organic papers
Acta Cryst.(2006). E62, o5–o7 doi:10.1107/S1600536805038791 Gokul Rajet al. C
6H10N3O2+C2F3O2
o5
Acta Crystallographica Section E Structure Reports
Online
ISSN 1600-5368
L
-Histidinium trifluoroacetate
Srinivasan Gokul Raj,a* Gubendiran Ramesh Kumar,a Rajagopal Mohanaand Ramasamy Jayavelb
aDepartment of Physics, Presidency College,
Chennai 600 005, India, andbCrystal Growth
Centre, Anna University, Chennai 600 025, India
Correspondence e-mail: gokulrajs@yahoo.com
Key indicators
Single-crystal X-ray study T= 293 K
Mean(C–C) = 0.005 A˚ Rfactor = 0.038 wRfactor = 0.108 Data-to-parameter ratio = 5.4
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2006 International Union of Crystallography
Printed in Great Britain – all rights reserved
The title compound, C6H10N3O2 +
C2F3O2
, crystallizes with two histidine cations and two trifluoroacetate anions in the asymmetric unit. The protonated cations and deprotonated anions are linked by a number of intermolecular N—H O hydrogen bonds to form a three-dimensional network.
Comment
Histidine is an interesting molecule from a crystal engineering point of view. It can act as a proton donor, a proton acceptor, a nucleophilic agent and a ligand for complexation with various metal ions. It exists as both orthorhombic and monoclinic polymorphic forms. The crystal structures of l-histidine
(Madden, McGandy & Seeman, 1972; Madden, McGandy, Seeman, Harding & Hoy, 1972) and its complexes, such asl
-histidinium hydrochloride (Fuess & Bartunik 1976; Donohue & Caron 1964), l-histidinium dinitrate (Benali-Cherif et al.,
2002), l-histidinium sulfate (Kumaret al., 2005) and l
-histi-dinium tetrafluorosuccinate (Akkurt et al., 2004), have been reported, and recently the crystal structure of l-histidinium
trichloroacetate (Gokul Raj et al., 2005) has also been published. In the present study, we report the crystal structure ofl-histidinium trifluoroacetate, (I).
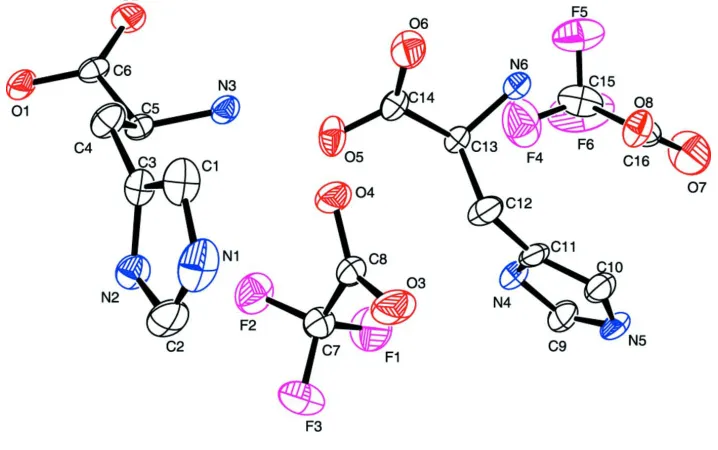
The molecular structure of (I) is illustrated in Fig. 1, and selected bond distances and angles are given in Table 1. Compound (I) crystallizes with two histidinium cations (Aand B) and two trifluoroacetate anions in the unit cell of the non-centrosymmetric triclinic space group P1. The -amino and imidazole groups of thel-histidinium cations are protonated
and positively charged, while the carboxyl groups are in the ionized state and are negatively charged. Moreover, the zwitterionic cation carries a net positive charge. The confor-mation angles of the histidine side chain (IUPAC–IUB Commission on Biochemical Nomenclature, 1970) are 1
[68.5 (4) and69.9 (4)], 21
[54.5 (5) and 54.1 (5)] and
22
[126.2 (4) and 132.0 (4)for cationsAandB, respectively]. The conformation 1is such that it is in agauche-II form for
both molecules, having a closed conformation (Pratap et al., 2000) (see Table 1). The difference in the absolute values of
21 and 22 is about 180, indicating the planarity of the
imidazole group. The deviations in the preferred torsion angles of21at90 and 90are due to the interactions of the
imidazole ring with other groups in the structure. The imida-zole group ofl-histidine is in atransconformation, for bothA
[C3—C4—C5—C6 = 170.3 (3)] and B [C11—C12—C13—
C14 = 168.9 (3)], with respect to the carboxylate group, and
gauchewith respect to the amino N atom. The corresponding gauche-II conformation angles observed in other complexes are60 and60.9inl-histidinium formate formic acid,60
and67.5 indl-histidinium formate monohydrate, 58.2 and
96.8 in l-histidinium glycolate,61.2 (5) and 99.2 (5) in
monoclinicl-histidinium acetate and 37.1 (6) and78.8 (6)
inl-histidinium trichloroacetate.
