metal-organic papers
m158
Lipstman and Goldberg [Zn(C44H28N4)(C3H7NO)] doi:10.1107/S1600536805042224 Acta Cryst.(2006). E62, m158–m160 Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
Unsolvated
(dimethylformamide)(tetraphenyl-porphyrinato)zinc(II)
Sophia Lipstman and Israel Goldberg*
School of Chemistry, Sackler Faculty of Exact Sciences, Tel-Aviv University, Ramat-Aviv, 69978 Tel-Aviv, Israel
Correspondence e-mail: goldberg@post.tau.ac.il
Key indicators
Single-crystal X-ray study T= 110 K
Mean(C–C) = 0.004 A˚ Rfactor = 0.052 wRfactor = 0.132
Data-to-parameter ratio = 17.8
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2006 International Union of Crystallography Printed in Great Britain – all rights reserved
The title compound, [Zn(C44H28N4)(C3H7NO)], is a
square-pyramidal five-coordinate zinc–porphyrin complex withN,N0
-dimethylformamide as an apical ligand. The crystal structure is characterized by a layered organization of the porphyrin species with tight stacking of the corrugated layers along theb
axis.
Comment
Numerous four-, five- and six-coordinate complexes of metallo-tetraphenylporphyrins have been reported with a wide variety of crystallization solvents (many of these compounds form clathrates) and axial/apical ligands coord-inated to the metal centre (Byrn,et al., 1991; Byrnet al., 1993). Porphyrin complexes with dimethylformamide as an axial or apical ligand include ZnII-tetra(4-hydroxyphenyl)porphyrin (Goldberget al., 1995), MnIII-tetraphenylporphyrin perchlor-ate (Hill & Williamson, 1985), ZrIV-tetraphenylporphyrin dichloride (Tutasset al., 2002), and ZnII-tetraphenylporphyrin (Droege et al., 1994). The latter crystallized as a dichloro-methane solvate. The present report provides information on the title five-coordinate complex, (I) (Fig. 1), determined at low temperature (ca110 K).
In the crystal structure, the porphyrin core is slightly saddled, with the inner pyrrole N atoms deviating
0.023 (1) A˚ from their mean plane. The zinc ion is displaced by 0.329 (1) A˚ from this N4 plane towards the apical ligand,
imparting to the metalloporphyrin entity a domed structure (Fig. 2), which is typical of many five-coordinate complexes of metallated tetraarylporphyrins (Allen, 2002).
Efficient crystal packing of the molecules of (I) (Fig. 3) is realized by corrugated layers of the metalloporphyrin species running perpendicular to the b axis. Adjacent
related layers fit tightly into one another; the apical ligands of one layer are inserted between the porphyrin frameworks of neighbouring layers. The parallel offset of these layers allows a close van der Waals contact between the concave surface of a given metalloporphyrin core and a phenyl ring from another layer. The corresponding shortest non-bonding distances include: C4 C35(1x, y, 2z) = 3.583 (2) A˚ , N22 C36(1x, y, 2z) = 3.459 (2) A˚ , and C6 C36(1x, y, 2z) = 3.463 (2) A˚ . This concave surface is in van der Waals contact with one of the methyl residues from the nearest layer translated alongb. The apical ligand from one layer penetrates through the nearest layer, approaching the porphyrin core [N24 C52(x, 1 +y, z) = 3.776 (3) A˚ ]. The geometry of the coordination around the zinc ion (Table 1) shows standard values (Allen, 2002, Vinodu & Goldberg, 2004).
Experimental
The crystals of the title compound were obtained as a side product by dissolving zinc–tetraphenylporphyrin (6.8 mg, 0.01 mmol) in N,N0
-dimethylformamide (2 ml) in the presence of tris(acetoneperoxide) (2.2 mg, 0.01 mmol) while attempting to generate new porphyrin complexes.
