Copyright © 1997, American Society for Microbiology
Identification of Candida Species by Randomly Amplified
Polymorphic DNA Fingerprinting of Colony Lysates
PAUL STEFFAN,1JOSE A. VAZQUEZ,2,3* DINA BOIKOV,2CHUN XU,1 JACK D. SOBEL,2ANDROBERT A. AKINS1
Department of Biochemistry and Molecular Biology1and Department of Medicine, Division of Infectious Diseases,2
Wayne State University School of Medicine, and Veterans Administration Medical Center,3Detroit, Michigan
Received 10 October 1996/Returned for modification 14 December 1996/Accepted 3 April 1997
We have characterized a method that produces simple yet diagnostic fingerprints that are unique to isolates of Candida species. DNA from individual colonies can be amplified from crude single-colony lysates. Randomly amplified polymorphic DNA (RAPD) fingerprints generated from a single primer correctly identified the species of most (>98%) of the isolates identified with CHROMagar Candida plates as non-Candida albicans Candida species. RAPD fingerprints were much more informative than the plates, since they distinguished between all tested species and required less time. Most (91%) of these identifications agreed with those assigned by API 20C tests. In almost every incident of species identity mismatch, electrophoretic karyotyping showed that the RAPD fingerprint was correct. This underscores the improved objectivity and reliability of this method over those of conventional diagnostic tools. The identities of approximately 30% of C. albicans isolates identified in clinical laboratories by positive germ tube tests are not verified by either testing on CHROMagar Candida plates or RAPD fingerprinting. Data suggest that clinical isolates conventionally identified as C. al-bicans in clinical settings are heterogeneous, consisting of both misidentified and atypical yeasts. RAPD fingerprints obtained from primary culture plate colonies allows for rapid, highly accurate determinations of Candida species, hence permitting earlier selection of appropriate antifungal agents in the clinical setting.
Candida species have become an important cause of
noso-comial infection, primarily affecting immunocompromised pa-tients. Candida species are now the fourth most common or-ganisms isolated from cultured blood (1), with crude mortality rates ranging from 70 to 100% (18, 24). The prognosis for the patient improves if the diagnosis of invasive fungal infection is established early and antifungal chemotherapy is initiated sooner. Thus, an early and accurate diagnosis of an invasive fungal infection is of utmost importance (5, 20).
Although Candida albicans remains the most common cause of fungemia and disseminated candidiasis, other Candida spe-cies are not uncommon, accounting for about 25 to 60% of systemic candidal infections in cancer patients (1, 5, 6, 19, 24, 26, 63). Candida lusitaniae (36, 42) and, occasionally, Candida
guilliermondii may develop in vivo resistance to amphotericin B
after prolonged therapy (10, 59) or after in vitro selection (unpublished data). Torulopsis (Candida) glabrata and Candida
krusei are also of particular importance because of their
in-creasing incidence and their intrinsic resistance to some of the azole antifungal agents (4, 21, 26, 34, 51). In addition, antifun-gal resistance has also been reported in some C. albicans strains recovered from AIDS patients (3, 34, 41, 43, 46). More-over, newly emerging fungal species may also be resistant to both azoles and amphotericin B (17). Candida species may also differ in their virulence characteristics (63). These consider-ations make it clear that rapid and reliable identification and delineation of individual fungal species are increasingly impor-tant as the number of immunocompromised patients increases. A large number of methods have been developed to differ-entiate strains of Candida species. Test kits, such as the API 20C Yeast Identification System (13, 15, 23, 27), can require
follow-up tests that take several days, even after the yeast has been recovered on fungal medium. Subjective scoring, inherent variations in colony morphologies and phenotypes among iso-lates within a species of Candida, and the inability of some clinical isolates to grow in vitro are also problematic, particu-larly for inexperienced users (9, 47, 62). Evaluations of related methods often rely on scores obtained by the API 20C system, making it difficult to get truly independent assessments (11). A final limitation may be that an increasing number of C. albicans isolates from AIDS patients are atypical and cannot be reliably identified with several commercial yeast identification kits (un-published data).
Differential agar media, notably Albicans ID and CHROMagar Candida, allow for the presumptive identifica-tion of C. albicans, Candida tropicalis, and C. krusei, based on colony color reactions (2, 14, 38, 40). Unfortunately, these media identify just a few limited species.
Molecular methods have been used to identify Candida iso-lates and to delineate subgroups within a species for epidemi-ological purposes (31, 49, 52, 60). PCR has been used to am-plify DNA of C. albicans directly from blood samples without culture, detecting as little as one cell per ml of blood (12, 30, 33, 53).
A special application of PCR detects randomly amplified polymorphic DNA (RAPD) by extending from short primers that anneal at multiple genomic loci (61). This approach does not depend on prior knowledge of species-specific sequences. Distinctive RAPD patterns were obtained from C. albicans, C.
lusitaniae, C. tropicalis, Candida parapsilosis, C. krusei, Candida haemulonii, and T. glabrata (3, 7, 22, 28, 29, 33, 35, 45, 50,
54–56, 62). Current limitations exist for routine use of these RAPD profiles for clinical diagnosis. The necessarily short primers require fastidious conditions for reproducible PCR (39). Furthermore, different products or no products may re-sult when using DNA templates of various purities, quantities, and qualities (25). Finally, preparation of DNA to serve as
* Corresponding author. Mailing address: Harper Professional Building, 4160 John R, Suite 2140, Detroit, MI 48201. Phone: (313) 745-9649. Fax: (313) 993-0203.
2031
on May 15, 2020 by guest
http://jcm.asm.org/
templates in the reaction is too laborious, technically demand-ing, and too likely to introduce contaminants that will be am-plified along with the clinical sample.
With these problems in mind, we have optimized primer and amplification procedures for RAPD fingerprinting of 10 of the most frequently encountered Candida species. We have devel-oped a method for the accurate, reproducible, and rapid screening of large numbers of clinical candidal isolates regard-less of species, which should also facilitate the use of RAPD fingerprinting as a clinical diagnostic and epidemiological tool.
