The importance of spatial scales to analysis of
fish diversity in Amazonian floodplain lakes and
implications for conservation
Carlos E.C. Freitas1, Flavia K. Siqueira-Souza1, Alexandro C. Florentino1, Lawrence E. Hurd2 1Department of Fisheries Sciences, Federal University of Amazonas, Manaus, Brazil
2Biology Department, Washington and Lee University, Lexington, VA USA Accepted for publication August 15, 2013
Abstract–The Amazon River Basin has the highest fish species diversity of any region in the world, but is under threat from anthropogenic perturbations including overharvesting, alien species and drought. We asked whether species diversity in this region is more a function of within-lake species richness (i.e.,adiversity) or differences among lakes (bdiversity). Although many studies have reported on species richness and diversity in single habitats, the importance of measuring diversity at different spatial scales is not yet well established. We collected fish in 10 floodplain lakes along the Solim~oes River (Brazil), divided evenly between two lake types: those on islands in the river channel (island lakes) and those on the margins of the river (coastal lakes) during 2006. We partitioned fish diversity into three spatial scales:a = within each lake;b1 =among lakes of the same type (coastal or island) and
b2 =between the two types of lakes, and compared their relative contributions to regional (c) diversity.b1+b2
contributed as much or more tocdiversity than dida. Although many of the 116 fish species were shared between lake types (S=72), 32 species were found exclusively in coastal lakes and 12 species were found exclusively in island lakes. Coastal lakes, which were deeper and cooler than island lakes, consistently had higher fish species richness than island lakes. We suggest that it will be necessary to set areas large enough to contain multiple lakes of both types to preserve regional fish diversity.
Key words: biodiversity measurement; fish species diversity; floodplain lakes; metacommunities; spatial scales in ecology
Introduction
The Amazon River Basin encompasses the world’s largest remaining tropical rainforest. This huge region has the highest fish species diversity of any region in the world with more than 2000 species identified so far (Reis et al. 2003). Biodiversity of freshwater eco-systems in general has decreased more than that of any other major ecosystem in modern times (Jenkins 2003; Pittock et al. 2008), and in the Amazon, the chief threats are commercial fishing (Batista et al. 1998), alien species (Latini & Petrere 2004) and droughts caused by global warming (Marengo et al. 2008). Freitas et al. (2013) examined the impact of an extraordinary drought on the diversity of fish assem-blages in floodplain lakes in the Brazilian Amazon
and found that regional species richness could not be characterised by species richness in a single lake, and suggested that this might be an important consider-ation in the design of conservconsider-ation areas.
The partitioning of regional species diversity, or landscape biodiversity (Veech et al. 2002), has proved to be especially fruitful in determining the contribution of different spatial scales and habitat types (Er€os 2007). Whittaker (1972) first proposed the existence of spatial components for diversity: a (alpha) diversity referring to the diversity within indi-vidual communities; b (beta) diversity based on dif-ferences among communities within a region and c (gamma) diversity, which represent total regional diversity, essentially a combination of a and b (Wilsey 2010).
Correspondence: C. E. C. Freitas, Federal University of Amazonas, Department of Fisheries Sciences, Av. Gen. Rodrigo Otavio, 3000. Manaus 69077-000, AM, Brazil. E-mail: cefreitas@ufam.edu.br
ECOLOGY OF FRESHWATER FISH
Alpha, beta and gamma components of diversity are among the fundamental descriptive variables of ecology and conservation biology (Jost 2007), because they allow us to look at the relative contribu-tion of local and regional patterns to biodiversity. We can also test different hypotheses about the processes that generate regional variety, that is, whether b diversity is a result of local biological interactions such as competition and predation that can result in uniform regional species composition, or of spatially limited dispersal resulting in nonrandom patchiness across a region (Legendre et al. 2005). Dispersal among habitat patches within a region is an important component of landscape ecology and models of meta-community dynamics (Holyoak et al. 2005).