Crystal data
[Zn(C44H28N4)(C3H7NO)]
Mr= 751.17
Monoclinic,P21=c a= 13.7419 (3) A˚
b= 9.2278 (2) A˚
c= 29.3016 (7) A˚ = 101.7186 (13)
V= 3638.22 (14) A˚3
Z= 4
Dx= 1.371 Mg m 3
MoKradiation Cell parameters from 8468
reflections = 2.3–28.2
= 0.72 mm1
T= 110 (2) K Prism, red
0.200.150.15 mm
Data collection
Nonius KappaCCD diffractometer ’and!scans
29748 measured reflections 8697 independent reflections
Rint= 0.059
max= 28.2
h=18!18
k=12!12
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.052
wR(F2) = 0.132
S= 1.03 8697 reflections 489 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.058P)2
+ 1.2438P]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.003 max= 0.85 e A˚
3 min=0.64 e A˚ 3
Table 1
Selected geometric parameters (A˚ ,).
Zn—N22 2.054 (2)
Zn—N24 2.065 (2)
Zn—N23 2.073 (2)
Zn—N21 2.091 (2)
Zn—O49 2.150 (2)
N22—Zn—N24 162.90 (9)
N22—Zn—N23 88.74 (8)
N24—Zn—N23 88.64 (8)
N22—Zn—N21 88.81 (8)
N24—Zn—N21 88.04 (8)
N23—Zn—N21 160.51 (9)
N22—Zn—O49 99.08 (8)
N24—Zn—O49 98.01 (8)
N23—Zn—O49 96.81 (8)
N21—Zn—O49 102.67 (8)
[image:2.610.47.295.69.247.2]C50—O49—Zn 126.0 (2)
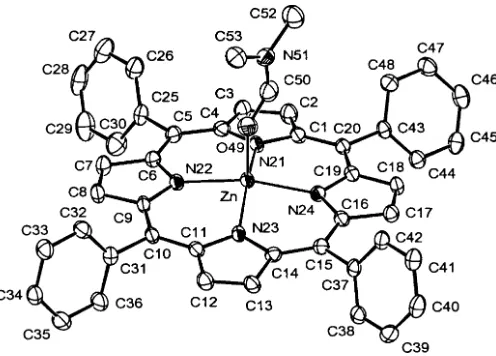
Figure 1
Molecular structure of the title compound, with the atom-labelling scheme. Displacement parameters are drawn at the 50% probablity level.
H atoms have been omitted. Figure 2
Stick illustration of the domed molecular structure. The Zn atom is represented by a small sphere. Colour code: C dark grey, H white, N blue, O red, Zn violet.
Figure 3
[image:2.610.312.563.73.263.2] [image:2.610.314.564.322.446.2]H atoms were placed in calculated positions and constrained to ride on their parent atoms, with C—H distances in the range 0.95– 0.98 A˚ and withUiso(H) = 1.2 or 1.5 timesUeq(C).
Data collection: COLLECT (Nonius, 1999); cell refinement: DENZO (Otwinowski & Minor, 1997); data reduction: DENZO; program(s) used to solve structure: SIR92 (Altomareet al., 1994); program(s) used to refine structure:SHELXL97(Sheldrick, 1997); molecular graphics: ORTEPIII (Burnett & Johnson, 1996) and MERCURY(Brunoet al., 2002); software used to prepare material for publication:SHELXL97(Sheldrick, 1997).
This research was supported in part by the Israel Science Foundation (grant No. 254/04).
References
Allen, F. H. (2002).Acta Cryst.B58, 380–388.
Altomare, A., Burla, M. C., Camalli, M., Cascarano, M., Giacovazzo, C., Guagliardi, A. & Polidori, G. (1994).J. Appl. Cryst.27, 435.
Bruno, I. J., Cole, J. C., Edgington, P. R., Kessler, M., Macrae, C. F., McCabe, P., Pearson, J. & Taylor, R. (2002).Acta Cryst.B58, 389–397.
Burnett, M. N. and Johnson, C. K. (1996).ORTEPIII. Oak Ridge National Laboratory Report ORNL-6895, Oak Ridge, Tennessee, USA.
Byrn, M. P., Curtis, C. J., Goldberg, I., Hsiou, Y.; Khan, S. I., Sawin, P. A., Tendick, S. K. & Strouse, C. E. (1991).J. Am. Chem. Soc.113, 6549– 6557.