MATERIALS AND METHODS
Candida isolates.Candida isolates were obtained from the American Type Culture Collection (ATCC) or from clinical specimens. The latter were recov-ered from immunocompromised hospitalized patients in either the Bone Marrow Transplant Unit (BMTU) or the Medical Intensive Care Unit (MICU) of Harper Hospital, a large tertiary-care hospital in Detroit, Mich. These were isolated from multiple anatomic sites and at separate times over a 1-year period from individual patients, as well as from different patients. Some isolates were recov-ered from the hands of hospital personnel or from environmental surfaces in both the BMTU and the MICU. In addition, several isolates were recovered from patients hospitalized at William Beaumont Hospital, Royal Oak, Mich. The reference isolates are described in Table 1. All standard, typically ATCC, yeast isolates were identified by germ tube production and chlamydospore formation on cornmeal agar and by the yeast API 20C method (bioMe´rieux Vitek, Inc., Hazelwood, Mo.) and were also verified with CHROMagar yeast plates and by electrophoretic karyotyping to verify the correct Candida species (38, 40, 57, 58). Clinical isolates were presumptively identified as C. albicans by germ tube for-mation, subject to confirmation by methods described in this report. Non-C. albicans Candida species were conventionally identified by the yeast API 20C method.
Yeasts were initially isolated on Sabouraud dextrose agar (SDA; Difco, De-troit, Mich.), stored in a 1:1 mixture of brain heart infusion broth (Difco) and glycerol, and frozen at270°C until analysis.
RAPD PCR conditions.PCRs were performed in a Robocycler 40
thermocy-cler (Stratagene, La Jolla, Calif.) with one of the following two programs (em-pirically modified from the program of Williams et al. [61]). Program A consisted of 5 cycles of 94°C for 30 s, 25°C for 2 min, and 72°C for 2 min; 45 cycles of 94°C for 30 s, 31°C for 2 min, and 72°C for 2 min; and 1 cycle of 72°C for 10 min. Program B consisted of 5 cycles of 94°C for 30 s, 25°C for 2 min, and 72°C for 2 min; 45 cycles of 94°C for 30 s, 34°C for 30 s, and 72°C for 2 min; and 1 cycle of 72°C for 10 min. Typical reactions, in mixtures of 25ml overlaid with mineral oil, were in 25 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 100mM deoxynucleoside triphosphates, 50 pmol of primer, and 2.5 U of Taq DNA polymerase, as provided by Bethesda Research Laboratories (Gaithersburg, Md.). Template DNAs were genomic DNA (,100 ng) or cells processed as described below. Reaction products were analyzed by ethidium bromide agarose gel electrophoresis (1.5% agarose). The sequence of primer CX5 is 59-ACACT
rpm and 30°C for 36 h. The Zymolyase-based method used to extract DNA has been described previously (58). Once the nucleic acids have been extracted and purified, the DNA was resuspended in 100ml of TE buffer (10 mM Tris [pH 7.5], 1 mM EDTA) and stored at 4°C. The DNA yield from this lysis procedure was typically about 1 mg per 200 ml of yeast cell culture.
Documentation.Polaroids of gels and autoradiograms were scanned with a
Logitech Scanner for printing at 300 dots per in.2. On occasion, exposures of lanes that were over- or underloaded were enhanced by the scanning software so that originally visible fragments were not lost in the reproduction.
RESULTS
Genomic DNA of Candida species can be amplified from single colonies by toothpick PCR.The conditions for releasing from single colonies DNA that was suitable for acting as a template for PCR were optimized for several reasons. First, they were optimized to arrive at a procedure that was rapid, simple, reproducible, and suitable for use ultimately in a clin-ical microbiology laboratory setting. Second, they were opti-mized to have a procedure that worked with all tested species of Candida. Our standard toothpick PCR protocol in its sim-plest form involves picking up a barely visible amount of cells from a single colony on a toothpick, suspending this in a buffer containing Zymolyase, and processing as described in Materi-als and Methods.
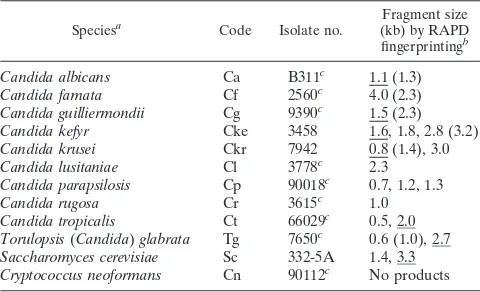
A number of PCR parameters were varied to test their influence on product patterns and yields. First, independent colonies of the same lineage of C. albicans gave the same amplification products (Fig. 1A to C). This and many other experiments demonstrate that the RAPD products obtained under these conditions are not idiosyncratic, and thus are po-tentially useful for species and strain identification. Second, similar RAPD products were obtained over a 10- to 100-fold range of starting template concentrations, thus reducing the chance that potential routine use of this method would be too sensitive to individual handling conditions. Third, similar am-plification products were obtained by using the colony lysates versus purified DNA (Fig. 1D and E). Varying the amount of primer in a series of 10-fold dilutions also did not dramatically alter the products unless the primer was present in excess (.100 pmol per 50-ml reaction mixture). Increasing the num-ber of cycles of amplification did not increase the yield. Alter-ing the temperature or the time of primer annealAlter-ing beyond that of programs A or B (see Materials and Methods) dramat-ically changed or eliminated RAPD products (data not shown). A number of alternate procedures for breaking open cells to release amplification-ready DNA were tried. We noted that some species could be amplified with no Zymolyase preincu-bation, presumably because some of the cells were broken by the initial near-boiling step of the PCR cycle. Other species of
Candida, however, required Zymolyase treatment. Boiling or
autoclaving the cell suspensions for 15 min before PCR also aSpecies were determined by conventional diagnostic methods.
bThe sizes of the PCR products were determined from Fig. 3 and 4. If multiple products are present, the major fragment is underlined; minor or variable frag-ments are given in parentheses.
cATCC isolate. ATCC number equivalents of some of the isolates are as follows: B3115ATCC 32354, 37785ATCC 34449, 36155ATCC 58964; 76505ATCC 2001.
on May 15, 2020 by guest
http://jcm.asm.org/
[image:2.612.58.298.82.228.2]sufficed for some but not all Candida species. All tested
Can-dida species generated suitable lysates for amplification after
vortexing for 1 min with 0.5-mm-diameter glass beads. Candida cells were incubated for 30 min in several organic solvents and then dried before they were diluted in TE as described above for PCR. This includes chloroform, ethanol, isopropanol, di-methyl sulfoxide, and mercaptoethanol. Again, variable yields and numbers of products resulted from these treatments in a species-dependent manner. Repeated cycles of freezing (270°C or dry ice-ethanol) and heating (50°C or boiling) did not work well for some Candida species, but was very efficient for high-quality templates from Aspergillus flavus and
Aspergil-lus fumigatus.