The trifluoroacetate anions play a vital role in the hydrogen bonding with the histidinium cationviathe amine N atom and the N atom of the imidazole ring. The three-dimensional network of N—H O bonds links the cations and
trifluoro-acetate anions, stabilizing the crystal packing as shown in Fig. 2.
Experimental
Crystals of the title compound, (I), were grown from a mixture ofl
-histidine and trifluoroacetic acid, in the stoichiometric ratio of 1:1, by slow evaporation at room temperature.
Crystal data
C6H10N3O2+C2F3O2
Mr= 269.19 Triclinic,P1
a= 5.1724 (6) A˚
b= 8.8183 (12) A˚
c= 12.481 (3) A˚
= 96.193 (17) = 99.853 (13) = 102.106 (13) V= 542.26 (16) A˚3
Z= 2
Dx= 1.649 Mg m
3 CuKradiation Cell parameters from 25
reflections
= 20–30 = 1.46 mm1
T= 293 (2) K Block, brown 0.20.20.1 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
!/2scans
Absorption correction: scan (Northet al., 1968)
Tmin= 0.790,Tmax= 0.864 2063 measured reflections 2063 independent reflections
2053 reflections withI> 2(I)
max= 68.0
h=6!6
k=10!10
l=14!15 2 standard reflections
frequency: 60 min intensity decay: none
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.038
wR(F2) = 0.108
S= 1.01 2063 reflections 382 parameters
H atoms treated by a mixture of independent and constrained refinement
w= 1/[2
(Fo2) + (0.0775P)2 + 0.22P]
whereP= (Fo2+ 2Fc2)/3 (/)max< 0.001
max= 0.42 e A˚
3
min=0.32 e A˚
3
Extinction correction:SHELXL97
Extinction coefficient: 0.055 (4) Absolute structure: Flack (1983), 97
[image:2.610.46.298.70.231.2]Friedel pairs Flack parameter: 0.04 (2)
Table 1
Selected geometric parameters (A˚ ,).
C1—N1 1.357 (6) C2—N1 1.317 (7) C2—N2 1.323 (5) C3—N2 1.378 (5) C5—N3 1.483 (4) C6—O2 1.226 (4)
C6—O1 1.270 (4) C7—F3 1.333 (5) C7—F1 1.333 (5) C7—F2 1.340 (5) C8—O3 1.231 (5) C8—O4 1.243 (5)
N1—C1—C3 107.6 (4) N1—C2—N2 108.7 (4) C1—C3—N2 105.8 (3) N2—C3—C4 124.3 (3) N3—C5—C6 109.6 (2) N3—C5—C4 111.1 (3) O2—C6—O1 125.9 (3) O2—C6—C5 119.4 (3) O1—C6—C5 114.6 (3) F3—C7—F1 106.8 (3)
F3—C7—F2 106.8 (3) F1—C7—F2 106.2 (3) F3—C7—C8 111.7 (3) F1—C7—C8 112.3 (3) F2—C7—C8 112.7 (3) O3—C8—O4 126.4 (4) O3—C8—C7 116.4 (3) O4—C8—C7 117.2 (3) C2—N1—C1 109.0 (3) C2—N2—C3 108.9 (3)
C1—C3—C4—C5 126.2 (4) N2—C3—C4—C5 54.1 (5) C3—C4—C5—N3 68.5 (4)
C3—C4—C5—C6 170.3 (3) C11—C12—C13—C14 168.9 (3)
organic papers
o6
Gokul Rajet al. C [image:2.610.47.293.282.467.2]6H10N3O2+C2F3O2 Acta Cryst.(2006). E62, o5–o7
Figure 2
The crystal packing of (I), viewed approximately down theaaxis. The intermolecular N—H O bonds are shown as broken lines.