The question of how different spatial scales con-tribute to regional biodiversity is an important consid-eration for conservation biology, because the relative importance of one or more hierarchical spatial levels can drive the development of strategies for preserva-tion of diversity. As discussed by Baselga et al. (2007), b diversity can be a result of two processes: nestedness and species turnover. Nestedness occurs when the fish assemblages of sites with lower diver-sity are subsets of fish assemblages of sites with higher diversity (Wright & Reeves 1992). Species turnover results from environmental, spatial and his-torical differences among sites (Baselga et al. 2007). Studies of b diversity may be particularly important for human-dominated landscapes, where habitat mod-ification can lead to homogenisation of ecological communities (Er€os 2007). However, quasi-natural events such as periodic drought exacerbated by glo-bal warming may promote a homogenisation of the landscape and consequently a reduction of beta diver-sity, as observed by Freitas et al. (2013) for Amazo-nian floodplain lakes.
There have been many studies aimed at measuring the alpha diversity (local species richness) of fish assemblages from Amazonian floodplain lakes (Merona & Bittencourt 1993; Saint-Paul et al. 2000; Merona & Rankin-de-Merona 2004; Siqueira-Souza & Freitas 2004). These studies have revealed species richness between 77 and 239 species, with a striking prevalence of species within the orders Characifor-mes and SiluriforCharacifor-mes. These relatively high values of local species richness have been considered to explain the high regional diversity of fish at the Ama-zon Basin, where half of the total number of fish spe-cies estimated for the entire Neotropic reside (Reis et al. 2003). However, the contribution of diversity at different spatial scales has yet to be examined.
Within the Amazon Basin ecosystem, floodplains adjacent to large white water rivers comprise a com-plex mosaic of lakes, floodplain channels, scroll bars and overbank deposits (Mertes et al. 1996). These
environments are distinguishable on the basis of geo-morphology and other environmental characteristics that produce different selective regimes that undoubt-edly account for much of the adaptive radiation of fish in this region (Soares et al. 2007; Freitas et al. 2010a; Hoorn et al. 2010). In addition to spatial het-erogeneity, the system is temporally dynamic, charac-terised by an annual hydrologic cycle with dramatic changes in water depth that create flooded forests in the floodplains adjacent to the rivers during high water, through which fish can move, and relatively isolated floodplain lakes during low water (Junk et al. 1989). We partitioned diversity into three spa-tial scales: a= diversity within each lake;
b1= diversity among lakes of the same type (coastal
or island locations); and b2= diversity between the
two types of lakes, such that b1+ b2 does not have
to equal ∑a. This approach is similar to that sug-gested by Legendre et al. (2005) that the variance estimated from a community composition table is a correct measure of beta diversity for a three-level framework to describe diversity.
Materials and methods Study area
The ten study lakes were situated at the lower stretch of the Solim~oes River (Amazonas – Brazil) and are included in the typology of wetlands subjected to pre-dictable, long-lasting, monomodal flood pulses (Junk et al. 2011). These are river floodplains with high flood amplitudes (up to 10 m near the confluence between the Solim~oes and Negro Rivers), adjacent to a large river, exhibiting high fertility (Sch€ongart & Junk 2007; Junk et al. 2011). Within the floodplain, there are two main lentic environments, called var-zea, with characteristic features: lakes on fluvial islands in the main river channel (island lakes) and lakes that are formed on the floodplains at the mar-gins of the river that also have connections to small streams originating on the floodplains (coastal lakes) (Mertes et al. 1996).
The five island lakes in our study were Sacambu, Preto, Piranha, Ressaca and Cacau, all of which are on the same island. These shallow lakes were formed behind scroll bars that were formed by the overbank deposition of fine material. By this procedure, the current in the main channel builds levees that tend to restrict the movement of the main channel and flood-plain channels (Mertes et al. 1996). The five coastal lakes were Preto, Iauara, Anana, Aracßa and Maraca (Fig. 1). All lakes were located along a 300 km stretch of the Solim~oes River between the mouth of the Coari River and the confluence of the Solim~oes and Negro Rivers in Brazil. All of these were typical
Amazonian floodplain lakes that contain water even during the low-water season of the hydrologic cycle (Junk et al. 1989).
Data collection
During 2006, collections of fish were made in all ten lakes, once during each of the four seasons of the hydrologic cycle: March (rising water), June (flood-ing), late August-early September (receding water) and late November-early December (low water).