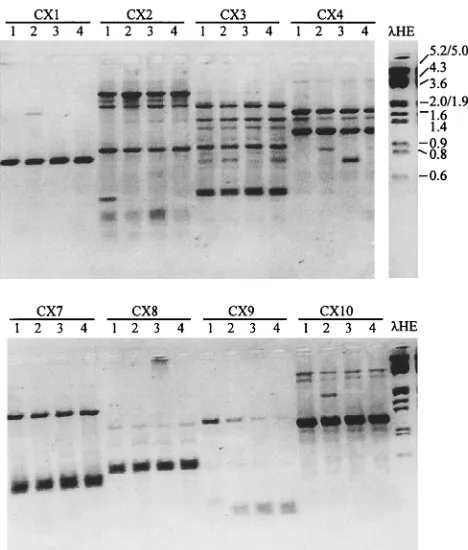
Ten different synthetic oligonucleotides, each with 10 bases of random sequence but with about 50% G1C content, were tested as primers to generate RAPD products from DNAs of major species of Candida. Most species generated at least one product. For example, four independent isolates of T. glabrata were amplified with each of eight primers (Fig. 2). Each primer generated a distinct fingerprint compared to those derived from other primers. For a given primer, each isolate generated basically the same fingerprint. These data suggest that the major RAPD products are potentially diagnostic for a species or at least for a subgroup of isolates of a species. A few RAPD products were polymorphic, e.g., the 0.4-kb fragment ated by CX2 from isolate X.35968, the 0.8-kb fragment gener-ated by CX4 from isolate W.40639, and the 1.9-kb fragment generated by CX10 from isolate CP.2B. The first two isolates were resistant to fluconazole, but with each primer they both generated the same profiles as the susceptible strains. Less intensely staining RAPD products were variable from one re-action to another and thus were not useful for strain delinea-tion.
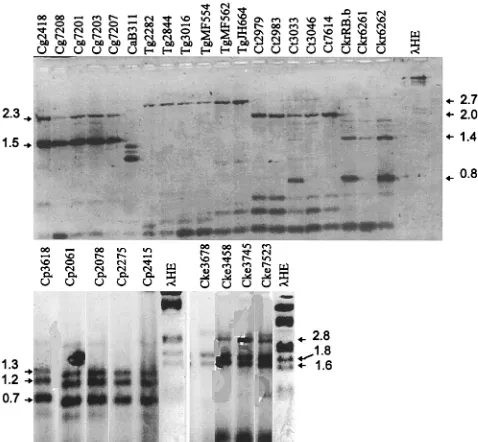
DNA of individual Candida species amplified by PCR to give distinct RAPD fragments.One primer, primer CX5, was cho-sen because of its capacity to prime multiple fragments in a reproducible manner from most Candida species. With this primer, RAPDs were generated from colony lysates of repre-sentative isolates of each major Candida species (Table 1). Each species generated from one to four major fragments (Fig. 3). Each isolate representative of a species generated different fragments from those of isolates of other species. Within a species, e.g., duplicate colonies of Candida kefyr 3458 and
independent isolates of C. krusei, C. lusitaniae, or
Saccharomy-ces cerevisiae, the RAPD profiles were identical or nearly
[image:3.612.109.508.74.228.2]iden-tical. Less intensely staining fragments were not always evident in independent PCRs of identical templates. More intensely staining fragments were reproducible, to the extent that spe-cies were identified from RAPD products in blind tests. Iden-tical or nearly idenIden-tical RAPD products were generated from FIG. 1. Characterization of products generated by toothpick PCR. (A to C) Three independent colonies of C. albicans B311 were toothpicked and incubated as described in the text. Instead of the standard dilution after Zymolyase treatment, the lysates were diluted as indicated from an initial estimated concentration of 105 cells perml. (D and E) Products derived from toothpicked colony lysates are compared to those from purified genomic DNA (10 ng) over the indicated range of primer concentrations. All reactions (rxn) were performed by PCR program A with primer CX5. Reactions products were analyzed on a 1.5% agarose–ethidium bromide gel. The size standards (in kilobases) in this and subsequent figures were generated by EcoRI-HindIII digestion of bacteriophage lambda (lanelHE).
FIG. 2. RAPD products of isolates of T. glabrata with eight primers. Single-colony lysates of four independent isolates of T. glabrata were amplified with the indicated CX primers by program A and were analyzed by 1.5% agarose– ethidium bromide gel electrophoresis. The isolates were X.35968 (lanes 1), CP 2B (lanes 2), W.40639 (lanes 3), and 7650 (lanes 4).lHE is described in the legend to Fig. 1.
on May 15, 2020 by guest
http://jcm.asm.org/
[image:3.612.318.552.398.673.2]the purified DNA of each isolate, as depicted in Fig. 3 (data not shown).
To test whether these fingerprints (Fig. 3) were unique for each isolate or were conserved among isolates within a species, we analyzed three to six isolates of six different Candida spe-cies. Each fingerprint was characteristic and specific for each of the Candida species tested (Fig. 4). Some fragments within a fingerprint were unique to an isolate, e.g., isolate 3033, but the fingerprint was easily recognized when it was superimposed on this polymorphism. The sizes of the diagnostic products for each species are listed in Table 1. To determine if these spe-cies-specific fingerprints were sufficiently conserved among larger numbers of isolates to allow them to be used as a diag-nostic tool for Candida species, we tested larger numbers of isolates of C. lusitaniae, T. glabrata, and C. albicans.
RAPD fingerprints of 37 C. lusitaniae isolates.DNAs of C.
lusitaniae isolates amplified by primer CX5 generated one of
two distinct RAPD fingerprints (Fig. 5A). The standard fin-gerprint was a single 2.3-kb fragment generated from 16 iso-lates, herein labeled type Cl-I. Note that Cl-I was reproduced independently in Fig. 3 from C. lusitaniae 3778.
A second fingerprint consisted of a preparation- and exper-iment-dependent pattern of either a faint 0.9-kb product or no product at all. This pattern, type Cl-II, could be interpreted in three ways. First, it may be artifactual due to poor or contam-inated templates. Second, Cl-II isolates may represent a sub-group of C. lusitaniae isolates that are missing one or both of the primer binding sites flanking the 2.1-kb sequence present in Cl-I isolates. Third, Cl-II isolates might constitute one or more species not previously characterized by CX5-primed RAPD fingerprints.
