organic papers
o1846
Whiteet al. C19H21N3 doi:10.1107/S1600536806012463 Acta Cryst.(2006). E62, o1846–o1848
Acta Crystallographica Section E Structure Reports Online
ISSN 1600-5368
9,9a-Diphenyl-1,3,4,6,7,9a-hexahydro-2
H
-pyrazino[1,2-
a
]pyrimidine at 130 K
Jonathan White,a* W. David McFadyen,aMichael Carland,a William A. Dennyband Vincent Murrayc
aSchool of Chemistry, University of Melbourne,
Melbourne, Victoria 3010, Australia,bAuckland
Cancer Society Research Centre, Faculty of Medicine and Health Science, University of Auckland, Auckland, New Zealand, and
cSchool of Biotechnology and Biomolecular
Sciences, University of New South Wales, Sydney, New South Wales 2052, Australia
Correspondence e-mail: whitejm@unimelb.edu.au
Key indicators
Single-crystal X-ray study T= 295 K
Mean(C–C) = 0.002 A˚ Rfactor = 0.038 wRfactor = 0.101
Data-to-parameter ratio = 10.0
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2006 International Union of Crystallography Printed in Great Britain – all rights reserved
The stereochemistry of the title compound, C19H21N3, has
been confirmed by a single-crystal X-ray analysis. The bicyclic ring system adopts a cis-decalin-like conformation, which presumably minimizes steric repulsion involving the bridge-head phenyl substituent. The conformation of the amino group appears to be dictated by a nitrogen anomeric effect.
Comment
Derivatives of 1,4,7-triazabicyclo[4.4.0]decane, (1), have attracted interest in recent years as substance P-binding inhibitors (Wonget al., 1993) and anti-inflammatories (Stasiak
et al., 1999), as well as for their potential synthetic use in the selective protection of linear triamines (Okawaraet al., 1990).
Substituted derivatives of (1), such as the title compound, (2), are conveniently prepared by the acid-catalysed conden-sation of an -diketone with N -(2-aminoethyl)-1,3-propane-diamine, (4) (see scheme). The related system, (6), is obtained by condensation of benzil with the dien, (5). In the case of the latter, only one bicyclic system may be generated. However, in the former case, two distinct bicyclic systems may reasonably be expected to form, namely the [4.4.0] system, (2), and the [5.3.0] structure, (3).
A single product is recovered in good yield from the reac-tion as a colourless crystalline solid. While this material has previously been identified as the [4.4.0] system, (2) (Okawara
et al., 1990), spectroscopic evidence in support of this claim has not been published. Here, we report the crystal structure of (2) in combination with spectroscopic data which demonstrate that the bicyclo[4.4.0] structure does indeed form, and as a single diastereomer.
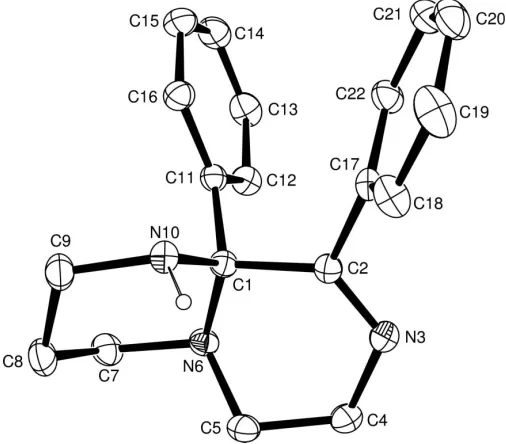
Crystals of (2) were grown from ethyl acetate and hexane. Selected bond distances, angles and dihedral angles for (2) are presented in Table 1, and a displacement ellipsoid plot is presented in Fig. 1.