We collected fish from populations at each location and season with nine monofilament gillnets of differ-ent mesh sizes: 20, 30, 40, 50, 60, 70, 80, 90 and 100 mm of stretch size. Nets of each mesh size were 20 m long 9 2 m deep and were attached to each
other, resulting in an overall length of 180 m per set for each lake. Nets were attached to floats so that they hung downward from the surface of the water, set out in the morning between 0500 and 0600 h, and brought in with the catch 12 h later. Fish caught during the morning hours were also collected from the nets at midday in order to reduce the damage made by piranhas and other predators that nibbled on the entangled fish. Fish were identified and preserved in formalin for permanent storage in the Zoological Collection of the National Institute for Amazonian Research (INPA) in Manaus, Brazil. Each time we collected fish from the nets, we measured dissolved oxygen and surface temperature with a multiparame-ters equipment model Hanna HI 9828. Depth was measured using a weighted rope. As we did not
Fig. 1. The 10 sampled lakes of the floodplain of the Solim~oes River in 2006, located between the mouth of the Coari and the confluence of the Solim~oes and Negro Rivers (Amazonas–Brazil). Above: The five coastal lakes are Preto, Iauara, Anana, Aracßa and Maraca. Below: The five island lakes are Sacambu, Preto, Piranha, Ressaca and Cacau.
evaluate seasonal effects, we used the mean of each measure taken at the two extreme seasons of the hydrological cycle, for example, mean depth =(depth at high water+ depth at low water)/2).
Data analysis
We measured a diversity for each lake as species richness (Sl) and used a global measure of beta diver-sity =S/a, whereSis the total number of species col-lected from all lakes, representing cdiversity, and a is the mean number of species observed at each lake (Whittaker 1972). This approach gives us a measure ofb-diversity that represents how many more species are present in the whole area than at an average site (Legendre et al. 2005). We also applied the multiple-site dissimilarity measures proposed by Baselga et al. (2007) and Baselga (2010), because of their ability to identify the predominance of species loss or species turnover in communities. In addition to species rich-ness, we calculated Shannon’s diversity index using logeand Pielou’s evenness using the software
Paleon-tological Statistic Software Package– PAST, version 2.07 (Hammer et al. 2001). After testing homogene-ity of variances (which was verified for our compari-sons), we compared species richness, abundance, Shannon index and evenness between the two types of lakes with a t-test using R software (R Develop-ment Core Team 2012).
We applied the package Vegan (Oksanen et al. 2010) from R software (R Development Core Team 2010) for analysis of fish diversity partitioning. The observed species richness among and within lakes provided the input data for an analysis of null models with 10,000 random assemblages (Crist et al. 2003). We used this method to test the hypothesis that the fish diversity at each level was a result of a random assemblage of individuals. Null hypothesis models are considered a powerful statistical tool for the anal-ysis of diversity partitioning across multiple scales (Gotelli & Graves 1996). Finally, we applied a discri-minant analysis to the environmental data to evaluate if environmental variables explain a significant pro-portion of the community composition variation.
Results
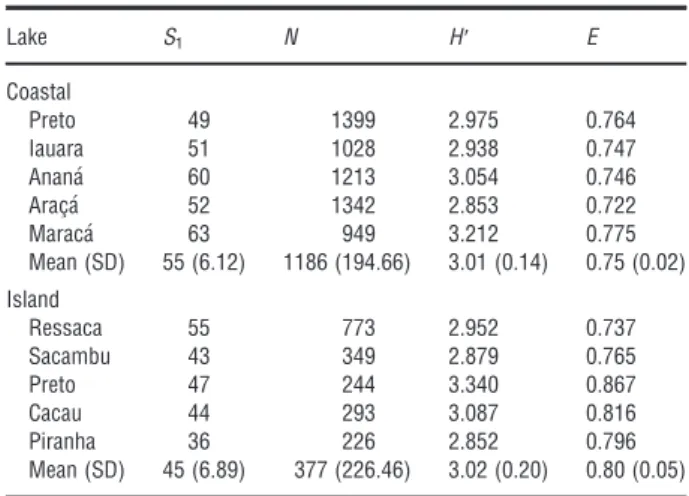
We collected a total of 116 species, belonging to six orders and 21 families, and a total of 7867 individu-als (Appendix S1). Species richness, pooled for the four hydrologic seasons, varied from 36 to 63 species and abundance varied between 226 and 1399, with values consistently higher in coastal lakes than in island lakes (t = 2.425, d.f. = 8, P = 0.041 and
t =6.059, d.f. = 8,P < 0.001, respectively: Table 1). The Shannon index was quite similar for both types of
lakes (t = 0.159, d.f. =8, P =0.875), with esti-mates between 2.852 and 3.340 (Table 1). Evenness also was quite similar (t = 1.892, d.f. =8, P =
0.095), with estimates between 0.722 and 0.867. Char-aciformes and Siluriformes were the most abundant groups, both in number of species and abundance, for both types of lakes. The serrasalmid,Pygocentrus nat-tereri, was the most abundant species in coastal lakes, and the small curimatid, Potamorhina altamazonica, was the most abundant species in island lakes (Appen-dix S1). There were 72 species shared between the two lake types; however, 32 species were exclusively found in coastal lakes and 12 species exclusively in island lakes.
