ␣-, -, ␥-, and ␦-tocopherol and their four corresponding unsaturated congeners, namely␣-,-,␥-, and␦-tocotrienol
[3]. Tocopherols are methyl-substituted hydroxychromans with a saturated phytyl side chain and the tocotrienols with an unsaturated side chain (Fig. 1). The main interest for studying Vitamin E is its natural antioxidant capabilities. It has been known as the most efficient antioxidant for break-ing free radical chain reactions and provides some natural
∗Corresponding author. Tel.: +607 5534517; fax: +607 5566162.
E-mail address: marsin@kimia.fs.utm.my (M.M. Sanagi).
oxidative protection to the oil. Most vegetable oils and biological fluids contain varying amounts of the dominant tocopherols. Nevertheless, tocotrienols can be found in palm oil, coconut oil, and cereal grains such as wheat, rye, oats, and barley[4].
Crude palm oil (CPO) is one of the major sources of Vitamin E and contains high quantities of tocopherols and tocotrienols in the range of 600–1000 ppm [5]. Recently, palm fatty acid distillate (PFAD), a by-product of the physical refining of palm oil, has been identified as a good raw material source for the recovery of Vitamin E. PFAD is relatively cheap and readily available throughout the refining industry[6].
Palm pressed fiber (PPF) is the fibrous residues separated from the mesocarp and kernel during palm oil extraction. PPF is usually burned as fuel to provide energy for the pro-cessing mill. Oil losses usually occur in various by-products, including the fiber, which remains after the mesocarp oil is extracted by a screw press. Because the screw press process for the extraction of oil from mesocarp is conventionally
done by single stage pressing, the oil loss is considerable
[7].
Residue oil obtained from the PPF has been known as a good source of Vitamin E from the middle of 1990s
[7]. Classical Soxhlet extraction had been utilized as the extraction method to obtain the residue oil from the PPF. Although the residue oil contains high quantities of minor components including the Vitamin E isomers, high quantities of impurities and degradation product were observed in the analysis of the oil by various analytical methods.
Pressurized liquid extraction (PLE) is a new extraction procedure that combines elevated temperature and high pressures with liquid solvents to achieve rapid and efficient extraction of analytes from various matrices. PLE has recently gained wide acceptance for the analysis of different pesticides, PAHs, PCBs in sediments and sludge [8–10]. Moreover, PLE has been shown to be equivalent to standard EPA extraction methodology in terms of recovery and precision[11]. However, no reports of the work performed to extract residue oil in PPF have been published to date.
In the present study, the optimization and validation of pressurized liquid extraction (PLE) was investigated as a new approach for the rapid extraction of residue oil in PPF. PLE method is compared with the Soxhlet extraction in determin-ing the valuable tocopherols and tocotrienols components in the recovered oil obtained from PPF. In addition, optimiza-tion of Vitamin E isomers separaoptimiza-tions using NPLC at different column temperature is also described and subsequently uti-lized as a separation technique in analyzing the Vitamin E contents in residue oil.
2. Experimental
2.1. Reagents
Tocopherols and tocotrienols were purchased as isomer kits from Merck (Darmstadt, Germany). ␣-Tocopherol ac-etate (␣-TAc) was obtained from Sigma–Aldrich (USA). Chloroform, n-hexane, 2-propanol, diethyl ether, ethyl ac-etate, absolute ethyl alcohol, and 1,4-dioxane were analytical grade obtained from various suppliers.
2.2. Chromatographic conditions
The LC systems consisted of a conventional LC system coupled with a column oven of a Perkin Elmer Autosystem Gas Chromatography (USA). NPLC separations were car-ried out on a 5m Hypersil silica column (200 mm×4.6 mm i.d.) (Sigma–Aldrich, Milwaukee, USA) using a Waters 515 HPLC pump (Milford, USA) for mobile phase delivery. A Rheodyne 7125 injection valve (Cotati, USA) fitted with a 5l loop was used for sample introduction. Analyte peaks were detected using a Shimadzu SPD-6A UV detector (Ky-oto, Japan) and were recorded on a Hewlett Packard HP 3396 Series II integrator (USA).