To determine which interpretation was correct, we repeated the RAPD analysis with an independent primer, primer PST, using the same lysate preparations that generated Cl-II pat-terns. Unlike CX5, PST primed a uniform, distinct set of RAPD fingerprints (1.3- and 0.7-kb fragments) that were iden-tical to those for the control Cl-I isolate, isolate 3778 (Fig. 5B). Several other RAPD fingerprints (data not shown) confirm that this fingerprint obtained with primer PST is not generated by any of the other Candida species from Fig. 3. By these criteria, Cl-II isolates constitute a subgroup of C. lusitaniae isolates. These data also suggest that the PST primer is a more inclusive diagnostic tool for this species than CX5, but that
fingerprint is a 2.7-kb DNA fragment. Some isolates also gen-erated small amounts of 0.6- and 1.0-kb products. The pres-ence and yield of these varied from experiment to experiment, even with the same isolate, and did not detract from identifying these isolates by their signature 2.7-kb product.
Only 3 among the 53 isolates did not generate the T.
gla-brata-specific product. Two of these, isolates 2123 and 2347,
instead generated comigrating 2.1-kb fragments characteristic of C. lusitaniae isolates (Fig. 3). Consistently, both of these isolates generated PST-primed fingerprints (data not shown) that were identical to those for known C. lusitaniae isolates (Fig. 5B). The third deviant, isolate 2474, generated a finger-print characteristic of the Candida guilliermondii standard fin-gerprints shown in Fig. 3 and 4. The latter fingerprint is char-acteristic of those of other clinical isolates that were identified as C. guilliermondii by conventional means.
These data suggest that CX5-primed RAPD fingerprints may be a useful diagnostic tool for identifying T. glabrata among clinical isolates.
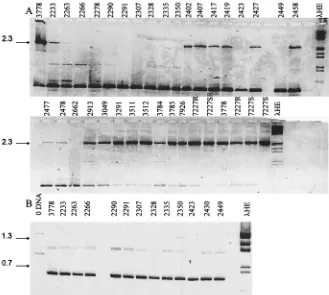
[image:4.612.79.279.71.201.2]RAPD fingerprints of 112 C. albicans isolates.In the first of two experiments, a set of 34 isolates identified as C. albicans by germ tube and chlamydospore formation and with the API 20C
FIG. 4. RAPD fingerprinting of independent isolates of seven Candida spe-cies. Colony lysates of the indicated isolates were amplified as described in the legend to Fig. 3 and analyzed on 1.5% agarose–ethidium bromide gels. Arrows indicate key species-specific products; the sizes of these are listed in Table 1.
lHE is described in the legend to Fig. 1. method and were amplified by program B (see Materials and Methods). RAPD
products were analyzed on 1% Separide–ethidium bromide gels (Bethesda Re-search Laboratories). Note that the smallest fragment is also seen in the no DNA (0 DNA) control lane and is thus a template-independent artifact. lHE is described in the legend to Fig. 1.
on May 15, 2020 by guest
http://jcm.asm.org/
[image:4.612.316.555.461.682.2]system were used in this study. These isolates were recovered from patients over a 2-year period and from three different hospital units. They were not randomly chosen from isolates identified as C. albicans; instead, these were chosen on the basis of their resistance to fluconazole (MICs, .20 mg/ml) compared to that of a well-characterized isolate of C. albicans (B311; MIC of fluconazole,,0.25mg/ml). Since these isolates were atypical by this criteria, we were rigorously testing whether the RAPD fingerprint derived from C. albicans B311 would be conserved in all isolates of the species.
Toothpick lysates were amplified and analyzed (Fig. 7). In most isolates (n 5 28), there was a predominant, species-specific 1.1-kb fragment that was shared by C. albicans B311, a well-known and well-characterized laboratory isolate of C.
al-bicans. Additional minor bands constituted subfamilies of
re-lated patterns A9, A0, A2, and A1. Thus, fluconazole-resistant isolates did not constitute a subgroup by their RAPD finger-prints.
The patterns of six isolates in Fig. 7 (isolates CPA, 286, A.31, 7227, 3343, and 3018) were substantially different from the A pattern, notably missing the signature 1.1-kb product. On the basis of a comparison of these to RAPD fingerprints of known species in Fig. 3, we predicted that five of these isolates were originally misidentified as C. albicans and were actually T.
glabrata (isolates CPA, 3343, and 3018), C. lusitaniae
(iso-late 7227), and C. tropicalis (iso(iso-late 286). That these new identities are correct was confirmed by CHROMagar Can-dida platings, by hybridization to species-specific DNA probes (51a), and by repeating the API 20C tests. Isolate A.31
gener-ated a RAPD fingerprint that did not conform to that for any other tested isolate and is presumably either none of the tested species or represents a subtype that has not yet been charac-terized.
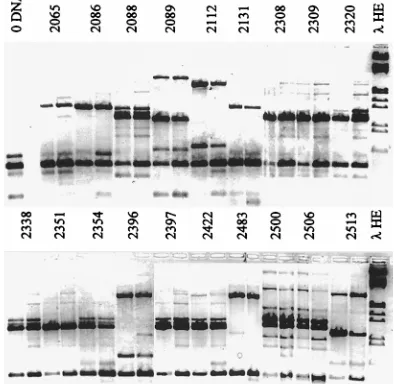
[image:5.612.142.471.69.364.2]In the second experiment, 19 randomly chosen isolates con-ventionally identified as C. albicans were fingerprinted (Fig. 8). Surprisingly, only 11 of these had C. albicans-specific finger-prints, consisting minimally of a 1.1-kb fragment and often forming a doublet or triplet with slightly larger products, as shown in Fig. 3. Deviant fingerprint patterns (found for eight
[image:5.612.317.556.563.675.2]FIG. 6. RAPD fingerprinting of T. glabrata isolates. Toothpick lysates of the indicated isolates were amplified with primer CX5 as described in the legend to Fig. 3 and were analyzed on 1% Separide–ethidium bromide gels. The arrow in-dicates the 2.5-kb product that defines the fingerprint. Fragments that seem slight-ly shifted in this figure, e.g., from isolates 2243 and 2287, were shown to comi-grate in other gels (data not shown).lHE is described in the legend to Fig. 1. FIG. 5. RAPD fingerprinting of C. lusitaniae isolates. Toothpick lysates of the indicated 12 isolates were amplified as described in the legend to Fig. 3 and analyzed on 1% Separide–ethidium bromide gels. (A) Primer CX5 was used. (B) Primer PST was used. Note that isolate 7227, originally misidentified as C. albicans, here verifies the interpretation in Fig. 4 that it is a C. lusitaniae isolate, along with derivative colonies 7227R, S, SR, and RS. Also note that the smallest RAPD product in panel A is template independent, as described in the legend to Fig. 3, and thus does not represent a C. lusitaniae-derived fragment shared by Cl-I and Cl-II subtypes. Arrows indicate the products that define the fingerprint.lHE is described in the legend to Fig. 1.