The bicyclic ring system in (2) exists in a cis-decalin-like conformation, which is preferred to the alternative trans -decalin conformation (which is attainable by inversion at the bridgehead atom N6) due to the presence of the bulky phenyl substituent at C1. Interestingly, the H atom attached at N10, which was located in a difference map and refined satisfac-torily without constraint, is axially oriented. This preferred axial orientation of atom H10 may have its origins in an anomeric interaction between the nitrogen lone pair on N10 and the C1—N6 bond (Alder et al., 1999). However, the alternative equatorial conformation would be disfavoured on steric grounds, as H10 would clash with the H atom attached to C16.
There is a weak intermolecular hydrogen bond between atoms H10 and N3 [N10—H10 = 0.89 (1) A˚ , H10 N3i = 2.50 (2) A˚ , N10 N3i = 3.350 (2) A˚ and N10—H10 N3i = 159.1 (8); symmetry code: (i) x+ 2,1
2 y, 1 2 z].
Experimental
A mixture of N-(2-aminoethyl)-1,3-propanediamine (5 ml, 46.3 mmol), benzil (9.73 g, 46.3 mmol), glacial acetic acid (0.5 ml) and ethanol (100 ml) was heated to reflux with stirring for 3 h, over which time a white precipitate formed. Volatiles were removed under reduced pressure and the residue was triturated with hexane and
dried. The crude product was recrystallized from ethyl acetate– hexane (1:1) to afford the title compound, (2), as large colourless blocks (yield 81%; m.p. 378–380 K). Elemental analysis, C19H21N3
requires: C 78.3, H 7.3, N 14.4%; found: C 78.0, H 7.4, N 14.7%.
Crystal data
C19H21N3 Mr= 291.39
Monoclinic,P21=c a= 10.4099 (7) A˚ b= 8.2868 (6) A˚ c= 19.1371 (14) A˚
= 103.483 (1) V= 1605.4 (2) A˚3
Z= 4
Dx= 1.206 Mg m 3
MoKradiation
= 0.07 mm 1 T= 295 (2) K Block, colourless 0.500.400.30 mm
Data collection
Bruker SMART CCD area-detector diffractometer
’and!scans
Absorption correction: none 8199 measured reflections
2833 independent reflections 2259 reflections withI> 2(I) Rint= 0.059
max= 25.0
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.039 wR(F2) = 0.102 S= 1.05 2833 reflections 284 parameters H atoms: see below
w= 1/[2
(Fo2) + (0.0552P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.002 max= 0.16 e A˚ 3 min= 0.14 e A˚ 3
Extinction correction:SHELXL97 (Sheldrick, 1997)
[image:2.610.44.297.73.295.2]Extinction coefficient: 0.0091 (18)
Table 1
Selected geometric parameters (A˚ ,).
C1—N10 1.4662 (16)
C1—N6 1.4748 (15)
C1—C11 1.5383 (16)
C1—C2 1.5441 (16)
C2—N3 1.2703 (15)
C2—C17 1.4994 (17)
C4—N3 1.4688 (18)
C4—C5 1.496 (2)
C5—N6 1.4551 (17)
C7—C8 1.515 (2)
C8—C9 1.516 (2)
C9—N10 1.4708 (16)
N10—C1—N6 115.47 (10)
N10—C1—C11 110.82 (10)
N6—C1—C11 107.90 (9)
N10—C1—C2 106.00 (9)
N6—C1—C2 109.26 (9)
C11—C1—C2 107.09 (9)
N3—C2—C17 116.59 (10)
N3—C2—C1 126.38 (11)
C17—C2—C1 116.90 (10)
N3—C4—C5 112.94 (12)
N6—C5—C4 107.84 (12)
N6—C7—C8 113.46 (12)
C7—C8—C9 109.55 (13)
N10—C9—C8 113.31 (12)
N10—C1—C2—N3 117.10 (13)
N6—C1—C2—N3 7.93 (16)
C11—C1—C2—N3 124.55 (12) N10—C1—C2—C17 58.73 (13) N6—C1—C2—C17 176.23 (9) C11—C1—C2—C17 59.62 (13)
N3—C4—C5—N6 54.85 (17)
N6—C7—C8—C9 54.90 (18)
C7—C8—C9—N10 50.40 (19) N10—C1—C11—C16 17.31 (15)
C1—C2—N3—C4 2.66 (18)
C5—C4—N3—C2 20.98 (19)
C4—C5—N6—C7 168.15 (12)
C4—C5—N6—C1 66.74 (14)
C8—C7—N6—C5 71.06 (16)
C8—C7—N6—C1 54.19 (16)
N10—C1—N6—C5 76.69 (13) C11—C1—N6—C5 158.74 (10)
C2—C1—N6—C5 42.64 (12)
N10—C1—N6—C7 49.52 (13) C11—C1—N6—C7 75.06 (12) C2—C1—N6—C7 168.85 (10) N6—C1—N10—C9 46.89 (15) C11—C1—N10—C9 76.15 (13) C2—C1—N10—C9 168.01 (11) C8—C9—N10—C1 47.26 (18)
The H atom attached to N10 was located by difference methods and its positional and isotropic displacement parameters were refined. Other H atoms were included in the refinement in calculated positions, with C—H(aromatic) = 0.95 A˚ and C—H(aliphatic) = 0.99 A˚ , and they were treated as riding, withUiso(H) = 1.2Ueq(C).
