organic papers
Acta Cryst.(2006). E62, o2393–o2394 doi:10.1107/S1600536806017764 Xiaet al. C
23H22O3
o2393
Acta Crystallographica Section E Structure Reports Online
ISSN 1600-5368
1-[4,5-Bis(benzyloxy)-2-methylphenyl]ethanone
Chun-Nian Xia,aGuang-Xiang Zhong,aWei-Xiao Hu,a* Wei Zhouaand Guo-Hong Wangb
aCollege of Pharmaceutical Science, Zhejiang
University of Technology, Hangzhou 310014, People’s Republic of China, andbZhejiang Shou
& Fu Chemicals Ltd, Dongdu Qiaotou, Jinyun, Zhejiang 321400, People’s Republic of China
Correspondence e-mail: huyang@mail.hz.zj.cn
Key indicators
Single-crystal X-ray study
T= 298 K
Mean(C–C) = 0.009 A˚
Rfactor = 0.061
wRfactor = 0.243
Data-to-parameter ratio = 14.0
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 25 April 2006 Accepted 12 May 2006
#2006 International Union of Crystallography
All rights reserved
Crystals of the title compound, C23H22O3, were conveniently synthesized according to the method of Patil, Matier & Khuong [(1990). Eur. Patent No. 373592]. The molecule consists of two planar segments that pack to give columns two molecules wide.
Comment
The title compound, (I), is useful in the synthesis of dihydroxybenzoate esters used as antiglaucoma and -blocking agents. They are useful for the treatment of glau-coma, or for lowering intraocular pressure, since they remain stable when applied topically to the eye but rapidly metabolize as they are absorbed (Patilet al., 1990).
Compound (I) consists of two major components. The C10– C15 phenyl ring is approximately perpendicular to the mean plane [0.008 (3) A˚ ] through the remaining non-H atoms [dihedral angle between the planes is 87.45 (18)]. The
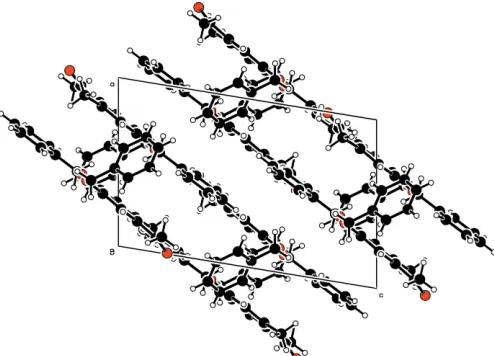
[image:1.610.267.394.335.421.2]mol-ecules pack into columns, two molmol-ecules wide, with the C8– C20 planar system forming the walls of the column and the C10–C15 phenyl rings extending into the middle of the columns (Fig. 2). Selected bond lengths and angles are listed in Table 1.
Experimental
Compound (I) was obtained by the method of Patil et al. (1990). Crystals suitable for structure analysis were obtained by slow evaporation of a solution in a mixture of petroleum ether and acetic acid (8:2) as brown crystalline cubes (m.p. 329–331 K). Elemental analysis calculated: C 79.74, H 6.40%; found: C 79.77, H 6.57%.
Crystal data
C23H22O3
Mr= 346.41
Monoclinic,P21=n
a= 9.1070 (17) A˚
b= 14.681 (3) A˚
c= 14.121 (3) A˚
= 99.320 (16) V= 1863.1 (7) A˚3
Z= 4
Dx= 1.235 Mg m 3
MoKradiation
= 0.08 mm 1
Data collection
Enraf–Nonius CAD-4 diffractometer
!/2scans
Absorption correction: scan (Northet al., 1968)
Tmin= 0.945,Tmax= 0.983
3771 measured reflections
3338 independent reflections 1184 reflections withI> 2(I)
Rint= 0.040 max= 25.2
3 standard reflections frequency: 60 min intensity decay: 0.3%
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.061
wR(F2) = 0.243
S= 1.02 3338 reflections 238 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.1108P)2
+ 0.1962P] whereP= (Fo
2
+ 2Fc 2
)/3 (/)max= 0.001
max= 0.37 e A˚ 3
min= 0.22 e A˚ 3
Extinction correction:SHELXL97
Extinction coefficient: 0.0107 (18)
Table 1
Selected geometric parameters (A˚ ,).