Our measure of overallb-diversity by the Whittak-er (1972) equation was 2.2, indicating that more than twice, the number of species is expected for the whole area than occurred at each lake. Our estimate of Simpson-based multiple-site dissimilarity index was 0.617, and the nested resultant multiple-site dis-similarity was 0.069. Partitioning analysis shows that the diversity within each lake and among the lakes of same type, a and b1, respectively, were the main
components of gamma diversity (Table 2, Fig. 2), with a total contribution of approximately 80%. The contribution of b2 was approximately 20% (see
Appendix S1).
The analysis by null models revealed that a diver-sity was not significantly different than for expected by random chance (P > 0.05). In contrast, b1
diver-sity was higher than expected (P <0.05), which is an indication of the high turnover at this spatial scale.
b2 was not different from random expectation
(P > 0.05). The importance of components aand b1
was higher in coastal lakes (91.7% of contribution to
Table 1. Species richness (Sl), abundance (N), Shannon’s index (H′) and evenness (E) of fish assemblages from two geomorphological types of Amazonian floodplain lakes of the Solim~oes River (Amazonas – Brazil, 2006). Comparisons of species richness, abundance, Shannon index and evenness between the two types of lakes with at-test using R software (R Development Core Team 2010).
Lake S1 N H′ E Coastal Preto 49 1399 2.975 0.764 Iauara 51 1028 2.938 0.747 Anana 60 1213 3.054 0.746 Aracßa 52 1342 2.853 0.722 Maraca 63 949 3.212 0.775 Mean (SD) 55 (6.12) 1186 (194.66) 3.01 (0.14) 0.75 (0.02) Island Ressaca 55 773 2.952 0.737 Sacambu 43 349 2.879 0.765 Preto 47 244 3.340 0.867 Cacau 44 293 3.087 0.816 Piranha 36 226 2.852 0.796 Mean (SD) 45 (6.89) 377 (226.46) 3.02 (0.20) 0.80 (0.05)
c diversity) than in island lakes (68.8% of contribu-tion for gamma diversity) (Fig. 2). By itself,a diver-sity in coastal lakes contributed 49.9% tocdiversity, and b1 contributed 41.8% (Fig. 2). The contribution
ofb2tocdiversity in coastal lakes was 8.3%. In
con-trast, the importance ofb2was higher in island lakes
(31.6% of contribution to c diversity) than coastal lakes, while contribution of b1 diversity was smaller
(36.7%, Fig. 2).
The physical parameters we measured show differ-ences by lake type (Table 3). Although there was no consistent difference in dissolved oxygen (DO) between lake types (except for greater variance in island lakes), both temperature and mean depth assorted according to lake type. Discriminant analysis showed significant effects of environmental variables (Wilks’ k=0.0168, F5,4 =46.775, P = 0.0012).
There is a clear distinction between the two types of lakes by variables temperature (Wilks’ k= 0.0549,
P = 0.039) and depth (Wilks’ k= 0.0629,
P = 0.029), where coastal lakes were deeper and cooler than island lakes (Fig. 3).
Discussion
It is clear from the results of our survey that (i) b diversity is as least as important a component of regional diversity as isaand that (ii) island lakes and
Table 2. Mean species richness of fish assemblages sampled in 10 floodplain lakes of the Solim~oes River (Amazonas–Brazil, 2006), partitioned betweena, b1, b2 andcspatial scales. Partitioning of species richness was analysed with the software package Vegan (Oksanen et al. 2010) from R software (R Development Core Team 2010). The observed species richness among and within lakes provided the input data for an analysis of null models in which estimated (expected) values were generated from 10,000 random assemblages (Crist et al. 2003).