Data collection:SMART(Bruker, 2000); cell refinement:SAINT (Bruker 1999); data reduction: SAINT; program(s) used to solve
organic papers
Acta Cryst.(2006). E62, o1846–o1848 Whiteet al. C
19H21N3
o1847
Figure 1 [image:2.610.313.567.403.653.2]structure: SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics: SHELXTL (Bruker, 2000); software used to prepare material for publication:WinGX(Farrugia, 1999).
The authors gratefully acknowledge financial support from the Australian Research Council.
References
Alder, R. W., Carniero, T. M. G., Mowlam, R., Orpen, A. G., Petillo, P., Vachon, D. J., Weisman, G. R. & White, J. M. (1999).J. Chem. Soc. Perkin Trans. 2, pp. 589–599.
Bruker (1999).SAINT. Version 6.02. Bruker AXS Inc., Madison, Wisconsin, USA.
Bruker (2000).SMART(Version 5.55) andSHELXTL. Bruker AXS Inc., Madison, Wisconsin, USA.
Farrugia, L. J. (1999).J. Appl. Cryst.32, 837–838.
Okawara, T., Uchiyama, K., Okamoto, Y., Yamasaki, T. & Furukawa, M. (1990).J. Chem. Soc. Chem. Commun.pp. 1385–1386.
Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
Stasiak, M., Eguchi, M., Mehlin, C. & Kahn, M. (1999).Proceedings of the American Peptide Symposium, pp. 223–234. Dordrecht: Kluwer Academic Publishers.
Wong, S. M., Musza, L., Gwendolyn, C., Kullnig, R., Gillum, A. M. & Cooper, R. (1993).J. Antibiot.46, 545–553.