Byrn, M. P., Curtis, C. J., Hsiou, Y., Khan, S. I., Sawin, P. A., Tendick, S. K., Terzis, A. & Strouse, C. E. (1993).J. Am. Chem. Soc.115, 9480– 9497.
Droege, P. A., Barnes, C. L. & Schlemper, E. O. (1994).J. Chem. Cryst.24, 111– 116.
Goldberg, I., Krupitsky, H., Stein, Z., Hsiou, Y. & Strouse, C. E. (1995).
Supramol. Chem.4, 203–221.
Hill, C. L. & Williamson, M. M. (1985).Inorg. Chem.24, 2836–2841. Nonius (1999).COLLECT. Nonius, Delft, The Netherlands.
Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276,
Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
Sheldrick, G. M. (1997).SHELXL97. University of Go¨ttingen, Germany. Tutass, A., Klopfer, M., Huckstadt, H. Cornelissen, U. & Homborg, H. (2002).
Z. Anorg. Allg. Chem.628, 1027–1044.
Vinodu, M. & Goldberg, I. (2004).Acta Cryst.E60, m579–m581.
metal-organic papers
m160
Lipstman and Goldberg [Zn(Csupporting information
Acta Cryst. (2006). E62, m158–m160 [doi:10.1107/S1600536805042224]
Unsolvated (dimethylformamide)(tetraphenylporphyrinato)zinc(II)
Sophia Lipstman and Israel Goldberg
S1. Comment
Numerous four-coordinate, five-coordinate and six-coordinate complexes of metallo-tetraphenylporphyrin have been
reported with a wide variety of crystallization solvents (many of these compounds form clathrates) and axial ligands
coordinated to the metal centre (Byrn, et al., 1991; Byrn et al., 1993). Porphyrin complexes with dimethylformamide as
an axial ligand include ZnII-tetra(4-hydroxyphenyl)porphyrin (Goldberg et al., 1995), MnIII-tetraphenylporphyrin
perchlorate (Hill & Williamson, 1985), ZrIV-tetraphenylporphyrin dichloride (Tutass et al., 2002), and ZnII
-tetraphenyl-porphyrin (Droege et al., 1994). The latter crystallized as a dichloromethane solvate. The present report provides
information on the five-coordinate complex of the title compound, (I) (Fig. 1), determined at low temperature (ca 110 K).
In the crystal structure, the porphyrin core is slightly saddled with the inner pyrrole N atoms deviating ±0.023 (1) Å
from their mean plane. The zinc ion is shifted by 0.329 (1) Å from this N4 plane towards the axial ligand, imparting to the
metalloporphyrin entity a domed structure (Fig. 2), which is typical of many five-coordinate complexes of metallated
tetraarylporphyrins (Allen, 2002).
Efficient crystal packing of the molecular units of (I) (Fig. 3) is realised by waved layers of the metalloporphyrin
species running perpendicular to the b axis. Adjacent inversion-related layers fit tightly into one another; the axial ligands
of one layer are inserted between the porphyrin frameworks of neighbouring layers. The parallel offset of these layers
allows a close van der Waals contact between the concave surface of a given metalloporphyrin core and a phenyl ring
from another layer. The corresponding shortest non-bonding distances include: C4···C35(1 − x, −y, 2 − z) = 3.583 (2) Å,
N22···C36(1 − x, −y, 2 − z) = 3.459 (2) Å, and C6···C36(1 − x, −y, 2 − z) = 3.463 (2) Å. This concave surface is in van der
Waals contact with one of the methyl residues from the nearest layer translated along b. The axial ligand from one layer
penetrates through the nearest layer approaching the porphyrin core [N24···C52(x, 1 + y, z) =3.776 (3) Å]. The geometry
of the coordination around the zinc ion (Table 1) shows already known values (Allen, 2002, Vinodu & Goldberg, 2004).
S2. Experimental
The crystals of the title compound were obtained as a side product by dissolving zinc-tetraphenylporphyrin in N,N
′-di-methylformamide in the presence of tris(acetoneperoxide) while attempting to formulate new porphyrin complexes.