2.3. Separation of tocopherols andtocotrienols by NPLC at different temperatures
Samples of eight natural Vitamin E isomers (␣-tocopherol, -tocopherol, ␥-tocopherol, ␦-tocopherol, ␣-tocotrienol, -tocotrienol, ␥-tocotrienol, and ␦-tocotrienol) and ␣ -tocopherol acetate were dissolved in the n-hexane and stored
at temperature−18◦C in darkness. Separation of prepared mixture was carried on a Hypersil silica column using mo-bile phase n-hexane:1,4-dioxane (96.0:4.0 v/v) at different column temperatures (30–60◦C). The prepared mixture was injected in triplicate onto the column and the solute concen-trations were 0.1–0.3 mg ml−1. UV detection of analytes for the comparison study was at 295 nm.
2.4. Palm pressedfiber samples
PPF samples were obtained from Felda Palm Industries Sdn. Bhd. at Kota Tinggi, Johor, Malaysia. Freshly collected samples were dried at 60–70◦C for 1 h. Homogenization was performed with a grinder.
2.5. Extraction of residue oil from palm pressed fiber
2.5.1. Soxhlet extraction
Separate extractions of dried fiber (5 g) were performed with different solvents, namely n-hexane and chloroform in a Soxhlet apparatus for 8 h. The residue oil obtained was weighed after rotary evaporation to remove solvents and pump-dried under vacuum.
2.5.2. Pressurizedliquidextraction
Pressurized liquid extraction of residue oil from PPF sam-ples was performed using a home-made accelerated solvent extractor (Fig. 2). Dried fiber samples (2, 4, 5, and 6 g) were accurately weighed into a 32 ml high-pressure extraction ves-sel. The vessel full of fiber was placed into the heated com-partment with its inlet and outlet connected to the preheating coil and the static valve C2, respectively. Extractions were carried out using 100% n-hexane. The extraction solvent was pumped through the vessel with static valve C2 open and the valve was closed once the solvent started to exit. Static valve C1 was closed after the system reached the expected pressure and subsequently the vessel was heated. Tempera-tures of 60, 80, 100, and 120◦C and pressures of 1000, 1500,
Fig. 2. Schematic diagram of home-made pressurized liquid extraction (PLE) system: A, HPLC pump; B, three-way switching valve; C1 and C2, static valves; D, pressure gauge; E, oven; F, preheating coil; G, extraction vessel; H, pressurized nitrogen gas; I, collection vial.
2000, and 2500 psi were tested with a heat-up time of 5 min. One-cycle extractions were performed for 10 min in static mode with flush volume 50% of the extraction cell volume (the volume of fresh n-hexane used for flushing of the extrac-tion cell after first 10 min static extracextrac-tion, i.e. approximately 16 ml, was collected with static valves C1 and C2 opened). Another 10 min static extraction was then carried out using same operating condition. The extracts were cooled to 40◦C by reducing oven temperature and were subsequently purged from the vessel using pressurized nitrogen for 1 min. The total volume of extract collected was in the range of 45–48 ml. The residue oil obtained was weighed after rotary evaporation to remove solvents and pump-dried under vacuum.
2.6. Determination of tocopherols, tocotrienols and
-carotene content
Tocopherols and tocotrienols contents were determined by NPLC. Residue oils obtained from Soxhlet and PLE extrac-tions were pretreated according to the method described by Tan and Brzuskiewicz [12]by dissolving the residue oil in
n-hexane and chill overnight at−20◦C, respectively. Sam-ples were then centrifuged at 4◦C to remove solids, and the lipid soluble supernatant was chromatographed. A solvent system of 99:4 v/v (n-hexane:1,4-dioxane) with a flow rate of 1 ml min−1 was applied to the system, and all the ana-lytes were detected using UV detector set at a wavelength of 295 nm. Identification of analyte was carried out by compar-ison of retention times in chromatogram with that of toco-pherols and tocotrienols standards.
The carotene content of residue oil is defined and calcu-lated as-carotene in mg ml−1by using PORIM test method p2.6 (1995)[13]. Residue oil obtained (0.1 g) was weighed into the 25 ml volumetric flask. The test portion was dissolved with n-hexane and diluted to the mark. The test portion was measured using a UV–vis detector with absorbance at 446 nm against the solvent used.