Figure 1
[image:2.610.312.567.536.725.2]Table 2
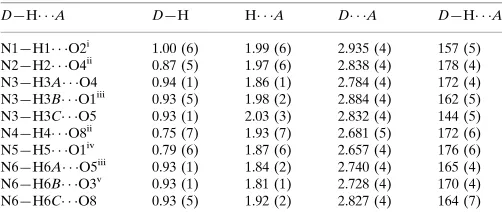
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
N1—H1 O2i
1.00 (6) 1.99 (6) 2.935 (4) 157 (5) N2—H2 O4ii
0.87 (5) 1.97 (6) 2.838 (4) 178 (4) N3—H3A O4 0.94 (1) 1.86 (1) 2.784 (4) 172 (4) N3—H3B O1iii
0.93 (5) 1.98 (2) 2.884 (4) 162 (5) N3—H3C O5 0.93 (1) 2.03 (3) 2.832 (4) 144 (5) N4—H4 O8ii
0.75 (7) 1.93 (7) 2.681 (5) 172 (6) N5—H5 O1iv
0.79 (6) 1.87 (6) 2.657 (4) 176 (6) N6—H6A O5iii 0.93 (1) 1.84 (2) 2.740 (4) 165 (4) N6—H6B O3v
0.93 (1) 1.81 (1) 2.728 (4) 170 (4) N6—H6C O8 0.93 (5) 1.92 (2) 2.827 (4) 164 (7)
Symmetry codes: (i)x1;y1;z; (ii)xþ1;y;z; (iii)x1;y;z; (iv)x1;y;zþ1; (v) x;yþ1;z.
The tertiary CH and secondary CH2H atoms of the histidinium cations were included in calculated positions and treated as riding atoms, with C—H = 0.97–0.98 A˚ and Uiso(H) = 1.2Ueq(parent C atom). All other H atoms were located in difference Fourier maps and refined freely [N—H = 0.75 (7)–1.00 (6) A˚ and C—H = 0.847 (11)–0.85 (6) A˚ ].
Data collection: CAD-4 Software (Enraf–Nonius, 1997); cell refinement: CAD-4 Software; data reduction: XCAD4 in WinGX (Farrugia, 1999); program(s) used to solve structure:SIR92(Burlaet al., 1989); program(s) used to refine structure: SHELXL97 (Shel-drick, 1997); molecular graphics: ORTEP32 (Farrugia, 1997); soft-ware used to prepare material for publication:SHELXL97.
SGR and GRK thank Professor A. K. Misra Dr Babu Varghese, and the Sophisticated Analytical Instrumentation Facility (SAIF), Indian Institute of Technology, Chennai 25, for use of the single-crystal X-ray diffraction facilities.
References
Akkurt, M., O¨ ztu¨rk, S., Ramajothi, J., Bu¨yu¨kgu¨ngor, O. & Dhanuskodi, S. (2004).Acta Cryst.E60, o481–o483.
Benali-Cherif, N., Benguedouar, L., Cherouana, A., Benjeddou, L. & Merazig, H. (2002).Acta Cryst.E58, o822–o824.
Burla, M. C., Camalli, M., Cascarano, G., Giacovazzo, C., Polidori, G., Spagna, R. & Viterbo, D. (1989).J. Appl. Cryst.22, 389–393.
Donohue, J. & Caron, A. (1964).Acta Cryst.17, 1178–1180. Farrugia, L. J. (1997).J. Appl. Cryst.30, 565.
Farrugia, L. J. (1999).J. Appl. Cryst.32, 837–838. Flack, H. D. (1983).Acta Cryst.A39, 876–881.
Fuess, H. & Bartunik, H. D. (1976).Acta Cryst.B32, 2803–2806.
Gokul Raj, S., Ramesh Kumar, G., Thenneti Raghavalu, Kumar, P., Mohan, R., Babu Varghese & Jayavel, R. (2005).Cryst. Growth Des.Submitted. IUPAC–IUB Commission on Biochemical Nomenclature (1970).J. Mol. Biol.
52, 1–17.
Kumar, R. P., Athimoolam, S., Bahadur S. A. & Rajaram, R. K. (2005).Acta Cryst.E61, o2419–o2421.
Madden, J. J., McGandy, E. L. & Seeman, N. C. (1972).Acta Cryst.B28, 2377– 2382.
Madden, J. J., McGandy, E. L., Seeman, N. C., Harding, M. M. & Hoy, A. (1972).Acta Cryst.B28, 2382–2389.
Enraf–Nonius (1997). CAD-4 Software. Enraf–Nonius BV, Delft, The Netherlands.
North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968).Acta Cryst.A24, 351– 359.