S3. Refinement
H atoms were placeded in calculated positions and were constrained to ride on their parent atoms, with C—H distances in
supporting information
[image:5.610.119.491.69.320.2]sup-2
Acta Cryst. (2006). E62, m158–m160Figure 1
Molecular structure of the title compound, with the atom-labelling scheme. Displacement parameters are drawn at the
50% probablity level.
Figure 2
Stick illustration of the domed molecular structure. The Zn atom is represented by a small sphere. Colour code: C dark
[image:5.610.130.484.377.648.2]Figure 3
Crystal packing of the title compound, illustrating the tight layered intermolecular organization. Hatoms are omitted for
clarity. Zn atoms are represented by small spheres. Colour code: C dark grey, N blue, O red, Zn violet.
(dimethylformamide)(tetraphenylporphyrinato)zinc(II)
Crystal data
[Zn(C44H28N4)(C3H7NO)] Mr = 751.17
Monoclinic, P21/c Hall symbol: -P 2ybc a = 13.7419 (3) Å b = 9.2278 (2) Å c = 29.3016 (7) Å β = 101.7186 (13)° V = 3638.22 (14) Å3
Z = 4
F(000) = 1560 Dx = 1.371 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 8468 reflections θ = 2.3–28.2°
µ = 0.72 mm−1 T = 110 K Prism, red
0.20 × 0.15 × 0.15 mm
Data collection Nonius KappaCCD
diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
Detector resolution: 56 microns pixels mm-1 φ and ω scans
29748 measured reflections
8697 independent reflections 5627 reflections with I > 2σ(I) Rint = 0.059
θmax = 28.2°, θmin = 2.3° h = −18→18
k = −12→12 l = −38→37
Refinement Refinement on F2 Least-squares matrix: full R[F2 > 2σ(F2)] = 0.052 wR(F2) = 0.132 S = 1.03 8697 reflections 489 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[σ2(F
o2) + (0.058P)2 + 1.2438P] where P = (Fo2 + 2Fc2)/3
supporting information
sup-4
Acta Cryst. (2006). E62, m158–m160Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Zn 0.27658 (2) 0.24348 (3) 0.894088 (10) 0.02084 (10) C1 0.04751 (18) 0.2034 (3) 0.88257 (9) 0.0231 (6) C2 −0.0296 (2) 0.1944 (3) 0.90945 (9) 0.0300 (7)
H2 −0.0977 0.1731 0.8977 0.036*
C3 0.0130 (2) 0.2218 (3) 0.95413 (9) 0.0293 (7)
H3 −0.0195 0.2233 0.9798 0.035*
C4 0.11684 (19) 0.2483 (3) 0.95587 (9) 0.0232 (6) C5 0.18809 (19) 0.2700 (3) 0.99681 (9) 0.0238 (6) C6 0.29151 (19) 0.2725 (3) 1.00014 (9) 0.0230 (6) C7 0.3634 (2) 0.2901 (3) 1.04308 (9) 0.0285 (7)
H7 0.3495 0.3074 1.0731 0.034*