3. Results and discussion
3.1. Separation of tocopherols andtocotrienols by NPLC at elevatedtemperature
Separations of tocopherols and tocotrienols were carried out at different column temperatures from 30, 40, 50 and 60◦C. It was noted that the elution times were inversely pro-portional to the column temperature. Based on the retention factors obtained in our study, it was observed that for each isomer, the retention factor decreased by a factor of 1.08 when the column temperature was increased by 10◦C. This observed trend was found consistently for the Vitamin E iso-mers at all column temperatures studied.
phase and the analytes with the increase in column tempera-ture. The resolution data gathered in our study demonstrated that the best separation was achieved at a column temperature of 40◦C. The repeatability and reproducibility of the method developed were also investigated. It was found that the results achieved on the same working day were generally acceptable, with R.S.D. value of <5%, and on three consecutive days, the R.S.D. values were lower than 7%. The detection limit val-ues, expressed as amount injected, were 5 ng (␦-tocopherol and␦-tocotrienol), 10 ng (␥-tocopherol,-tocopherol and -tocotrienol), 15 ng (␣-tocopherol), and 20 ng (␣-tocotrienol and␥-tocotrienol).
3.2. Determination of Vitamin E contents in residue oil from palm pressedfiber
3.2.1. Soxhlet extraction technique
Two types of solvent were used: n-hexane and chloroform. The PPF sample amount and total solvent usage were opti-mized based on the volume of the extraction thimble and size of the Soxhlet apparatus. Eight hours of Soxhlet extraction is generally adequate for exhaustive extraction of the residue oil in PPF sample. Hence, by using Soxhlet extraction as the reference method, the PLE method can be easily optimized based on the weight of the residue oil extracted from the PPF. It can be observed that the major forms of Vitamin E isomers present in residue oil from PPF using n-hexane as extraction solvent are␣-tocopherol (55.2%), followed by␥-tocotrienol (24.9%),␣-tocotrienol (17.2%), and␦-tocotrienol (2.8%). On the other hand, the major forms of Vitamin E isomers present in residue oil from PPF using chloroform as extraction sol-vent were␣-tocopherol (61.6%), followed by␣-tocotrienol (19.6%), ␥-tocotrienol (18.6%), and ␦-tocotrienol (0.2%). There was no significant deviation in the total quantities of tocopherols and tocotrienols obtained in PPF in relative with the results obtained by a previous study [7]. n-Hexane has been proven as a more suitable organic solvent to be utilized as the extraction solvent.
3.2.2. Pressurizedliquidextraction technique
Recoveries obtained by Soxhlet for the residue oil from PPF were compared with recoveries obtained by PLE in order to obtain optimal experimental conditions.
3.2.2.1. Choice of the sample amount. The optimization of
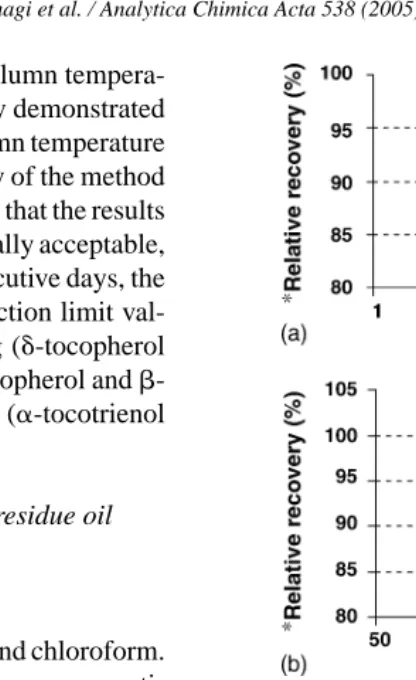
sample amount for extraction is important to achieve the best phase equilibration between sample matrices and extraction solvent in extraction vessel. In addition, the sample amount filled into the vessel will significantly reduce the total sol-vent usage for each extraction. In this work, optimization was performed on 2, 4, 5, and 6 g samples with a temperature of 100◦C, pressure of 1500 psi, and n-hexane as extraction solvent using the same extraction vessel.Fig. 3(a) showed the result obtained in the form of relative extraction recov-eries (for Soxhlet recovrecov-eries considered as equal to 100%). It can be observed that under these experimental conditions,
Fig. 3. Effect of (a) sample amount, (b) temperature, and (c) pressure on pressurized liquid extraction (PLE) efficiency (error bars represent standard deviation of results, n = 4);*(weight of residue oil obtained using PLE/weight of residue oil obtained using Soxhlet)×100.
recovery was highest for the 5 g sample. However, a decrease of recovery was noted for the 6 g sample. Therefore, 5 g of sample amount was chosen as the optimum sample amount for PLE in this study.