organic papers
o1848
Whiteet al. Csupporting information
sup-1
Acta Cryst. (2006). E62, o1846–o1848
supporting information
Acta Cryst. (2006). E62, o1846–o1848 [https://doi.org/10.1107/S1600536806012463]
9,9a-Diphenyl-1,3,4,6,7,9a-hexahydro-2
H
-pyrazino[1,2-
a
]pyrimidine at 130 K
Jonathan White, W. David McFadyen, Michael Carland, William A. Denny and Vincent Murray
9,9a-Diphenyl-1,3,4,6,7,9a-hexahydro-2H-pyrazino[1,2-a]pyrimidine
Crystal data
C19H21N3
Mr = 291.39 Monoclinic, P21/c Hall symbol: -P 2ybc
a = 10.4099 (7) Å
b = 8.2868 (6) Å
c = 19.1371 (14) Å
β = 103.483 (1)°
V = 1605.4 (2) Å3
Z = 4
F(000) = 624
Dx = 1.206 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 3216 reflections
θ = 2.5–27.0°
µ = 0.07 mm−1
T = 295 K Block, colourless 0.50 × 0.40 × 0.30 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: sealed tube Graphite monochromator
φ and ω scans
8199 measured reflections 2833 independent reflections
2259 reflections with I > 2σ(I)
Rint = 0.059
θmax = 25.0°, θmin = 2.0°
h = −9→12
k = −9→9
l = −22→18
Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.039
wR(F2) = 0.102
S = 1.05 2833 reflections 284 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(F
o2) + (0.0552P)2] where P = (Fo2 + 2Fc2)/3 (Δ/σ)max = 0.002
Δρmax = 0.16 e Å−3 Δρmin = −0.14 e Å−3
Extinction correction: SHELXL97 (Sheldrick, 1997), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 Extinction coefficient: 0.0091 (18)
Special details
Experimental. Spectroscopic analysis: 1H NMR (CDCl
supporting information
sup-2
Acta Cryst. (2006). E62, o1846–o1848
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
C1 0.76592 (11) 0.56603 (14) 0.16106 (6) 0.0376 (3)
C2 0.87028 (12) 0.66449 (14) 0.21485 (6) 0.0384 (3)
C4 1.01466 (16) 0.6782 (2) 0.13673 (9) 0.0576 (4)
C5 0.94319 (14) 0.53347 (18) 0.10020 (8) 0.0496 (4)
C7 0.72076 (16) 0.44324 (19) 0.04279 (8) 0.0550 (4)
C8 0.72399 (18) 0.27596 (19) 0.07505 (8) 0.0603 (4)
C9 0.68490 (16) 0.28547 (19) 0.14644 (8) 0.0547 (4)
C11 0.63407 (11) 0.65710 (16) 0.15083 (6) 0.0407 (3)
C12 0.61311 (14) 0.79593 (17) 0.10884 (7) 0.0508 (4)
C13 0.50115 (15) 0.8888 (2) 0.10474 (9) 0.0624 (4)
C14 0.40845 (16) 0.8451 (2) 0.14178 (9) 0.0667 (5)
C15 0.42783 (15) 0.7082 (2) 0.18299 (9) 0.0630 (4)
C16 0.54028 (13) 0.61408 (19) 0.18793 (8) 0.0507 (4)
C17 0.84523 (12) 0.69133 (14) 0.28802 (6) 0.0399 (3)