Pratap, J. V., Ravishankar, R. & Vijayan, M. (2000).Acta Cryst.B56, 690–696. Sheldrick, G. M. (1997).SHELXL97. University of Go¨ttingen, Germany.
organic papers
Acta Cryst.(2006). E62, o5–o7 Gokul Rajet al. C
supporting information
sup-1
Acta Cryst. (2006). E62, o5–o7
supporting information
Acta Cryst. (2006). E62, o5–o7 [doi:10.1107/S1600536805038791]
L
-Histidinium trifluoroacetate
Srinivasan Gokul Raj, Gubendiran Ramesh Kumar, Rajagopal Mohan and Ramasamy Jayavel
S1. Comment
Histidine is an interesting molecule from a crystal engineering point of view. It can act as a proton donor, a proton
acceptor, a nucleophilic agent and a ligand for complexation with various metal ions. It exists as both orthorhombic and
monclinic polymorphic forms. The crystal structures of L-histidine (Madden, McGandy & Seeman, 1972; Madden,
McGandy, Seeman, Harding & Hoy, 1972) and its complexes, such as L-histidinium hydrochloride (Fuess & Bartunik
1976; Donohue & Caron 1964), L-histidinium dinitrate (Benali-Cherif et al., 2002), L-histidinium sulfate (Kumar et al.,
2005) and L-histidinium tetrafluorosuccinate (Akkurt et al., 2004), have been reported, and recently the crystal structure
of L-histidinium trichloroacetate (Gokulraj et al., 2005) has also been published. In the present study, we report the
crystal structure of L-histidinium trifluoroacetate, (I).
The molecular structure of (I) is illustrated in Fig. 1, and selected bond distances and angles are given in Table 1.
Compound (I) crystallizes with two histidine cations (A and B) and two trifluoroacetate anions in the unit cell of the
non-centrosymmetric triclinic space group P1. The α-amino and imidazole groups of the L-histidine cations are protonated
and positively charged, while the carboxyl groups are in the ionized state and are negatively charged. Moreover, the
zwitterionic cation carries a net positive charge. The conformation angles of the histidine side chain (IUPAC–IUB
Commission on Biochemical Nomenclature, 1970) are χ1 [−68.5 (4) and −69.9 (4)°], χ21 [−54.5 (5) and 54.1 (5)°] and χ22
[126.2 (4) and 132.0 (4)° for cations A and B, respectively]. The conformation ψ1 is such that it is in a gauche II form for
both molecules, having a closed conformation (Pratap et al., 2000) (see Table 1). The difference in the absolute values of
χ21 and χ22 is about 180°, indicating the planarity of the imidazole group. The deviations in the preferred torsion angles of
χ21 at −90 and 90° are due to the interactions of the imidazole ring with other groups in the structure. The imidazole group
of L-histidine is in a trans conformation, for both A [C3—C4—C5—C6 = 170.3 (3)°] and B [C11—C12—C13—C14 =
168.9 (3)°], with respect to the carboxyl group, and gauche with respect to the amino N atom. The corresponding gauche
II conformation angles observed in other complexes are −60 and −60.9° in L-histidine formate formic acid, −60 and
−67.5° in DL-histidine formate monohydrate, −60.2 (3)° in L-histidine dinitrate, 58.2 and −96.8° in L-histidine glycolate,
−61.2 (5) and 99.2 (5)° in monoclinic L-histidine acetate and 37.1 (6) and −78.8 (6)° in L-histidinium trichloroacetate.
The trifluoroacetate anions play a vital role in the hydrogen bonding with the histidinium cation via the amine N atom
and the N atom of the imidazole ring. The three-dimensional network of N—H···O bonds links the cations and
trifluoro-acetate anions stabilizing the crystal packing as shown in Fig. 2.
S2. Experimental
Crystals of the title compound, (I), were grown from a mixture of L-histidine and trifluoroacetic acid, in the
supporting information
sup-2
Acta Cryst. (2006). E62, o5–o7
S3. Refinement
The tertiary CH and secondary CH2 H atoms of the histidinium cations were included in calculated positions and treated
as riding atoms, with C—H = 0.97–0.98 Å and Uiso(H) = 1.2Ueq(parent C atom). All other H atoms were located in
[image:5.610.126.485.143.368.2]difference Fourier maps and refined freely [N—H = 0.75 (7)–1.00 (6) Å and C—H = 0.847 (11)–0.85 (6) Å].
Figure 1
The molecular structure of (I), with the atomic numbering scheme and 50% probability displacement ellipsoids. H atoms
supporting information
sup-3
[image:6.610.133.485.73.342.2]Acta Cryst. (2006). E62, o5–o7
Figure 2
The crystal packing of (I), viewed down the b axis. The intramolecular N—H···O bonds are shown as broken lines.