3.2.2.2. Choice of the extraction temperature. Temperature
(∼100%). An increase of temperature to achieve its super-heated liquid state (above solvent boiling point) generally resulted in better mass transfer rates between the solvent and the sample matrices and directly increases the capacity of solvent to solubilize analytes. When the temperature was in-creased to 100 and 120◦C, no further increase of relative recoveries was observed. In contrast, slightly decreased re-coveries were noted. This phenomenon could be due to the vaporising of the hot solvent that led to a decrease in recovery. However, this phenomenon was proven not to give a marked effect on the actual extraction efficiency due to the low heat capacity of the organic solvent and rapid loss of heat along the adequately lengthy tubing between extraction vessel and collection vial (∼30 cm)[15]. Based on the results gathered, extraction temperature of 80◦C was chosen as the optimum temperature for the extractions in this study.
3.2.2.3. Choice of the extraction pressure. The main
pur-pose of high pressure in PLE is to maintain the solvents as liquids while above their atmospheric boiling points. In this study, extractions were performed on samples (5 g) under dif-ferent extraction pressures of 1000, 1500, 2000, and 2500 psi at a temperature of 80◦C with n-hexane as the solvent. Rel-ative extraction recoveries (Soxhlet recoveries considered as equal to 100%) are represented inFig. 3(c). Based on the re-sults obtained, recoveries are increased radically from 1000 to 1500 psi. The use of appropriate pressure will facilitate extractions from samples in which the analytes have been trapped in matrix pores. The pressure forces the solvent into areas of the matrices that would not normally be contacted by solvents under atmospheric conditions[15]. However, recov-eries were gradually decreased when higher pressures (2000 and 2500 psi) were applied onto the system. Based on the results on the relative recoveries obtained in this study, a pressure of 1500 psi was chosen as the optimum pressure for the extraction.
3.2.2.4. Quantitation of tocopherols andtocotrienols using optimizedPLE method. Based on the results obtained from
the optimization of PLE on PPF, the following optimum ex-traction conditions were chosen: sample amount, 5 g; extrac-tion temperature, 80◦C; extraction pressure, 1500 psi with a heat-up time of 5 min, one-cycle of extractions were per-formed during 10 min in static mode with 50% flush vol-ume of extraction solvent. The extracts were purged from the extraction vessel using pressurized nitrogen for 1 min.
Fig. 4shows the separation of PLE extraction yields of toco-pherols and tocotrienols in residue oil obtained from PPF. It can be observed that the major forms of Vitamin E isomers present in residue oil from PPF are␣-tocopherol (52.5%), fol-lowed by␥-tocotrienol (28.2%),␣-tocotrienol (17.4%), and ␦-tocotrienol (1.9%). The overall results showed that residue oil contain high quantities of Vitamin E isomers in a range from 3.7 to 4.0 mg ml−1, which is comparable or even higher than the results obtained using Soxhlet extraction.
Fig. 4. NPLC separations of residue oil extracts obtained by Soxhlet (A) and pressurized liquid extraction (B) using n-hexane as extraction solvent on a Hypersil silica column. Chromatographic conditions: mobile phase,
n-hexane:1,4-dioxane (96.0:4.0 v/v); flow rate, 1 ml min−1; temperature, 40◦C. Peaks: (I)␣-tocopherol, (II)␣-tocotrienol, (IV)␥-tocotrienol, (V) ␦-tocotrienol and (III and VI) unknown.