Diversity Spatial scale Observed Estimated Contribution toc(%) P
a Within Lakes 51.4 49.7 44.91 0.00023
b1 Among lakes 43.6 38.3 36 0.00006
b2 Between types 21 22 19.09 0.00087
c Total 116
Fig. 2. Partitioning ofcfish species diversity into three compo-nents in each of the two lake types assuming two nested spatial scales, whereais the diversity within each lake,b1is the diver-sity among lakes of the same type (coastal or island) andb2is the diversity between the two types of lakes located between the mouth of the Coari and the confluence of the Solim~oes and Negro Rivers (Amazonas–Brazil).
Table 3. Mean values of dissolved oxygen (DO), surface temperature, and depth of five coastal (C) and five island (I) lakes of the lower Solim~oes River (Amazonas –Brazil, 2006). Means of all values are calculated as follows: (value high water+value at low water)/2.
Lakes DO (ppm) Temp. (C) Depth (m) Coastal Iauara 0.94 29.1 6.29 Campina 1.23 29.8 9.76 Preto 1.11 29.4 6.84 Anana 1.14 29.6 9.35 Maraca 1.38 29.4 8.24 Mean 1.16 29.46 8.096 SD 0.145 0.233 1.357 Island Sacambu 2.96 31.9 1.8 Preto 0.97 32.1 4.3 Piranha 2.16 32.7 2.2 Ressaca 4.66 32.5 2.1 Cacau 0.96 31.3 2.2 Mean 2.342 32.1 2.52 SD 1.384 0.490 0.902 11 10 9 8 7 6 5 4 3 2 1 28.5 29.0 29.5 30.0 30.5 Temperature 31.0 Coastal Island 31.5 32.0 32.5 33.0 Depth
Fig. 3. Lake ordination by mean temperature (°C) and depth (m), showing that coastal lakes were generally cooler and deeper than island lakes.
coastal lakes differ substantially, both in fish species composition and with respect to the relative contribu-tion of beta diversity to regional diversity. Thus, a diversity by itself does not represent the diversity of fish species well, since at least half of regional diver-sity for both lake types is contributed byb-diversity.
According to one hypothesis, landscapes are mosa-ics where species composition is controlled by envi-ronmental characteristics peculiar to individual sites (Legendre et al. 2005; Willis et al. 2005). The high estimate of beta diversity revealed by our multiplica-tive approach, and the high estimates of Simpson-based multiple-site dissimilarity (i.e., high species turnover), coupled with the low value obtained for nestedness resultant multiple-site dissimilarity, tend to indicate that this may be true for Amazonian floodplain lakes. Our relatively high estimate of b1
diversity, mainly for coastal lakes, means that all lakes within a type are not equivalent. Therefore, large tracts of floodplain encompassing many lakes may have to be set aside to maintain regional diver-sity (Magurran et al. 2011).
The cyclical influence of the flood pulse (Junk et al. 1989) could create a situation such that the spe-cies composition of fish from Amazonian floodplain lakes fluctuates with both variation in pulse dynamics (abiotic force) and interaction potentials among spe-cies such as predation and competition (biotic force). The interconnectivity among lakes and rivers in which these forces fluctuate in importance could thus promote a metacommunity pattern with source and sink dynamics (Leibold et al. 2004; Urban 2004). Freitas et al. (2010b) observed the importance of interconnectedness to maintain the fish diversity of island lakes in the Solim~oes River during the flood season when floodplain lakes are connected with the main river channel. However, the effects of this high interconnection during the flood season can also include a prevalence of stochastic processes on com-munity structure and composition (Miyazono et al. 2010; Scarabotti et al. 2011). For example, Freitas & Garcez (2004) found fish assemblages to be stochas-tically structured at the channels between floodplain lakes and the Solim~oes River, hypothetically because these channels are temporary aquatic habitats. The relatively higher b2 diversity we found for island
lakes could be a consequence of their greater connec-tivity with the main river than coastal lakes have.
In the river stretch where our study lakes are located, scroll lakes are mixed with rounder, larger lakes (Mertes et al. 1996), increasing the spatial com-plexity of the system. These larger lakes are mainly coastal, where some of them are connected with the regional drainage network through small streams. Mojica et al. (2009) stated that the connection among floodplain lakes and forest streams may be
responsi-ble for the notably high fish species richness at these lakes. We believe that greater depth and the connec-tions with small streams in coastal lakes may be key factors to explain the observed differences between the two types of lakes.