on May 15, 2020 by guest
http://jcm.asm.org/
isolates) fell into three groups. The first deviant pattern, de-rived from isolates 2089 and 2513, contained the same three fragments generated by the standard strain T. glabrata 7650 (Fig. 3), but the relative intensities of the fragments differed. Hybridization data (data not shown) confirm that these frag-ments are homologous. The second deviant pattern, generated by isolates 2112, 2396, and 2483, duplicated the standard fin-gerprint of C. lusitaniae 3778 (Fig. 3). The third deviant pat-tern, a single 1.3-kb fragment, was generated from isolates 2065, 2086, and 2131. This fingerprint is not characteristic of any tested species (Fig. 3). Isolates with this atypical fingerprint may constitute a subgroup of C. albicans, since some isolates have the diagnostic green color on CHROMagar Candida plates typical of C. albicans. No isolates that were convention-ally identified as non-C. albicans Candida species generated unidentified fingerprints.
In addition to the 53 isolates whose fingerprints are pre-sented in Fig. 7 and 8, another 59 putative C. albicans isolates were also tested (data not shown). Among these, 42 had C.
al-bicans type A fingerprints and 17 fingerprinted as non-C. albi-cans Candida isolates, suggesting that their original
identifica-tion by germ tube formaidentifica-tion was unreliable. Among these 17 isolates, 3 had T. glabrata fingerprints, 2 had C. lusitaniae fin-gerprints, and 1 had a C. guilliermondii fingerprint. The other 11 isolates generated fingerprints atypical of those of any known species. Four of these generated a common 1.6- and 1.4-kb doublet. The remaining seven isolates generated single, unique products.
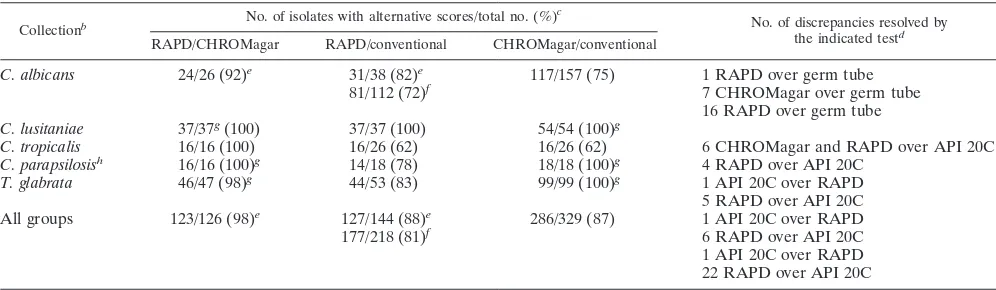
Comparison of RAPD fingerprinting to CHROMagar Can-dida plate tests and API 20C tests. Summary pairwise
com-compare favorably even to those generated by second-genera-tion diagnostic kits which require follow-up tests (12). Second, both fingerprints and CHROMagar Candida tests frequently disagree with API 20C results or germ tube formation studies, most notably (20 to 40%) among those isolates identified by the latter as C. albicans or C. tropicalis. Among those C.
albi-cans isolates that were tested by other methods, the identities
[image:6.612.58.298.72.307.2]of all but one were confirmed to be the identities assigned by the fingerprint. This suggests that the conventional identifica-tion methods, as applied in the clinical laboratories in this study, are not able to discriminate among a subset of isolates, some belonging to other Candida species and still others that are currently mislabeled incorrectly as C. albicans. All isolates designated C. tropicalis by API 20C but not by RAPD finger-printing were found to be non-C. tropicalis isolates by CHROMagar Candida tests. In some cases, the results of re-peat API 20C tests contradicted that of the first API 20C test and supported the result obtained by RAPD fingerprinting, again pointing out the propensity for human error in scoring these tests. Third, RAPD fingerprints are at least as specific among non-C. albicans Candida isolates as API 20C tests. It remains to be determined whether isolates of phenotypically similar Candida species, or indeed of isolates within a species
[image:6.612.337.534.480.672.2]FIG. 8. RAPD fingerprinting of fluconazole-susceptible C. albicans isolates. Duplicate colony lysates of the indicated isolates were amplified as described in the legend to Fig. 3 and were analyzed on 1% Separide–ethidium bromide gels. C. albicans isolates were randomly chosen for fingerprinting and secondarily were characterized as susceptible to azoles.lHE is described in the legend to Fig. 1.
FIG. 7. RAPD fingerprinting of fluconazole-resistant C. albicans isolates. Clinical isolates that were identified initially as C. albicans by conventional diagnostic methods were analyzed by toothpick PCR as described in the legend to Fig. 3. Twenty-eight of the 34 isolates generated highly similar RAPD frag-ment patterns (A patterns; the pattern type is listed after the isolate numbers). The intensely staining 1.1-kb fragment is the hallmark of the A pattern. Super-imposed on the basic A pattern are subgroups that differ primarily in the pres-ence or position of substoichiometric fragments (A9, A0, A2, A1). Asterisks or circles mark fragments that distinguish one subtype from another. Six isolates generated atypical patterns 1 to 4, as discussed in the text.lHE is described in the legend to Fig. 1.
on May 15, 2020 by guest
http://jcm.asm.org/
from a more geographically dispersed collection, will maintain the species-specific fingerprints generated by our collection.
Our conditions for RAPD fingerprinting are accurate, rapid, simple, and reproducible, thus allowing for the processing of very small single Candida colonies as soon as they are visible. After a 3-h reaction and a 1- or 2-hour gel electrophoresis, the species of Candida can be determined in most cases. Even CHROMagar Candida plates require 72 h of incubation for the specific identification of C. albicans (2). The principal asset of RAPD fingerprints is that they are species specific, yet they are still simple enough to obtain to give determinative identi-fication without computer-assisted analysis. What struck us as remarkable is that there were so few polymorphic products within species in this study. Most of those that were seen were additional, minor fragments in addition to the signature frag-ment defined in Table 1. The polymorphic pattern seen in some C. lusitaniae isolates is an exception to this that suggests that further studies will show small, but manageable, numbers of fingerprints that define a species. In its simplest form, one could envision the technician performing the toothpick sam-pling of 12- to 24-h-old colonies, PCR, and electrophoresis on a premade mini-gel with a supplied molecular weight standard consisting of combined RAPD products of the major Candida species. The unknown RAPD fingerprint in most cases could be visually determined instantly by comigration. By this meth-od, there are no requirements for DNA preparations, fastidi-ous spheroplasting, synthesis of probes, hybridizations, or film development. The RAPD fingerprints in the majority of cases are sufficiently simple that many fingerprint combinations are recognized when they are superimposed. This will allow for the identification of individual Candida species from mixed yeast cultures when the two species cannot be distinguished mor-phologically. Since the dilutions of the initial colony lysates required in this study are so large, our goal is to be able to fingerprint directly from biological samples.