C18 0.92079 (16) 0.60477 (18) 0.34463 (8) 0.0570 (4)
C19 0.9021 (2) 0.6243 (2) 0.41337 (9) 0.0719 (5)
C20 0.80995 (18) 0.7298 (2) 0.42663 (9) 0.0670 (5)
C21 0.73585 (15) 0.8181 (2) 0.37137 (8) 0.0617 (4)
C22 0.75340 (14) 0.79933 (18) 0.30254 (8) 0.0522 (4)
N3 0.97892 (11) 0.71750 (13) 0.20461 (6) 0.0489 (3)
N6 0.80224 (10) 0.56136 (13) 0.09106 (5) 0.0429 (3)
N10 0.75858 (11) 0.40874 (13) 0.19501 (5) 0.0420 (3)
H4A 0.9946 (15) 0.773 (2) 0.1041 (9) 0.074 (5)*
H4B 1.1098 (17) 0.666 (2) 0.1470 (8) 0.073 (5)*
H5A 0.9634 (13) 0.5249 (17) 0.0527 (8) 0.057 (4)*
H5B 0.9756 (12) 0.4346 (18) 0.1298 (7) 0.053 (4)*
H7A 0.6302 (15) 0.4812 (17) 0.0306 (7) 0.057 (4)*
H7B 0.7541 (13) 0.4425 (17) −0.0036 (8) 0.056 (4)*
H8A 0.6652 (16) 0.207 (2) 0.0436 (9) 0.075 (5)*
H8B 0.8146 (16) 0.2289 (19) 0.0836 (8) 0.066 (4)*
H9A 0.5883 (15) 0.3142 (17) 0.1382 (7) 0.057 (4)*
H9B 0.7010 (14) 0.180 (2) 0.1716 (8) 0.065 (4)*
H10 0.8396 (14) 0.3702 (16) 0.2135 (7) 0.048 (4)*
H12 0.6774 (14) 0.8249 (17) 0.0812 (7) 0.056 (4)*
H13 0.4914 (15) 0.982 (2) 0.0743 (8) 0.067 (5)*
supporting information
sup-3
Acta Cryst. (2006). E62, o1846–o1848
H15 0.3665 (16) 0.6776 (19) 0.2107 (8) 0.070 (5)*
H16 0.5542 (13) 0.5221 (18) 0.2191 (7) 0.056 (4)*
H18 0.9894 (16) 0.531 (2) 0.3336 (8) 0.069 (5)*
H19 0.9564 (19) 0.566 (2) 0.4505 (11) 0.099 (6)*
H20 0.7942 (15) 0.7411 (19) 0.4734 (8) 0.072 (5)*
H21 0.6734 (17) 0.896 (2) 0.3825 (9) 0.082 (5)*
H22 0.6993 (15) 0.863 (2) 0.2628 (9) 0.073 (5)*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C1 0.0396 (7) 0.0396 (7) 0.0336 (7) −0.0002 (5) 0.0087 (5) 0.0004 (5)
C2 0.0397 (7) 0.0331 (6) 0.0410 (7) 0.0027 (5) 0.0066 (5) 0.0028 (5)
C4 0.0523 (9) 0.0584 (10) 0.0694 (10) −0.0086 (7) 0.0291 (8) −0.0060 (8)
C5 0.0524 (8) 0.0523 (9) 0.0494 (9) 0.0035 (7) 0.0227 (6) −0.0010 (7)
C7 0.0600 (10) 0.0653 (10) 0.0378 (8) −0.0008 (7) 0.0075 (7) −0.0086 (7)
C8 0.0683 (11) 0.0549 (9) 0.0553 (9) −0.0130 (8) 0.0098 (8) −0.0173 (7)
C9 0.0610 (10) 0.0470 (9) 0.0556 (9) −0.0126 (7) 0.0125 (7) −0.0078 (7)
C11 0.0387 (7) 0.0469 (8) 0.0345 (7) −0.0002 (6) 0.0046 (5) −0.0052 (5)
C12 0.0508 (8) 0.0536 (9) 0.0462 (8) 0.0053 (6) 0.0079 (6) 0.0014 (6)
C13 0.0641 (10) 0.0549 (10) 0.0611 (10) 0.0152 (8) 0.0005 (8) 0.0008 (8)
C14 0.0489 (9) 0.0717 (11) 0.0741 (11) 0.0161 (8) 0.0036 (8) −0.0149 (9)
C15 0.0440 (9) 0.0788 (12) 0.0684 (10) 0.0003 (8) 0.0178 (7) −0.0117 (9)