Scarabotti et al. (2011) reported on the influence of environmental variables on the structure of fish assemblages in floodplain lakes of the Salado River, Argentina. Because of the inverse relationship between water depth and temperature, shallow lakes tend to be warmer than deeper lakes and deeper lakes tend to be colder than shallow lakes. Warmer lakes also tend to have lower oxygen concentrations than cooler ones. Rodriguez & Lewis (1997) identified four environmental descriptors associated with the structure of fish assemblages of the floodplain lakes at Orinoco River Basin: transparency, conductance, depth and area. They proposed that the first two de-scriptors (environmental variables) are a consequence of the second two (physical structure). Miranda (2011) also proposed that depth is the primary con-troller of the variability observed in several other environmental parameters of floodplain lakes, which in turn have some predictable effects on fish assem-blages: shallower lakes showed highest variability in physical environmental conditions and a high abun-dance of opportunistic species, while deeper lakes were more stable and exhibited a prevalence of resil-ient species and predators. However, in another study in which 11 environmental variables were monitored in floodplain lakes from the Middle Araguaia River, Tejerina-Garro et al. (1998) observed significant effects on the fish community only for transparency and maximum depth. In any case depth, which is determined by geological processes during lake for-mation, clearly is a key landscape variable to con-sider when attempting to explain variability in fish assemblages among lakes within a type, b1, and
among the different types of lakes (coastal or island),
b2.
Coastal lakes can be formed by at least two geo-morphological processes, while island lakes have just one geomorphological origin. Coastal lakes could arise either by anastomoses (changes at the river channel) or by hydraulic barriers formed by the tribu-taries of the main river. In the first case, the lakes are tiny, shallow and the long axis is in the same direc-tion as that of the river (Fig. 4a). This is also true for all island lakes. In the second case, lakes formed along the tributaries tend to be dendritic and deeper, and can run perpendicular to the river channel (Fig. 4b).
We believe that the conservation of fish diversity at the Amazonian floodplain lakes is dependent on the implementation of reserves that must be large enough to encompass different types of lakes and
multiple examples of each type. Strategies of multi-ple-use modules, as proposed by Saunders et al. (2002) and already implemented in some areas of the Amazon Basin (e.g., Mamiraua Reserve at the So-lim~oes River or Piagacßu-Purus Reserve at the Purus River) could be viable tools to the maintenance of Amazonian fish diversity.
Acknowledgements
We thank I. Santos, W. Dias and J. Pena for help in fieldwork. Supported by grants from the PIATAM Project–FINEP and by Grant 023/2009–PRONEX/FAPEAM-CNPq and by Grant 563073/2010-1– CNPq and by Lenfest grants to LEH from Washington and Lee University.
References
Baselga, A. 2010. Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeog-raphy 19: 134–143.
Baselga, A., Jimenez-Valverde, A. & Niccolini, G. 2007. A multiple-site similarity measure independent of richness. Biology Letters 3: 642–645.
Batista, V.S., Inhamuns, A.J., Freitas, C.E.C. & Freire-Brasil, D. 1998. Characterization of the fishery in river communi-ties in the low- Solim~oes/high-Amazon region. Fisheries Management and Ecology 5: 419–435.
Crist, T.O., Vech, J.A., Gering, J.C. & Summerville, K.S. 2003. Partitioning species diversity across landscapes and regions: a hierarchical analysis ofa,b, andcdiversity. The American Naturalist 162: 734–743.
Er€os, T. 2007. Partitioning the diversity of riverine fish: the roles of habitat types and non-native species. Freshwater Biology 52: 1400–1415.
Freitas, C.E.C. & Garcez, R.C.S. 2004. Fish communities of natural channels between floodplain lakes and Solim~ oes-Amazonas river. Acta Limnologica Brasiliensis 16: 273– 280.
Freitas, C.E.C., Siqueira-Souza, F.K., Prado, K.L.L., Yamam-oto, K.C. & Hurd, L.E. 2010a. Factors determining fish spe-cies diversity in Amazonian floodplain lakes. In: Rojas, N., Prieto, R., eds. Amazon basin: plant life, wildlife and envi-ronment. New York, NY: Nova Science Publishers, pp. 41– 76.