C8 0.4543 (2) 0.2772 (3) 1.03261 (9) 0.0281 (7)
H8 0.5163 0.2837 1.0539 0.034*
C9 0.43969 (18) 0.2517 (3) 0.98299 (8) 0.0221 (6) C10 0.51630 (18) 0.2214 (3) 0.95946 (9) 0.0222 (6) C11 0.50308 (18) 0.1851 (3) 0.91205 (9) 0.0227 (6) C12 0.58215 (19) 0.1554 (3) 0.88778 (9) 0.0271 (6)
H12 0.6512 0.1535 0.9011 0.033*
C13 0.54055 (18) 0.1309 (3) 0.84283 (9) 0.0257 (6)
H13 0.5746 0.1089 0.8185 0.031*
C14 0.43358 (18) 0.1444 (3) 0.83833 (9) 0.0218 (6) C15 0.36284 (18) 0.1337 (3) 0.79652 (9) 0.0219 (6) C16 0.25902 (18) 0.1433 (3) 0.79266 (8) 0.0216 (6) C17 0.18718 (18) 0.1212 (3) 0.74991 (9) 0.0239 (6)
H17 0.2012 0.1014 0.7201 0.029*
C18 0.09645 (18) 0.1340 (3) 0.76037 (8) 0.0234 (6)
H18 0.0345 0.1256 0.7391 0.028*
H26 0.0869 0.4795 1.0300 0.041* C27 0.0680 (2) 0.4127 (4) 1.09381 (11) 0.0427 (8)
H27 0.0321 0.4955 1.1003 0.051*
C28 0.0883 (2) 0.3026 (4) 1.12576 (11) 0.0463 (9)
H28 0.0672 0.3103 1.1546 0.056*
C29 0.1386 (2) 0.1820 (4) 1.11649 (11) 0.0475 (9)
H29 0.1520 0.1059 1.1387 0.057*
C30 0.1705 (2) 0.1706 (4) 1.07419 (10) 0.0411 (8)
H30 0.2050 0.0864 1.0676 0.049*
C31 0.62109 (19) 0.2259 (3) 0.98724 (9) 0.0237 (6) C32 0.6652 (2) 0.3566 (3) 1.00331 (10) 0.0318 (7)
H32 0.6284 0.4441 0.9975 0.038*
C33 0.76340 (19) 0.3599 (3) 1.02803 (10) 0.0315 (7)
H33 0.7934 0.4499 1.0385 0.038*
C34 0.8170 (2) 0.2336 (3) 1.03736 (9) 0.0288 (7)
H34 0.8841 0.2363 1.0537 0.035*
C35 0.7725 (2) 0.1032 (4) 1.02281 (10) 0.0375 (7)
H35 0.8084 0.0154 1.0301 0.045*
C36 0.6752 (2) 0.0996 (3) 0.99752 (10) 0.0344 (7)
H36 0.6455 0.0092 0.9872 0.041*
C37 0.39887 (17) 0.1146 (3) 0.75206 (9) 0.0227 (6) C38 0.45292 (17) −0.0066 (3) 0.74393 (9) 0.0255 (6)
H38 0.4690 −0.0788 0.7674 0.031*
C39 0.48369 (18) −0.0235 (3) 0.70193 (10) 0.0306 (7)
H39 0.5218 −0.1059 0.6972 0.037*
C40 0.45926 (19) 0.0787 (4) 0.66696 (10) 0.0330 (7)
H40 0.4785 0.0650 0.6379 0.040*
C41 0.4069 (2) 0.2004 (4) 0.67459 (10) 0.0317 (7)
H41 0.3915 0.2721 0.6510 0.038*
C42 0.37632 (19) 0.2189 (3) 0.71677 (9) 0.0262 (6)
H42 0.3399 0.3030 0.7216 0.031*
C43 −0.07116 (17) 0.1582 (3) 0.80790 (8) 0.0217 (6) C44 −0.10151 (18) 0.0328 (3) 0.78242 (9) 0.0250 (6)
H44 −0.0553 −0.0435 0.7820 0.030*
C45 −0.19850 (19) 0.0183 (3) 0.75757 (9) 0.0288 (6)
H45 −0.2179 −0.0669 0.7399 0.035*
C46 −0.2667 (2) 0.1274 (4) 0.75856 (10) 0.0329 (7)
H46 −0.3331 0.1171 0.7417 0.040*
C47 −0.2383 (2) 0.2517 (3) 0.78414 (10) 0.0325 (7)