C16 0.0442 (8) 0.0585 (9) 0.0492 (8) −0.0001 (6) 0.0102 (6) −0.0030 (7)
C17 0.0421 (7) 0.0368 (7) 0.0381 (7) −0.0067 (5) 0.0039 (5) −0.0029 (5)
C18 0.0755 (10) 0.0446 (8) 0.0457 (9) 0.0070 (7) 0.0036 (7) 0.0013 (6)
C19 0.1125 (14) 0.0542 (10) 0.0414 (9) 0.0019 (10) 0.0028 (9) 0.0073 (7)
C20 0.0933 (13) 0.0671 (11) 0.0443 (9) −0.0211 (9) 0.0235 (9) −0.0121 (8)
C21 0.0581 (9) 0.0770 (11) 0.0502 (9) −0.0025 (8) 0.0130 (7) −0.0175 (8)
C22 0.0500 (8) 0.0591 (9) 0.0439 (8) 0.0050 (7) 0.0034 (6) −0.0086 (7)
N3 0.0466 (6) 0.0469 (7) 0.0547 (7) −0.0070 (5) 0.0151 (5) −0.0034 (5)
N6 0.0459 (6) 0.0477 (6) 0.0362 (6) 0.0021 (5) 0.0117 (4) −0.0007 (5)
N10 0.0439 (7) 0.0397 (6) 0.0408 (6) −0.0033 (5) 0.0066 (5) 0.0003 (5)
Geometric parameters (Å, º)
C1—N10 1.4662 (16) C11—C16 1.3812 (18)
C1—N6 1.4748 (15) C11—C12 1.3911 (19)
C1—C11 1.5383 (16) C12—C13 1.384 (2)
C1—C2 1.5441 (16) C12—H12 0.975 (14)
C2—N3 1.2703 (15) C13—C14 1.373 (2)
C2—C17 1.4994 (17) C13—H13 0.956 (17)
C4—N3 1.4688 (18) C14—C15 1.369 (2)
C4—C5 1.496 (2) C14—H14 0.977 (17)
C4—H4A 0.992 (17) C15—C16 1.391 (2)
C4—H4B 0.969 (16) C15—H15 0.955 (16)
C5—N6 1.4551 (17) C16—H16 0.959 (15)
supporting information
sup-4
Acta Cryst. (2006). E62, o1846–o1848
C5—H5B 1.008 (14) C17—C22 1.3842 (19)
C7—N6 1.4716 (17) C18—C19 1.384 (2)
C7—C8 1.515 (2) C18—H18 0.998 (16)
C7—H7A 0.970 (14) C19—C20 1.365 (3)
C7—H7B 1.025 (15) C19—H19 0.93 (2)
C8—C9 1.516 (2) C20—C21 1.367 (2)
C8—H8A 0.945 (17) C20—H20 0.951 (15)
C8—H8B 0.999 (16) C21—C22 1.3802 (19)
C9—N10 1.4708 (16) C21—H21 0.973 (18)
C9—H9A 1.009 (15) C22—H22 0.987 (17)
C9—H9B 0.992 (16) N10—H10 0.894 (14)
N10—C1—N6 115.47 (10) C12—C11—C1 119.64 (11)
N10—C1—C11 110.82 (10) C13—C12—C11 120.63 (15)
N6—C1—C11 107.90 (9) C13—C12—H12 120.5 (8)
N10—C1—C2 106.00 (9) C11—C12—H12 118.8 (8)
N6—C1—C2 109.26 (9) C14—C13—C12 120.53 (17)
C11—C1—C2 107.09 (9) C14—C13—H13 122.7 (9)
N3—C2—C17 116.59 (10) C12—C13—H13 116.8 (9)
N3—C2—C1 126.38 (11) C15—C14—C13 119.30 (15)
C17—C2—C1 116.90 (10) C15—C14—H14 121.9 (9)
N3—C4—C5 112.94 (12) C13—C14—H14 118.7 (9)
N3—C4—H4A 108.9 (9) C14—C15—C16 120.80 (16)
C5—C4—H4A 109.2 (9) C14—C15—H15 120.8 (10)
N3—C4—H4B 107.5 (9) C16—C15—H15 118.4 (10)
C5—C4—H4B 112.7 (10) C11—C16—C15 120.33 (16)
H4A—C4—H4B 105.3 (13) C11—C16—H16 120.2 (8)
N6—C5—C4 107.84 (12) C15—C16—H16 119.4 (8)