The utility of this approach depends on two kinds of exten-sions of our current analysis. First, more RAPD fingerprints
must be collected and recorded for those Candida species which are underrepresented in this study, especially, C. krusei,
C. kefyr, and Candida rugosa. Second, further analysis is
need-ed of those isolates in this study whose conventional identifi-cations are in apparent discrepancy with those of the RAPD fingerprint. The frequency of this occurrence must be evalu-ated in a larger study with isolates from diverse sources since errors in conventional identifications will probably vary greatly with the experience of the individuals performing the tests.
In our case, conventional identification of C. albicans was clearly the most problematic, since the identities of almost 30% of isolates were in dispute. This is a lower percentage than reported in other comparable studies (2). This difference is a result of our original identifications of C. albicans, which relied mostly on positive germ tube tests. Half of the 30% of isolates whose identities were in dispute were identified by our own conventional biochemical tests as non-C. albicans Candida spe-cies. We suspect that the source of the original misidentifica-tion was human error (e.g., sample labeling or contaminamisidentifica-tion). A more interesting issue, however, is raised by the other 15% of the disputed C. albicans identifications in which the RAPD fingerprints were unrecognizable. These numbered less than 5% of the total fingerprinted isolates, indicating a high degree of sensitivity as well as specificity. These isolates, all of which were identified as C. albicans by positive germ tube formation, had either of two fingerprints: a 1.3-kb fragment or a 1.6- and 1.4-kb doublet. Neither had any products that comi-grated with the products of standard Candida species. The isolates in this group that were tested on CHROMagar Can-dida plates were green. This generally indicates a strain of C.
albicans, so they may indeed be C. albicans isolates that have
[image:7.612.58.556.81.226.2]undergone extensive rearrangements. Although CHROMagar testing is not the “gold standard,” the results were verified by electrophoretic karyotyping. In addition, there is evidence for this type of variability and alteration among C. albicans isolates by electrophoretic karyotyping (32, 37). We are currently an-alyzing isolates from AIDS patients which have a range of
TABLE 2. Comparison of yeast identifications made by RAPD fingerprinting, with CHROMagar Candida plates, and by conventional testsa
Collectionb No. of isolates with alternative scores/total no. (%) c
No. of discrepancies resolved by the indicated testd RAPD/CHROMagar RAPD/conventional CHROMagar/conventional
C. albicans 24/26 (92)e 31/38 (82)e 117/157 (75) 1 RAPD over germ tube
81/112 (72)f 7 CHROMagar over germ tube
16 RAPD over germ tube C. lusitaniae 37/37g(100) 37/37 (100) 54/54 (100)g
C. tropicalis 16/16 (100) 16/26 (62) 16/26 (62) 6 CHROMagar and RAPD over API 20C C. parapsilosish 16/16 (100)g 14/18 (78) 18/18 (100)g 4 RAPD over API 20C
T. glabrata 46/47 (98)g 44/53 (83) 99/99 (100)g 1 API 20C over RAPD
5 RAPD over API 20C All groups 123/126 (98)e 127/144 (88)e 286/329 (87) 1 API 20C over RAPD
177/218 (81)f 6 RAPD over API 20C
1 API 20C over RAPD 22 RAPD over API 20C
aConventional tests include API 20C and germ tube formation; for most C. albicans isolates, identifications were based initially on positive germ tube tests. Data include RAPD fingerprints from previous figures and data not previously shown.
bCollections include only those for which RAPD fingerprints were available.
cNumbers indicate those isolates of each species, grouped by conventional tests, for which alternative test scores were available/total number of isolates tested (percent identical or consistent identifications of pairs of tests).
dPairwise discrepancies in assigned identities were resolved by the third method or independently by electrophoretic karyotype and in some cases by a repeat API 20C test.
eExcludes a set of C. albicans isolates that were not tested on CHROMagar Candida plates and that were atypical by drug susceptibility testing or electrophoretic karyotype.
fIncludes a set of C. albicans isolates that were not tested on CHROMagar Candida plates and that were atypical by drug susceptibility testing or electrophoretic karyotype.
gRatios indicate consistent identifications, not identities, since these CHROMagar Candida plate scores do not distinguish among several species. hIsolates identified by API 20C as C. parapsilosis were atypical C. lusitaniae isolates (data not shown).
on May 15, 2020 by guest
http://jcm.asm.org/
priately under normal stringency conditions.
A final validation for RAPD fingerprinting as a method for diagnosing the species responsible for fungal diseases is the emergence of new and atypical varieties and species of oppor-tunistic fungal pathogens as a consequence of the improved rate of survival of immunocompromised patients (16, 44). By this same technology, RAPD fingerprints along with definitive DNA probes can be rapidly developed and used as these mi-crobes appear in clinical situations.
In conclusion, the RAPD methodology has the capability to identify Candida species rapidly, accurately, reliably, and sim-ply early on in the disease process. This capacity enables the clinician to use the most appropriate antifungal agent early enough to possibly affect the morbidity and mortality resulting from fungal infections. In addition to its rapid diagnosis and
Candida identification roles, this process also enables the use
of RAPD fingerprints to assist with the determination of the molecular epidemiology of nosocomial fungal infections by having the ability to delineate among strains of the various
Candida species which were evaluated.
REFERENCES
1. Banerjee, S. N., T. G. Emori, D. H. Culver, G. P. Gaynes, W. R. Jarvis, and
T. Huran.1991. Secular trends in nosocomial primary bloodstream
infec-tions in the United States. Am. J. Med. 91(Suppl. 3b):86S–89S.
2. Baumgartner, C., A. Freydiere, and Y. Gille. 1996. Direct identification and recognition of yeast species from clinical material by using Albicans ID and CHROMagar Candida plates. J. Clin. Microbiol. 34:454–456.