H47 −0.2853 0.3265 0.7851 0.039*
C48 −0.14061 (19) 0.2674 (3) 0.80847 (10) 0.0283 (6)
H48 −0.1213 0.3537 0.8256 0.034*
O49 0.29027 (15) 0.4680 (2) 0.87671 (7) 0.0377 (5) C50 0.2233 (2) 0.5474 (4) 0.86032 (10) 0.0376 (8)
H50 0.1587 0.5066 0.8519 0.045*
N51 0.23272 (17) 0.6899 (3) 0.85300 (8) 0.0305 (5) C52 0.1458 (2) 0.7731 (4) 0.83234 (12) 0.0443 (9)
supporting information
sup-6
Acta Cryst. (2006). E62, m158–m160H52B 0.1559 0.8169 0.8032 0.066*
H52C 0.1350 0.8496 0.8540 0.066*
C53 0.3246 (2) 0.7675 (4) 0.86449 (12) 0.0428 (8)
H53A 0.3795 0.6987 0.8733 0.064*
H53B 0.3229 0.8331 0.8906 0.064*
H53C 0.3343 0.8238 0.8374 0.064*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C38 0.0164 (12) 0.0334 (17) 0.0253 (14) −0.0013 (11) 0.0011 (11) −0.0036 (12) C39 0.0201 (13) 0.0369 (19) 0.0349 (17) 0.0015 (12) 0.0060 (12) −0.0108 (14) C40 0.0280 (14) 0.049 (2) 0.0227 (15) −0.0069 (14) 0.0076 (12) −0.0090 (14) C41 0.0319 (15) 0.0414 (19) 0.0218 (15) −0.0058 (14) 0.0052 (12) −0.0023 (13) C42 0.0234 (13) 0.0348 (18) 0.0200 (14) −0.0007 (12) 0.0037 (11) −0.0035 (12) C43 0.0197 (12) 0.0301 (17) 0.0156 (13) −0.0004 (11) 0.0040 (10) 0.0033 (11) C44 0.0256 (13) 0.0305 (17) 0.0195 (13) 0.0002 (12) 0.0057 (11) 0.0028 (12) C45 0.0322 (14) 0.0340 (18) 0.0200 (14) −0.0105 (13) 0.0049 (12) 0.0001 (12) C46 0.0225 (13) 0.046 (2) 0.0284 (15) −0.0091 (13) 0.0005 (12) 0.0043 (14) C47 0.0203 (13) 0.042 (2) 0.0341 (16) 0.0052 (13) 0.0032 (12) −0.0002 (15) C48 0.0231 (13) 0.0314 (18) 0.0297 (15) 0.0024 (12) 0.0035 (12) −0.0017 (13) O49 0.0475 (12) 0.0383 (14) 0.0270 (11) 0.0116 (11) 0.0073 (10) 0.0032 (10) C50 0.0302 (15) 0.054 (2) 0.0288 (17) −0.0022 (15) 0.0059 (13) −0.0073 (15) N51 0.0428 (14) 0.0229 (14) 0.0255 (13) 0.0003 (11) 0.0062 (11) 0.0015 (11) C52 0.0319 (16) 0.056 (2) 0.044 (2) 0.0019 (15) 0.0048 (14) −0.0003 (17) C53 0.0323 (16) 0.049 (2) 0.0438 (19) 0.0049 (15) 0.0003 (14) −0.0047 (16)
Geometric parameters (Å, º)
Zn—N22 2.054 (2) C27—H27 0.9500
Zn—N24 2.065 (2) C28—C29 1.367 (5)
Zn—N23 2.073 (2) C28—H28 0.9500
Zn—N21 2.091 (2) C29—C30 1.400 (4)
Zn—O49 2.150 (2) C29—H29 0.9500
C1—N21 1.376 (3) C30—H30 0.9500
C1—C20 1.404 (3) C31—C36 1.383 (4)
C1—C2 1.445 (4) C31—C32 1.388 (4)
C2—C3 1.345 (4) C32—C33 1.396 (4)
C2—H2 0.9500 C32—H32 0.9500
C3—C4 1.438 (4) C33—C34 1.376 (4)
C3—H3 0.9500 C33—H33 0.9500