L-Histidinium trifluoroacetate
Crystal data
C6H10N3O2+·C2F3O2−
Mr = 269.19
Triclinic, P1 Hall symbol: P 1
a = 5.1724 (6) Å
b = 8.8183 (12) Å
c = 12.481 (3) Å
α = 96.193 (17)°
β = 99.853 (13)°
γ = 102.106 (13)°
V = 542.26 (16) Å3
Z = 2
F(000) = 276
Dx = 1.649 Mg m−3
Cu Kα radiation, λ = 1.54180 Å Cell parameters from 25 reflections
θ = 20–30°
µ = 1.46 mm−1
T = 293 K Block, brown 0.2 × 0.2 × 0.1 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω/2θ scans
Absorption correction: ψ scan (North et al., 1968)
Tmin = 0.790, Tmax = 0.864 2064 measured reflections
2063 independent reflections 2053 reflections with I > 2σ(I)
Rint = 0.001
θmax = 68.0°, θmin = 3.6°
h = −6→6
k = −10→10
l = −14→15
supporting information
sup-4
Acta Cryst. (2006). E62, o5–o7 Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.038
wR(F2) = 0.108
S = 1.01 2063 reflections 382 parameters 13 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(F
o2) + (0.0775P)2 + 0.22P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001 Δρmax = 0.42 e Å−3 Δρmin = −0.32 e Å−3
Extinction correction: SHELXL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 Extinction coefficient: 0.055 (4)
Absolute structure: Flack (1983), 92 Friedel pairs
Absolute structure parameter: 0.04 (2)
Special details
Experimental. psi-scan absorption correction: North et al. (1968) Number of psi-scan sets used was 4 Theta correction was applied. Averaged transmission function was used. No Fourier smoothing was applied.
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
C1 0.8441 (8) 0.2285 (5) −0.2787 (4) 0.0460 (9) C2 1.1001 (11) 0.1445 (4) −0.1522 (4) 0.0524 (11) C3 1.0878 (7) 0.3327 (4) −0.2536 (3) 0.0331 (7) C4 1.1832 (8) 0.4790 (4) −0.2996 (3) 0.0379 (8)
H4A 1.0329 0.4972 −0.3507 0.045*
H4B 1.3199 0.4626 −0.3411 0.045*
C5 1.3010 (6) 0.6273 (3) −0.2138 (3) 0.0272 (6)
H5A 1.4381 0.6052 −0.1568 0.033*
C6 1.4345 (6) 0.7612 (4) −0.2695 (3) 0.0289 (6) C7 1.0914 (8) 0.4240 (4) 0.0842 (3) 0.0412 (8) C8 0.8156 (7) 0.3716 (4) 0.0061 (3) 0.0369 (7) C9 0.9856 (9) 0.9431 (6) 0.5115 (4) 0.0523 (10) C10 0.6365 (9) 0.7805 (5) 0.4043 (4) 0.0467 (9) C11 0.8024 (8) 0.8432 (4) 0.3391 (3) 0.0402 (8) C12 0.7871 (10) 0.8073 (4) 0.2184 (3) 0.0462 (9)
H12A 0.6122 0.7387 0.1858 0.055*
H12B 0.9228 0.7497 0.2067 0.055*
C13 0.8268 (6) 0.9487 (4) 0.1569 (3) 0.0303 (6)
H13 0.9902 1.0259 0.1958 0.036*
supporting information
sup-5
Acta Cryst. (2006). E62, o5–o7