O1—C7 1.253 (6) O2—C3 1.378 (5) O2—C9 1.420 (5) O3—C4 1.351 (5)
O3—C16 1.435 (5) C7—C8 1.457 (7) C9—C10 1.483 (6) C16—C17 1.489 (6) C6—C1—C2 120.1 (4)
C6—C1—C7 123.4 (5) O1—C7—C8 119.7 (5) O1—C7—C1 119.6 (5)
C8—C7—C1 120.7 (5) O2—C9—C10 113.9 (4) O3—C16—C17 108.9 (4)
H atoms bonded to C atoms were placed in calculated positions and refined using a riding model, withUiso(H) = 1.2Ueq(parent atom)
[or 1.5Ueq(methyl C)], with C—H distances of 0.96–0.97 A˚ , and
0.93 A˚ for those bonded to benzene rings.
Data collection: CAD-4 EXPRESS (Enraf–Nonius, 1994); cell refinement: CAD-4 EXPRESS; data reduction: XCAD4, PSI and
EAC (Enraf–Nonius, 1994); program(s) used to solve structure:
SHELXS97(Sheldrick, 1997); program(s) used to refine structure:
SHELXL97 (Sheldrick, 1997); molecular graphics: ORTEP-3 for Windows (Version 1.05; Farrugia, 1999); software used to prepare material for publication:SHELXL97.
We are very grateful to the National Natural and Scientific Foundation (grant No. 20272053). We also acknowledge financial support by the Science and Technology Department of Zhejiang Province (grant No. 2005 C23022).
References
Enraf–Nonius (1994).XCAD4,PSI, EACandCAD-4 EXPRESS. Enraf– Nonius, Delft, The Netherlands.
Farrugia, L. J. (1999).J. Appl. Cryst.32, 837–838.
North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968).Acta Cryst.A24, 351– 359.
Patil, G., Matier, W. L. & Khuong, H. X. (1990). Eur. Patent No. 373592. Sheldrick, G. M. (1997). SHELXL97 and SHELXS97. University of
[image:2.610.316.563.71.249.2]Go¨ttingen, Germany.
Figure 1
The structure of (I), shown with 30% probability displacement ellipsoids.
Figure 2
[image:2.610.47.298.72.208.2]supporting information
sup-1 Acta Cryst. (2006). E62, o2393–o2394
supporting information
Acta Cryst. (2006). E62, o2393–o2394 [https://doi.org/10.1107/S1600536806017764]
1-[4,5-Bis(benzyloxy)-2-methylphenyl]ethanone
Chun-Nian Xia, Guang-Xiang Zhong, Wei-Xiao Hu, Wei Zhou and Guo-Hong Wang
1-[4,5-Bis(benzyloxy)-2-methylphenyl]ethanone
Crystal data
C23H22O3
Mr = 346.41 Monoclinic, P21/n
Hall symbol: -P 2yn
a = 9.1070 (17) Å
b = 14.681 (3) Å
c = 14.121 (3) Å
β = 99.320 (16)°
V = 1863.1 (7) Å3
Z = 4
F(000) = 736
Dx = 1.235 Mg m−3
Melting point = 329–331 K Mo Kα radiation, λ = 0.71073 Å Cell parameters from 25 reflections
θ = 9.7–12.4°
µ = 0.08 mm−1
T = 298 K Cube, brown
0.30 × 0.30 × 0.30 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω/2θ scans
Absorption correction: ψ scan (North et al., 1968)
Tmin = 0.945, Tmax = 0.983
3771 measured reflections
3338 independent reflections 1184 reflections with I > 2σ(I)
Rint = 0.040
θmax = 25.2°, θmin = 2.0°
h = −10→10
k = 0→17
l = −1→16
3 standard reflections every 60 min intensity decay: 0.3%
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.061
wR(F2) = 0.243
S = 1.02 3338 reflections 238 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.1108P)2 + 0.1962P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 0.37 e Å−3
Δρmin = −0.22 e Å−3
Extinction correction: SHELXL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4
Special details
Experimental. IR (KBr, cm-1): 1668, 1601, 1568, 1519, 1371, 1267, 1254, 1204, 1153, 1054, 748, 733. 1H NMR
(CDCl3): 7.46–7.31 (m, 12H, Ph—H), 5.20 (s, 2H, CH2), 5.16 (s, 2H, CH2), 2.47 (s, 3H, CH3), 2.45 (s, 3H, CH3). EIMS
m/z (%): 347 (3.2, M + 1), 346 (6.2, M+), 255 (3.4), 181 (8.4), 92 (9.5), 91?(100), 65 (13.8), 43 (6.1).