Freitas, C.E.C., Siqueira-Souza, F.K., Guimar~aes, A.R., San-tos, F.A. & SanSan-tos, I.L.A. 2010b. Interconnectedness during high waters maintains similarity in fish assemblages of island floodplain lakes in the Amazonian basin. Zoologia 27: 931–938.
Freitas, C.E.C., Siqueira-Souza, F.K., Humston, R. & Hurd, L.E. 2013. An initial assessment of drought sensitivity in Amazonian fish communities. Hydrobiologia 705: 159–171. Gotelli, N.J. & Graves, G.R. 1996. Null models in ecology.
Washington, DC: Smithsonian Institute.
Hammer, O., Harper, D.A.T. & Ryan, P.D. 2001. PAST: pale-ontological Statistics Software Package for Education and Data Analysis. Paleontological Electronica 4: 1–9.
Holyoak, M., Leibold, M.A. & Holt, R.D. 2005. Metacommu-nities: spatial dynamics and ecological communities. Chi-cago: University of Chicago Press.
Hoorn, C., Wesselingh, F.P., ter Steege, H., Bermudez, M.A., Mora, A., Sevink, J., Sanmartin, I., Sanchez-Meseguer, A., Anderson, C.L., Figueiredo, J.P., Jaramillo, C., Riff, D., Ne-gri, F.R., Hooghiemstra, H., Lundberg, J., Stadler, T., S€ arki-nen, T. & Antonelli, A. 2010. Amazonia through time: andean uplift, climate change, landscape evolution, and bio-diversity. Science 330: 927–931.
Jenkins, M. 2003. Prospects for biodiversity. Science 302: 1175–1177.
Jost, L. 2007. Partitioning diversity into independent alpha and beta components. Ecology 88: 2427–2439.
Junk, W.J., Bayley, P.B. & Sparks, R.E. 1989. The flood pulse concept in river-floodplain systems. In: Dodge, D.P.. ed. Proceedings of the International Large River Sympo-sium. Canadian Special Publication of Fishery and Aquatic Science 106, pp. 110–127.
Junk, W.J., Piedade, M.T.F., Sch€ongart, J., Chon-Haft, M., A-deeney, J.M. & Wittmann, M. 2011. A classification of major naturally-occurring Amazonian lowland Wetlands. Wetland 31: 623–640.
Latini, A.O. & Petrere, M. 2004. Reduction of a native fish fauna by alien species: an example from Brazilian freshwa-ter tropical lakes. Fisheries Management and Ecology 11: 71–79.
Legendre, P., Borcard, D. & Peres-Neto, P.R. 2005. Analyzing beta diversity: partioning the spatial variation of community composition data. Ecological Monographs 75: 435–450. Leibold, M.A., Holyoak, M., Mouquet, N., Amarasekare, P.,
Chase, J.M., Hoopes, M.F., Holt, D., Shurin, J.B., Law, R., Tilman, D., Loreau, M. & Gonzalez, A. 2004. The meta-community concept: a framework for multi-scale meta-community ecology. Ecology Letters 7: 601–613.
Magurran, A.E., Khachonpisitsak, S. & Ahmad, A.B. 2011. Biological diversity of fish communities: pattern and pro-cess. Journal of Fish Biology 79: 1393–1412.
Marengo, J.A., Nobre, C.A., Tomasella, J., Oyama, M.D., de Oliveira, G.S., de Oliveira, R., Carmago, H., Alves, L.M. & Brown, I.F. 2008. The drought of Amazonia in 2005. Journal of Climatology 21: 495–516. 62°23’30”W (b) (b) (a) 62°18’0”W 62°12’30”W 62°7’0”W 3°44’30”S 3°50’0”S 3°55’30”S 62°7’0”W 62°12’30”W 62°18’0”W 62°23’30”W 3°55’30”S 3°50’0”S 3°44’30”S
Fig. 4. Typical coastal lakes of the Solim~oes River (Amazonas– Brazil) formed by (a) hydraulic barriers at the mouths of tributar-ies and by (b) anastomoses with the river channel.
Merona, B. & Bittencourt, M.M. 1993. Les peuplements de poisons du lago do Rei, un lac d’inondation d’Amazonie Centrale: description generale. Amazoniana 7: 415–441. Merona, B. & Rankin-de-Merona, J. 2004. Food resource
par-titioning in a fish community of the central Amazon flood-plain. Neotropical Ichthyology 2: 75–84.