3. Berenguer, J., T. M. Diaz-Guerra, B. Ruiz-Diaz, J. C. L. B. de Quiros, J. L.
Rodriguez-Tudela, and J. V. Martinez-Suarez.1996. Genetic dissimilarity of
two fluconazole resistant Candida albicans strains causing meningitis and oral candidiasis in the same AIDS patient. J. Clin. Microbiol. 34:1542–1545. 4. Berrouane, Y. F., R. J. Hollis, and M. A. Pfaller. 1996. Strain variation among and antifungal susceptibilities of isolates of Candida krusei. J. Clin. Microbiol. 34:1856–1858.
5. Bodey, G. P. 1986. Candidiasis in cancer patients. Am. J. Med. 77S:13–19. 6. Bodey, G. P. 1993. Candidiasis: pathogenesis, diagnosis, and treatment, 2nd
ed. Raven Press, Ltd., New York, N.Y.
7. Bostock, A., M. N. Khattak, R. Matthews, and J. Burnie. 1993. Comparison of PCR fingerprinting, by random amplification of polymorphic DNA, with other molecular typing methods for Candida albicans. J. Gen. Microbiol.
139:2179–2184.
8. Carlotti, A., R. Grillot, A. Couble, and J. Villard. 1994. Typing of C. krusei clinical isolates by restriction endonuclease analysis and hybridization with CkF1,2 DNA probe. J. Clin. Microbiol. 32:1691–1699.
9. de Repentigny, L., and E. Reiss. 1984. Current trends in immunodiagnosis of candidiasis and aspergillosis. Rev. Infect. Dis. 6:301–312.
10. Dick, J. D., B. R. Rosengard, W. G. Merz, R. K. Stuart, G. M. Hutchins, and
R. Saral.1985. Fatal disseminated candidiasis due to amphotericin
B-resis-tant Candida guilliermondii. Ann. Intern. Med. 102:67–68.
11. El-Zaatari, M., L. Pasarell, M. R. McGinnis, J. Buckner, G. A. Land, and
I. F. Salkin.1990. Evaluation of the updated Vitek yeast identification data
base. J. Clin. Microbiol. 28:1938–1941.
12. Engleberg, N. C., and B. I. Eisenstein. 1992. Detection of microbial nucleic acids for diagnostic purposes. Annu. Rev. Med. 43:147–155.
13. Fenn, J., H. Segal, B. Barland, D. Denton, J. Whisenant, H. Chun, K.
Christofferson, L. Hamilton, and K. Carroll.1994. Comparison of updated
patients with hematologic malignancies. Eur. J. Microbiol. Infect. Dis. 14: 768–774.
20. Jones, J. M. 1990. Laboratory diagnosis of invasive candidiasis. Clin. Micro-biol. Rev. 3:32–45.
21. Karyotakis, N., M. Dignani, and E. Anaissie. 1995. SCH 51048, a new antifungal triazole active against hematogenous Candida krusei infections in neutropenic mice. Antimicrob. Agents Chemother. 39:775–777.
22. King, D., J. Rhine-Chalberg, M. A. Pfaller, S. A. Moser, and W. G. Merz. 1995. Comparison of four DNA-based methods for strain delineation of Candida lusitaniae. J. Clin. Microbiol. 33:1467–1470.
23. Kitch, T. T., M. R. Jacobs, M. R. McGinnis, and P. C. Appelbaum. 1996. Ability of RapID yeast system to identify 304 clinically significant yeasts within 5 hours. J. Clin. Microbiol. 34:1069–1071.
24. Komshian, S. V., A. Uwaydah, J. D. Sobel, and L. R. Crane. 1989. Fungemia caused by Candida species and Torulopsis in hospitalized patients. Fre-quency, characteristics, and evaluation of factors influencing outcome. Rev. Infect. Dis. 3:379–390.
25. Kubelik, A. R., and L. J. Szabo. 1995. High GC-primers are useful in RAPD analysis of fungi. Curr. Genet. 28:384–389.
26. Kullberg, B. J., and A. Voss. 1996. The changing pattern of Candida infec-tions: different species and increased resistance. Ned. Tijdschr. Geneeskd.
140:148–151.
27. Land, G., J. Burke, C. Shelby, J. Rhodes, J. Collett, I. Bennett, and J.
Johnson.1996. Screening protocol for Torulopsis (Candida) glabrata. J. Clin.
Microbiol. 34:2300–2303.
28. Lehmann, P. F., D. Lin, and B. A. Lasker. 1992. Genotypic identification and characterization of species and strains within the genus Candida by using random amplified polymorphic DNA. J. Clin. Microbiol. 30:3249–3254. 29. Lin, D., and P. Lehmann. 1995. Random amplified polymorphic DNA for
strain delineation within Candida tropicalis. J. Med. Vet. Mycol. 33:241–246. 30. Magee, B. B., E. H. A. Rikkerink, and P. T. Magee. 1988. Methods for the genetics and molecular biology of Candida albicans. Anal. Biochem. 175: 361–372.
31. Magee, B. B., T. M. D’Souza, and P. T. Magee. 1987. Strain and species identification by restriction fragment length polymorphisms in the ribosomal DNA repeat of Candida species. J. Bacteriol. 169:1639–1643.
32. Magee, P. T. 1993. Variations in chromosome size and organization in Candida albicans and Candida stellatoidea. Trends Microbiol. 1:338–342. 33. Maiwald, M., R. Kappe, and H. G. Sonntag. 1994. Rapid presumptive
iden-tification of medically relevant yeasts to the species level by polymerase chain reaction and restriction enzyme analysis. J. Med. Vet. Mycol. 32:115–122. 34. Marichal, P., J. Gorrens, M. C. Coene, L. Le Jeune, and H. Vanden Bossche.
1995. Origin of differences in susceptibility of Candida krusei to azole anti-fungal agents. Mycoses 38:111–117.
35. McCullough, M. J., B. C. Ross, B. D. Dwyer, and P. C. Reade. 1994. Geno-type and phenoGeno-type of oral Candida albicans from patients infected with the human immunodeficiency virus. Microbiology 140:1195–1202.
36. Merz, W. G. 1980. Candida lusitaniae: frequency of recovery, colonization, infection, and amphotericin B resistance. J. Clin. Microbiol. 20:1194–1195. 37. Merz, W. G., C. Connelly, and P. Hieter. 1988. Variation of electrophoretic karyotypes among clinical isolates of Candida albicans. J. Clin. Microbiol.
26:842–845.
38. Odds, F. C., and R. Bernaerts. 1994. CHROMagar Candida, a new differ-ential isolation medium for presumptive identification of clinically important Candida species. J. Clin. Microbiol. 32:1923–1929.