3.3. Comparison of Soxhlet extraction with pressurized liquidextraction
By using the optimized method obtained, a study of the repeatability of PLE was carried out. The contents of residue oil obtained from PPF were analyzed and compared with those obtained using Soxhlet extraction. Fig. 5 shows the average values and their corresponding standard deviations for the two extraction methods studied. The overall results
Table 1
Comparison of results obtained on the determination of residue oil contents using various extraction methods
Parameter Extraction method
Soxhlet (n-hexane) Soxhlet (chloroform) Pressurized liquid extraction (PLE)
Total extraction time (h) 8 8 25 min
Total solvent usage (ml) ∼200 ∼200 ∼45
Total Vitamin E contents (mg ml−1) 3.1–3.5 3.3–3.6 3.7–4.0
Total-carotene contents (mg ml−1) 3.1–3.3 2.8–3.0 3.3–3.5
Method repeatability (R.S.D., %) (n = 4) 3.0–6.0 3.0–6.0 <3.0
demonstrated that PLE method developed in this study is comparable with the Soxhlet extraction. In addition, by using same batch of sample, higher concentrations of ␥-tocotrienol and␣-tocotrienol in residue oil were obtained under PLE optimized conditions compared to those obtained by using the Soxhlet extraction. Based on the t-test results, no significant differences of the concentration of␣-tocopherol and ␦-tocotrienol were observed using various extraction methods in our study. The total quantity of carotene contents in the residue oil was also determined using the PORIM standard procedure. Based on the results illustrated in
Fig. 5, the highest value of -carotene was obtained by using n-hexane as solvent in PLE system. Soxhlet extraction generally resulted in lower values of -carotene content and the values using n-hexane as extraction solvent were slightly higher than those using chloroform as extraction solvent. The overall RSD percent for each Vitamin E isomers detected and -carotene concentrations using PLE system were less than 15%. Table 1 lists some of the extraction conditions and results for the three extraction methods used in this study. The results demonstrate that PLE gives total -carotene and vitamin contents comparable to the Soxhlet extraction methods but offers the advantages of lower total organic solvent requirement and rapid extraction.
4. Conclusions
A novel and rapid method for the determination of Vita-min E isomers in PPF has been developed using home-made PLE extraction system and NPLC. The detailed comparison of Soxhlet with PLE showed the acceptable efficiency of PLE in terms of total extraction time, total solvent usage, total Vitamin E isomers contents as well as the method repeatabil-ity. PLE method demonstrated an outstanding performance with a total of four-fold reduction in total organic solvent consumption and up to 16-fold reduction in the total extrac-tion time required against the Soxhlet extracextrac-tion without significantly loss in extraction efficiency. Although the rela-tive residue oil recovery for optimized PLE system (99.2%) is slightly lower than that for Soxhlet extraction (nominal value of 100%), slightly higher concentration of Vitamin E
isomers and-carotene contents were observed. This could probably be due to the shorter extraction time afforded by PLE that consequently reduced the analytes degradation. Current work provides a great interest to further investigate on the applicability of the PLE method to the analysis of other minor components in the residue oil obtained from PPF such as carotenoids, sterols, phospholipids, and glycolipids.
Acknowledgements
The authors would like to thank Universiti Teknologi Malaysia and the Ministry of Science, Technology and In-novation, Malaysia, for financial support through the IRPA program, a studentship for H.H. See, and the Felda Palm In-dustries Sdn. Bhd. for PPF samples.
References
[1] H.M. Evans, Vitamin Horm. 20 (1962) 379.
[2] O.H. Emerson, G.A. Emerson, A. Mohammed, H.M. Evans, J. Biol. Chem. 122 (1937) 99–107.
[3] S.L. Abidi, J. Chromatogr. A 881 (2000) 197–216.
[4] F.J. Ruperez, D. Martin, E. Herera, C. Barbas, J. Chromatogr. A 935 (2001) 45–69.
[5] S.H. Goh, Y.M. Choo, S.H. Ong, JAOCS 62 (2) (1985) 237–240. [6] K. Sundram, T. Thiagarajan, A. Gapor, Y. Basiron, Health Nutr. 13
(2002) 634–640.
[7] Y.M. Choo, S.C. Yap, C.K. Ooi, A.N. Ma, S.H. Goh, A.S.H. Ong, JAOCS 73 (5) (1996) 599–602.
[8] A.D.L. Cal, E. Eljarrat, D. Barcel´o, J. Chromatogr. A 1021 (2003) 165–173.
[9] J.D. Berset, M. Ejem, R. Holzer, P. Lischer, Anal. Chim. Acta 383 (1999) 263–275.
[10] V. Flotron, J. Houessou, A. Bosio, C. Delteil, A. Bermond, V. Camel, J. Chromatogr. A 999 (2003) 175–184.
[11] Test Methods for Evaluating Solid Waste, Method 3545. USEPA SW-846, Update III, 3rd ed., U.S. GPO, Washington, DC, 1995.
[12] B. Tan, L. Brzuskiewicz, Anal. Biochem. 180 (1989) 368–373. [13] PORIM Test Method, Palm Oil Research Institute of Malaysia, 1995,
pp. 2.6, 43–44.
[14] Application Note 208, Dionex, Sunnyvale, CA, 1996.