Mertes, L.A.K., Dunne, T. & Martinelli, L.A. 1996. Channel-floodplain geomorphology along the Solim~oes-Amazon River, Brazil. Geological Society of America Bulletin 108: 1089–1107.
Miranda, L.E. 2011. Depth as an organizer of fish assem-blages in floodplain lakes. Aquatic Sciences 73: 211–221. Miyazono, S., Aycock, J.N., Miranda, L.E. & Tietjen, T.E.
2010. Assemblage patterns of fish functional groups relative to habitat connectivity and conditions in floodplain lakes. Ecology of Freshwater Fish 19: 578–585.
Mojica, J.L., Castellanos, C. & Lobon-Cervia, J. 2009. High temporal species turnover enhances the complexity of fish assemblages in Amazonianterra firme streams. Ecology of Freshwater Fish 18: 520–526.
Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., O’Hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H. & Wagner, H. 2010. Vegan: Community Ecology Package. R package version 1.17-6. http://CRAN.Rproject.org/package=vegan. Pittock, J., Hansen, L.J. & Abell, R. 2008. Running dry:
fresh-water biodiversity, protected areas and climate change. Bio-diversity 9: 30–39.
R Development Core Team. 2012. R: A language and envir-onment for statistical computing. R Foundation for Statisti-cal Computing: Vienna, Austria. ISBN 3-900051-07-0. Available at: http://CRAN.Rproject.org.
Reis, R.E., Kullander, S.O. & Ferraris, C.J. Jr 2003. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs.
Rodriguez, M.A. & Lewis, W.M. 1997. Structure of fish assem-blages along environmental gradients in floodplain lakes of the Orinoco River. Ecological Monographs 67: 109–128. Saint-Paul, L., Zuanon, J.A., Villacorta Correa, M.A., Garcia,
M., Fabre, N.N., Berger, U. & Junk, W.J. 2000. Fish com-munities in central Amazonian white- and blackwater flood-plains. Environmental Biology of Fishes 57: 235–250. Saunders, D.L., Meeuwig, J.J. & Vincent, C.J. 2002.
Freshwa-ter protected areas: strategies for conservation. Conservation Biology 16: 30–41.
Scarabotti, P.A., Lopez, J.A. & Pouilly, M. 2011. Flood pulse and the dynamics of fish assemblage structure from neotropical floodplain lakes. Ecology of Freshwater Fish 20: 605–618.
Sch€ongart, J. & Junk, W.J. 2007. Forecasting the flood pulse in central Amazonia by ENSO-indices. Journal of Hydrol-ogy 335: 124–132.
Siqueira-Souza, F.K. & Freitas, C.E.C. 2004. Fish diversity of floodplain lakes on the lower stretch of the Solim~oes River. Brazilian Journal of Biology 64: 501–510.
Soares, M.G.M., Costa, E.L., Siqueira-Souza, F.K., Anjos, H.D.B., Yamamoto, K.C. & Freitas, C.E.C. 2007. Peixes de lagos do medio rio Solim~oes. Manaus, Brazil: EDUA. Tejerina-Garro, F.L., Rejean, F. & Rodrõguez, M.A. 1998.
Fish community structure in relation to environmental varia-tion in floodplain lakes of the Araguaia River, Amazon Basin. Environmental Biology of Fishes 51: 399–410. Urban, M. 2004. Disturbance heterogeneity determines
fresh-water metacommunity structure. Ecology 85: 2971–2978. Veech, J.A., Summerville, K.S., Crist, T.O. & Gering, J.C.
2002. The additive partitioning of species diversity: recent revival of an old idea. Oikos 99: 3–9.
Whittaker, R. 1972. Evolution and measurement of species diversity. Taxon 21: 213–215.
Willis, S.C., Winemiller, K.O. & Lopez-Fernandez, H. 2005. Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecolo-gia 142: 284–295.
Wilsey, B.J. 2010. An empirical comparison of beta diversity indices in establishing prairies. Ecology 91: 1984–1988. Wright, D.H. & Reeves, J.H. 1992. On the meaning and
mea-surement of nestedness of fish assemblages. Oecologia 92: 416–428.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Appendix S1. List of fish species sampled at 10 floodplain lakes (five each of island and coastal lake types) in the floodplain of the Solim~oes River (Amazonas–Brazil, 2006). Order (O), family (F) and italicized genus and species are given.