39. Penner, G. A., A. Bush, R. Wise, W. Kim, L. Domier, K. Kasha, A. Laroche,
G. Scoles, S. J. Molnar, and G. Fedak.1993. Reproducibility of random
amplified polymorphic DNA (RAPD) analysis among laboratories. PCR Methods Appl. 2:341–345.
40. Pfaller, M. A., A. Houston, and S. Coffman. 1996. Application of CHROMa-gar Candida for rapid screening of clinical specimens for Candida albicans, C. krusei, and Candida (Torulopsis) glabrata. J. Clin. Microbiol. 34:58–61. 41. Pfaller, M. A., J. Rhine-Chalberg, S. W. Redding, J. Smith, G. Farinacci,
on May 15, 2020 by guest
http://jcm.asm.org/
A. W. Fothergill, and M. G. Rinaldi.1994. Variations in fluconazole suscep-tibility and electrophoretic karyotype among oral isolates of Candida albi-cans from patients with AIDS and oral candidiasis. J. Clin. Microbiol. 32: 59–64.
42. Powderly, W., G. Kobayashi, G. Herzig, and G. Medoff. 1988. Amphotericin B resistant yeast infection in severely immunocompromised patients. Am. J. Med. 84:826–832.
43. Redding, S., J. Smith, G. Farinacci, M. G. Rinaldi, A. Fothergill, J.
Rhine-Chalberg, and M. A. Pfaller.1994. Resistance of Candida albicans to
flu-conazole during treatment of oropharyngeal candidiasis in a patient with AIDS: documentation by in vitro susceptibility testing and DNA subtype analysis. Clin. Infect. Dis. 18:240–242.
44. Rinaldi, M. G. 1989. Emerging opportunists, p. 65–76. In D. Drutz (ed.), Systemic fungal infections: diagnosis and treatment II. The W. B. Saunders Company, Philadelphia, Pa.
45. Robert, F., F. Lebreton, M. E. Bougnoux, A. Paugam, D. Wassermann, M.
Schlotterer, C. Tourte-Schaefer, and J. Dupouy-Camet.1995. Use of random
amplified polymorphic DNA as a typing method for Candida albicans in epidemiological surveillance of a burn unit. J. Clin. Microbiol. 33:2366–2371. 46. Ruhnke, M., A. Eigler, I. Tennagen, B. Geiseler, E. Engelmann, and M.
Trautmann.1994. Emergence of fluconazole-resistant strains of Candida
albicans in patients with recurrent oropharyngeal candidosis and human immunodeficiency virus infection. J. Clin. Microbiol. 32:2092–2098. 47. Rustchenko-Bulgac, E. P., F. Sherman, and J. B. Hicks. 1990. Chromosomal
rearrangements associated with morphological mutants provide a means for genetic variation of Candida albicans. J. Bacteriol. 172:1276–1283. 48. Sandhu, G. S., B. C. Kline, L. Stockman, and G. D. Roberts. 1995. Molecular
probes for diagnosis of fungal infections. J. Clin. Microbiol. 33:2913–2919. 49. Scherer, S., and D. A. Stevens. 1987. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J. Clin. Microbiol. 25:675– 679.
50. Schonian, G., O. Meusel, H. J. Tietz, W. Meyer, Y. Graser, I. Tausch, W.
Presber, and T. G. Mitchell.1993. Identification of Candida albicans by
DNA fingerprinting with the polymerase chain reaction. Mycoses 36:171– 179.
51. Spinillo, A., E. Capuzzo, T. O. Egbe, F. Baltaro, S. Nicola, and G. Piazzi. 1995. Torulopsis glabrata vaginitis. Obstet. Gynecol. 85:993–998.
51a.Steffan, P., J. A. Vazquez, and R. A. Akins. Unpublished data.
52. Stevens, D. A., F. C. Odds, and S. Scherer. 1990. Application of DNA typing methods to Candida albicans epidemiology and correlations with phenotype. Rev. Infect. Dis. 12:258–266.
53. Sullivan, D., M. Henman, G. Moran, L. O’Neill, D. Bennett, D. Shanley, and
D. Coleman.1996. Molecular genetic approaches to identification,
epidemi-ology and taxonomy of non-albicans Candida species. J. Med. Microbiol.
44:399–408.
54. Thanos, M., G. Schonian, W. Meyer, C. Schweynoch, Y. Graser, T. G.
Mitchell, W. Presber, and H. J. Tietz.1996. Rapid identification of Candida
species by DNA fingerprinting with PCR. J. Clin. Microbiol. 34:615–621. 55. Tietz, H. J., A. Kussner, M. Thanos, M. P. de Andrade, W. Presber, and G.
Schonian.1995. Phenotypic and genotypic characterization of unusual
vag-inal isolates of Candida albicans from Africa. J. Clin. Microbiol. 33:2462– 2465.
56. van Belkum, A., W. Melchers, B. E. de Pauw, S. Scherer, W. Quint, and J. F.
Miles.1994. PCR-mediated genotyping of Candida albicans strains from
bone marrow transplant patients. Bone Marrow Transplant. 13:811–815. 57. Vazquez J. A., A. Beckley, S. Donabedian, J. D. Sobel, and M. J. Zervos.
1993. Comparison of restriction enzyme analysis versus pulse-field gradient electrophoresis as a typing system for Torulopsis and Candida species other than C. albicans. J. Clin. Microbiol. 31:2021–2030.
58. Vazquez, J. A., A. Beckley, J. D. Sobel, and M. J. Zervos. 1991. Comparison of restriction enzyme analysis versus pulsed-field gradient gel electrophoresis as a typing system for Candida albicans. J. Clin. Microbiol. 29:962–967. 59. Vazquez, J. A., T. Lundstrom, L. Dembry, P. Chandrasekar, D. Boikov, and
M. B. Perri.1995. Invasive Candida guilliermondii infection: in vitro
suscep-tibility studies and molecular analysis. Bone Marrow Transplant. 16:849–853. 60. Weissman, A., I. Berdicevsky, and B. Cavari. 1995. Molecular identification
of Candida albicans. J. Med. Vet. Mycol. 33:205–207.
61. Williams, A. G., A. R. Kubelik, K. J. Livak, J. A. Rafalski, and S. V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 18:6531–6535.
62. Williams, D. W., M. J. Wilson, M. A. O. Lewis, and A. J. C. Potts. 1995. Identification of Candida species by PCR and restriction fragment length poly-morphism analysis of intergenic spacer regions of ribosomal DNA. J. Clin. Microbiol. 33:2476–2479.
63. Wingard, J. R. 1995. Importance of Candida species other than C. albicans as pathogens in oncology. Clin. Infect. Dis. 20:115–125.