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
O1 0.0061 (4) 0.1097 (3) 0.1888 (3) 0.1207 (15)
O2 0.3573 (4) 0.2071 (2) −0.1227 (2) 0.0834 (10)
O3 0.3898 (3) 0.0341 (2) −0.1416 (2) 0.0731 (9)
C6 0.1873 (5) 0.0278 (4) 0.0639 (3) 0.0706 (12)
C1 0.1645 (5) 0.1201 (4) 0.0727 (3) 0.0686 (13)
C2 0.2211 (5) 0.1827 (3) 0.0100 (3) 0.0726 (13)
H2 0.2070 0.2449 0.0172 0.087*
C3 0.2959 (5) 0.1522 (3) −0.0603 (3) 0.0655 (12)
C4 0.3153 (5) 0.0588 (3) −0.0703 (3) 0.0652 (12)
C5 0.2615 (5) −0.0003 (3) −0.0079 (3) 0.0692 (13)
H5 0.2764 −0.0624 −0.0150 0.083*
C7 0.0846 (6) 0.1599 (4) 0.1454 (4) 0.0859 (15)
C8 0.0962 (7) 0.2568 (4) 0.1678 (4) 0.107 (2)
H8A 0.0483 0.2912 0.1136 0.161*
H8B 0.1992 0.2737 0.1820 0.161*
H8C 0.0488 0.2692 0.2223 0.161*
C9 0.3638 (6) 0.3026 (3) −0.1064 (3) 0.0756 (14)
H9A 0.2636 0.3252 −0.1067 0.091*
H9B 0.4023 0.3317 −0.1590 0.091*
C10 0.4576 (6) 0.3294 (3) −0.0149 (3) 0.0681 (13)
C11 0.4160 (7) 0.4016 (4) 0.0368 (4) 0.1027 (19)
H11 0.3285 0.4326 0.0136 0.123*
C12 0.4992 (14) 0.4293 (6) 0.1212 (6) 0.156 (4)
H12 0.4700 0.4788 0.1548 0.187*
C13 0.6222 (15) 0.3839 (10) 0.1537 (8) 0.186 (7)
H13 0.6786 0.4034 0.2109 0.223*
C14 0.6733 (11) 0.3095 (8) 0.1087 (9) 0.161 (5)
H14 0.7589 0.2780 0.1349 0.194*
C15 0.5858 (7) 0.2842 (4) 0.0195 (5) 0.1042 (19)
H15 0.6166 0.2363 −0.0157 0.125*
supporting information
sup-3 Acta Cryst. (2006). E62, o2393–o2394
C17 0.4799 (5) −0.0734 (3) −0.2445 (3) 0.0639 (12)
C18 0.5077 (6) −0.0020 (4) −0.3012 (4) 0.0886 (16)
H18 0.4780 0.0563 −0.2869 0.106*
C19 0.5784 (7) −0.0148 (4) −0.3786 (5) 0.116 (2)
H19 0.5962 0.0351 −0.4158 0.139*
C20 0.6231 (7) −0.0989 (5) −0.4024 (5) 0.114 (2)
H20 0.6711 −0.1074 −0.4551 0.137*
C21 0.5957 (7) −0.1690 (5) −0.3476 (5) 0.112 (2)
H21 0.6255 −0.2270 −0.3631 0.134*
C22 0.5252 (6) −0.1588 (4) −0.2690 (4) 0.0942 (16)
H22 0.5082 −0.2093 −0.2326 0.113*
C23 0.1385 (6) −0.0460 (4) 0.1308 (3) 0.0925 (17)
H23A 0.2129 −0.0519 0.1869 0.139*
H23B 0.1266 −0.1032 0.0975 0.139*
H23C 0.0457 −0.0285 0.1494 0.139*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
O1 0.120 (3) 0.147 (4) 0.109 (3) −0.030 (3) 0.060 (3) −0.011 (3)
O2 0.123 (3) 0.068 (2) 0.066 (2) −0.0134 (19) 0.0372 (19) −0.0058 (17)
O3 0.094 (2) 0.066 (2) 0.0640 (19) −0.0006 (17) 0.0249 (17) 0.0006 (16)
C6 0.066 (3) 0.076 (3) 0.066 (3) −0.015 (3) 0.000 (2) −0.002 (3)
C1 0.056 (3) 0.104 (4) 0.048 (3) −0.010 (3) 0.013 (2) −0.007 (3)