C15 0.7585 (11) 1.3651 (5) 0.3768 (5) 0.0661 (14) C16 0.5426 (10) 1.2437 (6) 0.4187 (4) 0.0568 (12) N1 0.8581 (8) 0.1136 (4) −0.2154 (4) 0.0566 (10) N2 1.2439 (7) 0.2766 (3) −0.1733 (3) 0.0378 (6) N3 1.0882 (6) 0.6752 (3) −0.1614 (2) 0.0313 (6) N4 1.0179 (8) 0.9436 (5) 0.4079 (3) 0.0476 (8) N5 0.7568 (8) 0.8435 (5) 0.5115 (3) 0.0503 (9) N6 0.5937 (6) 1.0230 (3) 0.1517 (2) 0.0339 (6) O1 1.6392 (5) 0.7386 (3) −0.3067 (2) 0.0394 (6) O2 1.3431 (5) 0.8785 (3) −0.2730 (2) 0.0411 (6) O3 0.6610 (6) 0.2548 (4) 0.0239 (3) 0.0614 (9) O4 0.7704 (5) 0.4498 (3) −0.0692 (2) 0.0475 (7) O5 1.0912 (5) 0.8762 (4) 0.0320 (2) 0.0484 (7) O6 0.6702 (6) 0.8824 (4) −0.0369 (2) 0.0556 (8) O7 0.4911 (11) 1.2820 (8) 0.5071 (3) 0.1013 (17) O8 0.4445 (7) 1.1223 (4) 0.3506 (3) 0.0530 (7) F1 1.0849 (7) 0.5183 (4) 0.1742 (2) 0.0693 (8) F2 1.2823 (5) 0.5032 (3) 0.0376 (2) 0.0600 (7) F3 1.1793 (6) 0.3021 (3) 0.1180 (3) 0.0637 (7) F4 0.9327 (8) 1.2950 (5) 0.3359 (5) 0.1025 (14) F5 0.6422 (8) 1.4234 (5) 0.2938 (3) 0.0916 (11) F6 0.8936 (13) 1.4847 (5) 0.4500 (4) 0.130 (2) H1 0.710 (12) 0.017 (7) −0.223 (5) 0.058 (14)* H1′ 0.694 (9) 0.218 (10) −0.323 (6) 0.11 (3)* H2 1.407 (11) 0.327 (6) −0.141 (4) 0.044 (12)* H2′ 1.183 (9) 0.101 (6) −0.104 (3) 0.050 (13)* H3A 0.996 (8) 0.596 (4) −0.128 (3) 0.036 (10)* H3B 0.970 (9) 0.714 (6) −0.210 (4) 0.063 (15)* H3C 1.163 (10) 0.758 (4) −0.104 (3) 0.059 (14)* H4 1.141 (12) 0.986 (7) 0.389 (5) 0.057 (16)* H5 0.727 (11) 0.816 (6) 0.567 (5) 0.055 (14)* H6A 0.440 (5) 0.965 (4) 0.103 (3) 0.033 (10)* H6B 0.614 (9) 1.110 (3) 0.115 (3) 0.037 (10)* H6C 0.578 (15) 1.067 (8) 0.221 (3) 0.09 (2)* H9′ 1.104 (10) 0.982 (7) 0.569 (3) 0.075 (18)* H10′ 0.489 (6) 0.713 (5) 0.377 (4) 0.054 (14)*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-6
Acta Cryst. (2006). E62, o5–o7
C9 0.057 (2) 0.074 (3) 0.0308 (19) 0.018 (2) 0.0151 (18) 0.0149 (19) C10 0.059 (2) 0.044 (2) 0.045 (2) 0.0155 (18) 0.0230 (19) 0.0195 (17) C11 0.059 (2) 0.0395 (17) 0.0339 (18) 0.0221 (16) 0.0195 (16) 0.0208 (15) C12 0.076 (3) 0.0374 (18) 0.0330 (18) 0.0198 (18) 0.0196 (18) 0.0114 (15) C13 0.0346 (15) 0.0320 (15) 0.0250 (15) 0.0060 (12) 0.0091 (12) 0.0054 (12) C14 0.0380 (17) 0.0360 (16) 0.0265 (16) 0.0012 (13) 0.0086 (13) 0.0025 (12) C15 0.072 (3) 0.045 (2) 0.067 (3) 0.012 (2) −0.017 (3) −0.001 (2) C16 0.067 (3) 0.075 (3) 0.036 (2) 0.039 (2) 0.0035 (19) 0.002 (2) N1 0.064 (2) 0.0285 (15) 0.072 (3) −0.0132 (15) 0.032 (2) 0.0000 (16) N2 0.0411 (16) 0.0304 (13) 0.0410 (16) 0.0032 (12) 0.0113 (13) 0.0069 (12) N3 0.0371 (14) 0.0268 (12) 0.0321 (14) 0.0051 (10) 0.0120 (11) 0.0101 (11) N4 0.054 (2) 0.064 (2) 0.0331 (17) 0.0189 (17) 0.0187 (15) 0.0212 (15) N5 0.068 (2) 0.064 (2) 0.0354 (17) 0.0283 (18) 0.0267 (16) 0.0268 (16) N6 0.0382 (14) 0.0367 (14) 0.0284 (14) 0.0082 (12) 0.0094 (11) 0.0079 (11) O1 0.0350 (12) 0.0497 (14) 0.0389 (13) 0.0068 (10) 0.0166 (10) 0.0215 (11) O2 0.0462 (13) 0.0314 (11) 0.0503 (14) 0.0079 (10) 0.0146 (11) 0.0208 (11) O3 0.0518 (16) 0.0565 (18) 0.080 (2) 0.0037 (13) 0.0146 (16) 0.0421 (17) O4 0.0412 (13) 0.0536 (15) 0.0528 (16) 0.0075 (11) 0.0136 (12) 0.0299 (13) O5 0.0405 (13) 0.0647 (17) 0.0360 (14) 0.0072 (12) 0.0134 (11) −0.0089 (12) O6 0.0502 (16) 0.083 (2) 0.0282 (13) 0.0158 (15) 0.0020 (12) −0.0051 (13) O7 0.115 (3) 0.161 (5) 0.0402 (19) 0.070 (3) 0.017 (2) −0.010 (2) O8 0.0631 (17) 0.0536 (16) 0.0461 (15) 0.0097 (14) 0.0271 (14) 0.0060 (13) F1 0.0872 (19) 0.0697 (17) 0.0504 (15) 0.0236 (15) 0.0151 (14) −0.0059 (13) F2 0.0432 (13) 0.0721 (17) 0.0599 (15) −0.0005 (11) 0.0103 (11) 0.0160 (13) F3 0.0614 (15) 0.0599 (15) 0.0738 (18) 0.0265 (12) 0.0020 (13) 0.0210 (13) F4 0.078 (2) 0.079 (2) 0.160 (4) 0.0144 (18) 0.047 (3) 0.031 (3) F5 0.107 (3) 0.082 (2) 0.070 (2) 0.0078 (19) −0.0181 (19) 0.0230 (17) F6 0.189 (5) 0.072 (2) 0.079 (3) −0.006 (3) −0.047 (3) −0.0087 (19)
Geometric parameters (Å, º)
C1—N1 1.357 (6) C10—H10′ 0.85 (5)
C1—C3 1.360 (5) C11—N4 1.363 (6)
C1—H1′ 0.85 (6) C11—C12 1.491 (5)
C2—N1 1.317 (7) C12—C13 1.530 (5)
C2—N2 1.323 (5) C12—H12A 0.9700
C2—H2′ 0.847 (11) C12—H12B 0.9700
C3—N2 1.378 (5) C13—N6 1.483 (4)
C3—C4 1.492 (5) C13—C14 1.544 (4)
C4—C5 1.539 (4) C13—H13 0.9800
C4—H4A 0.9700 C14—O6 1.222 (5)
C4—H4B 0.9700 C14—O5 1.255 (5)
C5—N3 1.483 (4) C15—F6 1.303 (6)
C5—C6 1.535 (4) C15—F5 1.316 (6)
C5—H5A 0.9800 C15—F4 1.333 (7)
C6—O2 1.226 (4) C15—C16 1.577 (8)
C6—O1 1.270 (4) C16—O7 1.208 (6)
supporting information
sup-7
Acta Cryst. (2006). E62, o5–o7
C7—F1 1.333 (5) N1—H1 1.00 (6)
C7—F2 1.340 (5) N2—H2 0.87 (5)
C7—C8 1.533 (5) N3—H3A 0.93 (5)
C8—O3 1.231 (5) N3—H3B 0.93 (5)
C8—O4 1.243 (5) N3—H3C 0.93 (5)
C9—N5 1.318 (7) N4—H4 0.75 (7)
C9—N4 1.332 (6) N5—H5 0.79 (6)
C9—H9′ 0.85 (5) N6—H6A 0.93 (5)
C10—C11 1.357 (6) N6—H6B 0.93 (5)
C10—N5 1.377 (6) N6—H6C 0.93 (5)
N1—C1—C3 107.6 (4) C11—C12—H12B 108.3
N1—C1—H1′ 115 (6) C13—C12—H12B 108.3
C3—C1—H1′ 137 (6) H12A—C12—H12B 107.4
N1—C2—N2 108.7 (4) N6—C13—C12 111.0 (3)
N1—C2—H2′ 136 (4) N6—C13—C14 109.4 (3)
N2—C2—H2′ 115 (4) C12—C13—C14 109.9 (3)
C1—C3—N2 105.8 (3) N6—C13—H13 108.8
C1—C3—C4 129.9 (4) C12—C13—H13 108.8
N2—C3—C4 124.3 (3) C14—C13—H13 108.8
C3—C4—C5 115.1 (3) O6—C14—O5 126.7 (3)
C3—C4—H4A 108.5 O6—C14—C13 118.4 (3)
C5—C4—H4A 108.5 O5—C14—C13 114.9 (3)
C3—C4—H4B 108.5 F6—C15—F5 106.3 (4)
C5—C4—H4B 108.5 F6—C15—F4 108.7 (6)
H4A—C4—H4B 107.5 F5—C15—F4 104.6 (5)
N3—C5—C6 109.6 (2) F6—C15—C16 115.0 (5)
N3—C5—C4 111.1 (3) F5—C15—C16 110.5 (4)