C4—N21 1.381 (3) C34—C35 1.378 (4)
C4—C5 1.400 (4) C34—H34 0.9500
C5—C6 1.405 (4) C35—C36 1.391 (4)
C5—C25 1.506 (3) C35—H35 0.9500
C6—N22 1.374 (3) C36—H36 0.9500
C6—C7 1.442 (4) C37—C38 1.389 (4)
C7—C8 1.350 (4) C37—C42 1.401 (4)
C7—H7 0.9500 C38—C39 1.389 (4)
C8—C9 1.446 (4) C38—H38 0.9500
C8—H8 0.9500 C39—C40 1.383 (4)
C9—N22 1.375 (3) C39—H39 0.9500
C9—C10 1.399 (4) C40—C41 1.376 (4)
C10—C11 1.404 (4) C40—H40 0.9500
C10—C31 1.504 (3) C41—C42 1.395 (4)
C11—N23 1.382 (3) C41—H41 0.9500
C11—C12 1.441 (4) C42—H42 0.9500
supporting information
sup-8
Acta Cryst. (2006). E62, m158–m160C12—H12 0.9500 C43—C44 1.394 (4)
C13—C14 1.454 (3) C44—C45 1.389 (4)
C13—H13 0.9500 C44—H44 0.9500
C14—N23 1.381 (3) C45—C46 1.380 (4)
C14—C15 1.404 (3) C45—H45 0.9500
C15—C16 1.411 (3) C46—C47 1.383 (4)
C15—C37 1.495 (3) C46—H46 0.9500
C16—N24 1.377 (3) C47—C48 1.393 (4)
C16—C17 1.443 (4) C47—H47 0.9500
C17—C18 1.348 (3) C48—H48 0.9500
C17—H17 0.9500 O49—C50 1.199 (3)
C18—C19 1.435 (3) C50—N51 1.342 (4)
C18—H18 0.9500 C50—H50 0.9500
C19—N24 1.377 (3) N51—C53 1.431 (4)
C19—C20 1.411 (3) N51—C52 1.445 (4)
C20—C43 1.501 (3) C52—H52A 0.9800
C25—C26 1.383 (4) C52—H52B 0.9800
C25—C30 1.386 (4) C52—H52C 0.9800
C26—C27 1.390 (4) C53—H53A 0.9800
C26—H26 0.9500 C53—H53B 0.9800
C27—C28 1.371 (5) C53—H53C 0.9800
N22—Zn—N24 162.90 (9) C27—C26—H26 119.6
N22—Zn—N23 88.74 (8) C28—C27—C26 119.7 (3)
N24—Zn—N23 88.64 (8) C28—C27—H27 120.1
N22—Zn—N21 88.81 (8) C26—C27—H27 120.1
N24—Zn—N21 88.04 (8) C29—C28—C27 120.6 (3)
N23—Zn—N21 160.51 (9) C29—C28—H28 119.7
N22—Zn—O49 99.08 (8) C27—C28—H28 119.7
N24—Zn—O49 98.01 (8) C28—C29—C30 119.9 (3)
N23—Zn—O49 96.81 (8) C28—C29—H29 120.0
N21—Zn—O49 102.67 (8) C30—C29—H29 120.0
N21—C1—C20 125.6 (2) C25—C30—C29 120.2 (3)
N21—C1—C2 109.5 (2) C25—C30—H30 119.9
C20—C1—C2 124.8 (2) C29—C30—H30 119.9
C3—C2—C1 107.3 (2) C36—C31—C32 118.7 (2)
C3—C2—H2 126.3 C36—C31—C10 120.5 (2)
C1—C2—H2 126.3 C32—C31—C10 120.8 (2)
C2—C3—C4 107.4 (2) C31—C32—C33 120.3 (3)
C2—C3—H3 126.3 C31—C32—H32 119.8
C4—C3—H3 126.3 C33—C32—H32 119.8
N21—C4—C5 125.4 (2) C34—C33—C32 120.4 (3)
N21—C4—C3 109.6 (2) C34—C33—H33 119.8
C5—C4—C3 124.8 (2) C32—C33—H33 119.8
C4—C5—C6 125.7 (2) C33—C34—C35 119.5 (3)
C4—C5—C25 117.6 (2) C33—C34—H34 120.3
C6—C5—C25 116.6 (2) C35—C34—H34 120.3
N22—C6—C7 109.7 (2) C34—C35—H35 119.9
C5—C6—C7 124.5 (2) C36—C35—H35 119.9