C2 0.076 (3) 0.077 (3) 0.064 (3) −0.010 (3) 0.010 (2) −0.003 (3)
C3 0.072 (3) 0.081 (4) 0.047 (2) −0.008 (3) 0.019 (2) 0.003 (3)
C4 0.073 (3) 0.065 (3) 0.056 (3) −0.011 (2) 0.007 (2) −0.002 (3)
C5 0.076 (3) 0.071 (3) 0.060 (3) −0.007 (2) 0.009 (3) 0.001 (3)
C7 0.076 (3) 0.101 (4) 0.083 (4) −0.015 (3) 0.023 (3) 0.001 (3)
C8 0.121 (5) 0.088 (4) 0.125 (5) 0.011 (4) 0.057 (4) −0.008 (4)
C9 0.095 (4) 0.067 (4) 0.068 (3) 0.007 (3) 0.025 (3) 0.007 (3)
C10 0.081 (4) 0.070 (3) 0.055 (3) −0.016 (3) 0.019 (3) 0.004 (3)
C11 0.141 (5) 0.091 (4) 0.085 (4) −0.030 (4) 0.046 (4) −0.017 (3)
C12 0.244 (11) 0.144 (8) 0.095 (6) −0.100 (8) 0.070 (7) −0.040 (5)
C13 0.167 (12) 0.259 (17) 0.119 (8) −0.134 (13) −0.018 (8) 0.055 (10)
C14 0.093 (6) 0.200 (11) 0.185 (11) −0.039 (7) 0.003 (7) 0.116 (9)
C15 0.074 (4) 0.119 (5) 0.119 (5) −0.014 (4) 0.013 (4) 0.041 (4)
C16 0.071 (3) 0.064 (3) 0.075 (3) 0.000 (2) 0.002 (3) 0.003 (2)
C17 0.054 (3) 0.066 (3) 0.069 (3) −0.001 (2) 0.002 (2) −0.011 (3)
C18 0.112 (4) 0.076 (4) 0.086 (4) 0.006 (3) 0.042 (3) −0.002 (3)
C19 0.152 (5) 0.088 (4) 0.122 (5) 0.007 (4) 0.064 (5) −0.005 (4)
C20 0.121 (5) 0.119 (6) 0.111 (5) 0.016 (4) 0.047 (4) −0.012 (4)
C21 0.122 (5) 0.091 (5) 0.128 (5) 0.020 (4) 0.038 (4) −0.033 (4)
C22 0.108 (4) 0.063 (3) 0.111 (4) 0.005 (3) 0.013 (4) −0.001 (3)
Geometric parameters (Å, º)
O1—C7 1.253 (6) C11—H11 0.9300
O2—C3 1.378 (5) C12—C13 1.320 (14)
O2—C9 1.420 (5) C12—H12 0.9300
O3—C4 1.351 (5) C13—C14 1.381 (13)
O3—C16 1.435 (5) C13—H13 0.9300
C6—C5 1.369 (6) C14—C15 1.427 (12)
C6—C1 1.381 (6) C14—H14 0.9300
C6—C23 1.549 (7) C15—H15 0.9300
C1—C2 1.428 (6) C16—C17 1.489 (6)
C1—C7 1.472 (6) C16—H16A 0.9700
C2—C3 1.368 (6) C16—H16B 0.9700
C2—H2 0.9300 C17—C18 1.367 (6)
C3—C4 1.393 (6) C17—C22 1.383 (6)
C4—C5 1.382 (6) C18—C19 1.370 (7)
C5—H5 0.9300 C18—H18 0.9300
C7—C8 1.457 (7) C19—C20 1.359 (7)
C8—H8A 0.9599 C19—H19 0.9300
C8—H8B 0.9599 C20—C21 1.336 (8)
C8—H8C 0.9599 C20—H20 0.9300
C9—C10 1.483 (6) C21—C22 1.377 (8)
C9—H9A 0.9700 C21—H21 0.9300
C9—H9B 0.9700 C22—H22 0.9300
C10—C15 1.361 (7) C23—H23A 0.9599
C10—C11 1.375 (7) C23—H23B 0.9599
C11—C12 1.367 (10) C23—H23C 0.9599
C3—O2—C9 119.1 (3) C13—C12—H12 120.9
C4—O3—C16 117.6 (3) C11—C12—H12 120.9
C5—C6—C1 117.5 (4) C12—C13—C14 124.7 (13)
C5—C6—C23 117.8 (5) C12—C13—H13 117.6
C1—C6—C23 124.7 (5) C14—C13—H13 117.6