C6—C5—C4 109.4 (3) F4—C15—C16 111.1 (4)
N3—C5—H5A 108.9 O7—C16—O8 130.2 (6)
C6—C5—H5A 108.9 O7—C16—C15 117.4 (5)
C4—C5—H5A 108.9 O8—C16—C15 112.4 (4)
O2—C6—O1 125.9 (3) C2—N1—C1 109.0 (3)
O2—C6—C5 119.4 (3) C2—N1—H1 127 (3)
O1—C6—C5 114.6 (3) C1—N1—H1 124 (3)
F3—C7—F1 106.8 (3) C2—N2—C3 108.9 (3)
F3—C7—F2 106.8 (3) C2—N2—H2 128 (3)
F1—C7—F2 106.2 (3) C3—N2—H2 123 (3)
F3—C7—C8 111.7 (3) C5—N3—H3A 113 (3)
F1—C7—C8 112.3 (3) C5—N3—H3B 112 (4)
F2—C7—C8 112.7 (3) H3A—N3—H3B 112 (4)
O3—C8—O4 126.4 (4) C5—N3—H3C 111 (3)
O3—C8—C7 116.4 (3) H3A—N3—H3C 104 (4)
O4—C8—C7 117.2 (3) H3B—N3—H3C 105 (5)
N5—C9—N4 108.4 (4) C9—N4—C11 109.5 (4)
N5—C9—H9′ 123 (5) C9—N4—H4 126 (5)
N4—C9—H9′ 127 (5) C11—N4—H4 124 (4)
supporting information
sup-8
Acta Cryst. (2006). E62, o5–o7
C11—C10—H10′ 121 (4) C9—N5—H5 120 (4)
N5—C10—H10′ 132 (4) C10—N5—H5 130 (4)
C10—C11—N4 106.2 (4) C13—N6—H6A 113 (3)
C10—C11—C12 130.8 (4) C13—N6—H6B 113 (3)
N4—C11—C12 122.8 (4) H6A—N6—H6B 96 (4)
C11—C12—C13 116.0 (3) C13—N6—H6C 112 (5)
C11—C12—H12A 108.3 H6A—N6—H6C 119 (5)
C13—C12—H12A 108.3 H6B—N6—H6C 102 (5)
N1—C1—C3—N2 −0.5 (4) C11—C12—C13—C14 168.9 (3) N1—C1—C3—C4 179.2 (3) N6—C13—C14—O6 −19.2 (4) C1—C3—C4—C5 126.2 (4) C12—C13—C14—O6 102.9 (4) N2—C3—C4—C5 −54.1 (5) N6—C13—C14—O5 159.8 (3) C3—C4—C5—N3 −68.5 (4) C12—C13—C14—O5 −78.1 (4) C3—C4—C5—C6 170.3 (3) F6—C15—C16—O7 11.0 (7) N3—C5—C6—O2 −5.9 (4) F5—C15—C16—O7 −109.4 (5) C4—C5—C6—O2 116.2 (3) F4—C15—C16—O7 135.0 (5) N3—C5—C6—O1 172.8 (3) F6—C15—C16—O8 −171.2 (5) C4—C5—C6—O1 −65.1 (3) F5—C15—C16—O8 68.4 (5) F3—C7—C8—O3 33.6 (5) F4—C15—C16—O8 −47.2 (5) F1—C7—C8—O3 −86.3 (4) N2—C2—N1—C1 −0.2 (5) F2—C7—C8—O3 153.9 (4) C3—C1—N1—C2 0.5 (5) F3—C7—C8—O4 −145.9 (4) N1—C2—N2—C3 −0.1 (4)
F1—C7—C8—O4 94.2 (4) C1—C3—N2—C2 0.4 (4)
F2—C7—C8—O4 −25.6 (5) C4—C3—N2—C2 −179.3 (3) N5—C10—C11—N4 −0.5 (4) N5—C9—N4—C11 0.9 (5) N5—C10—C11—C12 173.7 (4) C10—C11—N4—C9 −0.2 (5) C10—C11—C12—C13 132.0 (4) C12—C11—N4—C9 −175.0 (4) N4—C11—C12—C13 −54.6 (5) N4—C9—N5—C10 −1.2 (5) C11—C12—C13—N6 −69.9 (4) C11—C10—N5—C9 1.0 (5)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
N1—H1···O2i 1.00 (6) 1.99 (6) 2.935 (4) 157 (5) N2—H2···O4ii 0.87 (5) 1.97 (6) 2.838 (4) 178 (4) N3—H3A···O4 0.94 (1) 1.86 (1) 2.784 (4) 172 (4) N3—H3B···O1iii 0.93 (5) 1.98 (2) 2.884 (4) 162 (5) N3—H3C···O5 0.93 (1) 2.03 (3) 2.832 (4) 144 (5) N4—H4···O8ii 0.75 (7) 1.93 (7) 2.681 (5) 172 (6) N5—H5···O1iv 0.79 (6) 1.87 (6) 2.657 (4) 176 (6) N6—H6A···O5iii 0.93 (1) 1.84 (2) 2.740 (4) 165 (4) N6—H6B···O3v 0.93 (1) 1.81 (1) 2.728 (4) 170 (4) N6—H6C···O8 0.93 (5) 1.92 (2) 2.827 (4) 164 (7)