N6—C5—H5A 109.2 (8) C18—C17—C22 118.15 (13)
C4—C5—H5A 107.4 (8) C18—C17—C2 117.64 (12)
N6—C5—H5B 113.1 (7) C22—C17—C2 124.19 (11)
C4—C5—H5B 108.9 (8) C17—C18—C19 120.27 (15)
H5A—C5—H5B 110.2 (11) C17—C18—H18 117.1 (9)
N6—C7—C8 113.46 (12) C19—C18—H18 122.7 (9)
N6—C7—H7A 108.7 (8) C20—C19—C18 120.84 (16)
C8—C7—H7A 108.8 (9) C20—C19—H19 121.1 (12)
N6—C7—H7B 106.6 (8) C18—C19—H19 118.0 (12)
C8—C7—H7B 111.6 (8) C19—C20—C21 119.56 (15)
H7A—C7—H7B 107.5 (11) C19—C20—H20 121.4 (10)
C7—C8—C9 109.55 (13) C21—C20—H20 119.0 (10)
C7—C8—H8A 110.2 (10) C20—C21—C22 120.12 (16)
C9—C8—H8A 109.7 (10) C20—C21—H21 118.1 (10)
C7—C8—H8B 110.8 (9) C22—C21—H21 121.8 (10)
C9—C8—H8B 108.4 (9) C21—C22—C17 121.04 (14)
H8A—C8—H8B 108.3 (13) C21—C22—H22 119.8 (9)
N10—C9—C8 113.31 (12) C17—C22—H22 119.2 (9)
N10—C9—H9A 106.8 (8) C2—N3—C4 118.56 (11)
supporting information
sup-5
Acta Cryst. (2006). E62, o1846–o1848
N10—C9—H9B 107.5 (9) C5—N6—C1 111.09 (10)
C8—C9—H9B 109.9 (8) C7—N6—C1 110.82 (10)
H9A—C9—H9B 109.4 (12) C1—N10—C9 114.25 (10)
C16—C11—C12 118.41 (12) C1—N10—H10 110.5 (9)
C16—C11—C1 121.64 (12) C9—N10—H10 107.9 (8)
N10—C1—C2—N3 117.10 (13) C1—C2—C17—C22 −75.05 (16)
N6—C1—C2—N3 −7.93 (16) C22—C17—C18—C19 1.3 (2)
C11—C1—C2—N3 −124.55 (12) C2—C17—C18—C19 −179.94 (13)
N10—C1—C2—C17 −58.73 (13) C17—C18—C19—C20 −0.5 (2)
N6—C1—C2—C17 176.23 (9) C18—C19—C20—C21 −0.4 (3)
C11—C1—C2—C17 59.62 (13) C19—C20—C21—C22 0.6 (2)
N3—C4—C5—N6 54.85 (17) C20—C21—C22—C17 0.2 (2)
N6—C7—C8—C9 54.90 (18) C18—C17—C22—C21 −1.2 (2)
C7—C8—C9—N10 −50.40 (19) C2—C17—C22—C21 −179.82 (12)
N10—C1—C11—C16 17.31 (15) C17—C2—N3—C4 173.19 (12)
N6—C1—C11—C16 144.62 (11) C1—C2—N3—C4 −2.66 (18)
C2—C1—C11—C16 −97.86 (13) C5—C4—N3—C2 −20.98 (19)
N10—C1—C11—C12 −169.18 (11) C4—C5—N6—C7 168.15 (12)
N6—C1—C11—C12 −41.87 (14) C4—C5—N6—C1 −66.74 (14)
C2—C1—C11—C12 75.65 (13) C8—C7—N6—C5 71.06 (16)
C16—C11—C12—C13 0.29 (19) C8—C7—N6—C1 −54.19 (16)
C1—C11—C12—C13 −173.42 (12) N10—C1—N6—C5 −76.69 (13)
C11—C12—C13—C14 −0.3 (2) C11—C1—N6—C5 158.74 (10)
C12—C13—C14—C15 0.0 (2) C2—C1—N6—C5 42.64 (12)
C13—C14—C15—C16 0.3 (2) N10—C1—N6—C7 49.52 (13)
C12—C11—C16—C15 0.06 (19) C11—C1—N6—C7 −75.06 (12)
C1—C11—C16—C15 173.64 (12) C2—C1—N6—C7 168.85 (10)
C14—C15—C16—C11 −0.4 (2) N6—C1—N10—C9 −46.89 (15)
N3—C2—C17—C18 −69.94 (15) C11—C1—N10—C9 76.15 (13)
C1—C2—C17—C18 106.31 (13) C2—C1—N10—C9 −168.01 (11)