C6—C1—C2 120.1 (4) C13—C14—C15 115.5 (11)
C6—C1—C7 123.4 (5) C13—C14—H14 122.3
C2—C1—C7 116.5 (5) C15—C14—H14 122.3
C3—C2—C1 120.8 (4) C10—C15—C14 120.8 (7)
C3—C2—H2 119.6 C10—C15—H15 119.6
C1—C2—H2 119.6 C14—C15—H15 119.6
C2—C3—O2 125.0 (4) O3—C16—C17 108.9 (4)
C2—C3—C4 118.9 (4) O3—C16—H16A 109.9
O2—C3—C4 116.1 (4) C17—C16—H16A 109.9
O3—C4—C5 125.5 (4) O3—C16—H16B 109.9
O3—C4—C3 115.2 (4) C17—C16—H16B 109.9
C5—C4—C3 119.3 (4) H16A—C16—H16B 108.3
C6—C5—C4 123.5 (4) C18—C17—C22 117.3 (5)
supporting information
sup-5 Acta Cryst. (2006). E62, o2393–o2394
O1—C7—C8 119.7 (5) C17—C18—C19 121.2 (5)
O1—C7—C1 119.6 (5) C17—C18—H18 119.4
C8—C7—C1 120.7 (5) C19—C18—H18 119.4
C7—C8—H8A 109.5 C20—C19—C18 121.4 (6)
C7—C8—H8B 109.5 C20—C19—H19 119.3
H8A—C8—H8B 109.5 C18—C19—H19 119.3
C7—C8—H8C 109.5 C21—C20—C19 117.7 (6)
H8A—C8—H8C 109.5 C21—C20—H20 121.1
H8B—C8—H8C 109.5 C19—C20—H20 121.1
O2—C9—C10 113.9 (4) C20—C21—C22 122.6 (6)
O2—C9—H9A 108.8 C20—C21—H21 118.7
C10—C9—H9A 108.8 C22—C21—H21 118.7
O2—C9—H9B 108.8 C21—C22—C17 119.9 (5)
C10—C9—H9B 108.8 C21—C22—H22 120.1
H9A—C9—H9B 107.7 C17—C22—H22 120.1
C15—C10—C11 118.7 (5) C6—C23—H23A 109.5
C15—C10—C9 121.4 (5) C6—C23—H23B 109.5
C11—C10—C9 119.8 (5) H23A—C23—H23B 109.5
C12—C11—C10 122.0 (7) C6—C23—H23C 109.5
C12—C11—H11 119.0 H23A—C23—H23C 109.5
C10—C11—H11 119.0 H23B—C23—H23C 109.5
C13—C12—C11 118.2 (11)
C5—C6—C1—C2 −1.7 (6) C2—C1—C7—C8 −15.2 (7)
C23—C6—C1—C2 176.7 (4) C3—O2—C9—C10 63.0 (5)
C5—C6—C1—C7 179.0 (4) O2—C9—C10—C15 36.9 (6)
C23—C6—C1—C7 −2.6 (7) O2—C9—C10—C11 −143.5 (4)
C6—C1—C2—C3 1.2 (7) C15—C10—C11—C12 0.0 (8)
C7—C1—C2—C3 −179.4 (4) C9—C10—C11—C12 −179.5 (5)
C1—C2—C3—O2 −178.5 (4) C10—C11—C12—C13 −0.8 (11)
C1—C2—C3—C4 0.3 (7) C11—C12—C13—C14 −0.4 (16)
C9—O2—C3—C2 9.3 (7) C12—C13—C14—C15 2.1 (15)
C9—O2—C3—C4 −169.5 (4) C11—C10—C15—C14 1.8 (8)
C16—O3—C4—C5 6.1 (6) C9—C10—C15—C14 −178.6 (5)
C16—O3—C4—C3 −175.1 (4) C13—C14—C15—C10 −2.8 (11)
C2—C3—C4—O3 179.8 (4) C4—O3—C16—C17 176.6 (4)
O2—C3—C4—O3 −1.3 (6) O3—C16—C17—C18 −9.9 (6)
C2—C3—C4—C5 −1.3 (7) O3—C16—C17—C22 170.7 (4)
O2—C3—C4—C5 177.6 (4) C22—C17—C18—C19 −0.4 (8)
C1—C6—C5—C4 0.8 (7) C16—C17—C18—C19 −179.7 (5)
C23—C6—C5—C4 −177.8 (4) C17—C18—C19—C20 0.3 (10)
O3—C4—C5—C6 179.6 (4) C18—C19—C20—C21 0.0 (10)
C3—C4—C5—C6 0.8 (7) C19—C20—C21—C22 −0.2 (10)
C6—C1—C7—O1 −15.6 (8) C20—C21—C22—C17 0.0 (9)
C2—C1—C7—O1 165.1 (5) C18—C17—C22—C21 0.3 (7)