C8—C7—C6 107.0 (2) C31—C36—C35 120.8 (3)
C8—C7—H7 126.5 C31—C36—H36 119.6
C6—C7—H7 126.5 C35—C36—H36 119.6
C7—C8—C9 107.3 (2) C38—C37—C42 118.2 (2)
C7—C8—H8 126.4 C38—C37—C15 121.8 (2)
C9—C8—H8 126.4 C42—C37—C15 120.0 (2)
N22—C9—C10 126.2 (2) C39—C38—C37 120.8 (3)
N22—C9—C8 109.4 (2) C39—C38—H38 119.6
C10—C9—C8 124.3 (2) C37—C38—H38 119.6
C9—C10—C11 125.2 (2) C40—C39—C38 120.6 (3)
C9—C10—C31 117.5 (2) C40—C39—H39 119.7
C11—C10—C31 117.3 (2) C38—C39—H39 119.7
N23—C11—C10 125.2 (2) C41—C40—C39 119.5 (3)
N23—C11—C12 109.7 (2) C41—C40—H40 120.3
C10—C11—C12 125.1 (2) C39—C40—H40 120.3
C13—C12—C11 107.6 (2) C40—C41—C42 120.4 (3)
C13—C12—H12 126.2 C40—C41—H41 119.8
C11—C12—H12 126.2 C42—C41—H41 119.8
C12—C13—C14 107.2 (2) C41—C42—C37 120.6 (3)
C12—C13—H13 126.4 C41—C42—H42 119.7
C14—C13—H13 126.4 C37—C42—H42 119.7
N23—C14—C15 125.1 (2) C48—C43—C44 118.5 (2) N23—C14—C13 109.3 (2) C48—C43—C20 119.9 (2) C15—C14—C13 125.5 (2) C44—C43—C20 121.7 (2) C14—C15—C16 125.1 (2) C45—C44—C43 120.8 (3)
C14—C15—C37 118.4 (2) C45—C44—H44 119.6
C16—C15—C37 116.5 (2) C43—C44—H44 119.6
N24—C16—C15 126.0 (2) C46—C45—C44 120.1 (3)
N24—C16—C17 109.7 (2) C46—C45—H45 119.9
C15—C16—C17 124.3 (2) C44—C45—H45 119.9
C18—C17—C16 107.0 (2) C45—C46—C47 119.9 (3)
C18—C17—H17 126.5 C45—C46—H46 120.0
C16—C17—H17 126.5 C47—C46—H46 120.0
C17—C18—C19 107.5 (2) C46—C47—C48 120.0 (3)
C17—C18—H18 126.2 C46—C47—H47 120.0
C19—C18—H18 126.2 C48—C47—H47 120.0
N24—C19—C20 125.1 (2) C43—C48—C47 120.7 (3)
N24—C19—C18 109.7 (2) C43—C48—H48 119.6
C20—C19—C18 125.2 (2) C47—C48—H48 119.6
C1—C20—C19 125.1 (2) C50—O49—Zn 126.0 (2)
C1—C20—C43 117.5 (2) O49—C50—N51 124.9 (3)
C19—C20—C43 117.4 (2) O49—C50—H50 117.6
C1—N21—C4 106.2 (2) N51—C50—H50 117.6
C1—N21—Zn 127.01 (16) C50—N51—C53 124.2 (3) C4—N21—Zn 126.48 (17) C50—N51—C52 119.3 (3)
supporting information
sup-10
Acta Cryst. (2006). E62, m158–m160C6—N22—Zn 127.12 (17) N51—C52—H52A 109.5
C9—N22—Zn 126.17 (16) N51—C52—H52B 109.5
C14—N23—C11 106.22 (19) H52A—C52—H52B 109.5 C14—N23—Zn 126.31 (16) N51—C52—H52C 109.5 C11—N23—Zn 126.21 (17) H52A—C52—H52C 109.5 C19—N24—C16 106.2 (2) H52B—C52—H52C 109.5 C19—N24—Zn 127.51 (16) N51—C53—H53A 109.5 C16—N24—Zn 125.43 (16) N51—C53—H53B 109.5 C26—C25—C30 118.8 (3) H53A—C53—H53B 109.5
C26—C25—C5 121.4 (3) N51—C53—H53C 109.5
C30—C25—C5 119.7 (3) H53A—C53—H53C 109.5