Introduction: We conducted a phase II study of combination chemo-therapy with irinotecan (CPT) and cisplatin (CDDP) in patients with advanced large-cell neuroendocrine carcinoma (LCNEC) of the lung.
Methods: Patients received irinotecan (60 mg/m2, days 1, 8, and 15)
and cisplatin (60 mg/m2, day 1) every 4 weeks for up to four cycles.
The primary endpoint was the response rate. Expected and threshold values for the primary endpoint were 50% and 30%.
Results: Forty-four patients were enrolled between January 2005 and November 2011. The response rate (RR) was 54.5% (95% confi-dence interval [CI], 38.8–69.6%). The median progression-free sur-vival time was 5.9 months (95% CI, 5.5–6.3), and the median sursur-vival time was 15.1 months (95% CI, 11.2–19.0). A central pathological review of specimens from 41 patients demonstrated that 30 patients had LCNEC but that 10 patients had small-cell lung cancer (SCLC) and one had non–small-cell lung cancer with a neuroendocrine struc-ture. The RR was 46.7% (95% CI, 28.3–65.7%) in the LCNEC group and 80% (95% CI, 44.4–97.5%) in the SCLC group (p = 0.0823). The median survival time was 12.6 months (95% CI, 9.3–16.0) in the
LCNEC group and 17.3 months (95% CI, 11.2–23.3) in the SCLC group (p = 0.047).
Conclusions: Combination chemotherapy with irinotecan and cis-platin was active in patients with LCNEC, but the RR and the over-all survival period among the patients with LCNEC seemed to be inferior to those among the patients with SCLC. Small numbers of patients were a major limitation in this study.
Key Words: Cisplatin, Irinotecan, Large-cell neuroendocrine carci-noma, Lung.
(J Thorac Oncol. 2013;8: 980-984)
L
arge-cell neuroendocrine carcinoma (LCNEC) and small-cell lung cancer (SCLC) are recognized as high-grade neuroendocrine carcinoma of the lung, and account for approximately 15% of all forms of lung cancer. A consider-able overlap in nuclear size was observed between LCNEC and SCLC in a morphometric analysis.1 Thus, a differential diagnosis between LCNEC and SCLC using tiny biopsy specimens is often difficult. Consequently, prospective clin-ical trials on chemotherapy in advanced LCNEC have not been reported. LCNEC shares many similarities with SCLC in terms of not only structure, immunohistochemistry, and molecular biology, but also treatment;2 several studies have shown that LCNEC responds to cisplatin-based chemothera-peutic regimens similar to those used for SCLC.3,4 However, as LCNEC is a poorly recognized and underdiagnosed entity, it is frequently mistaken for poorly differentiated non–small-cell lung cancer (NSCLC), atypical carcinoid, or intermediate cell-type SCLC.5,6 In one previous study that included 75 patients, only 44 (53%) were correctly diag-nosed as having LCNEC at the outset, whereas 31 (47%) were misdiagnosed as having other NSCLCs.7 Such diffi-culty is attributable to the obscure structure of neuroendo-crine tumors at the light microscopy level, especially when cytology or small biopsy samples are being examined.5 As a result, no optimal treatment for patients with LCNEC has been indicated as yet, and no evidence exists as to whether affected patients might benefit from chemotherapeutic pro-tocols designed for NSCLC or SCLC.Copyright © 2013 by the International Association for the Study of Lung Cancer
ISSN: 1556-0864/13/0807-0980
Combination Chemotherapy with Irinotecan and Cisplatin
for Large-Cell Neuroendocrine Carcinoma of the Lung
A Multicenter Phase II Study
Seiji Niho, MD, PhD,* Hirotsugu Kenmotsu, MD,† Ikuo Sekine, MD, PhD,‡ Genichiro Ishii, MD, PhD,§
Yuichi Ishikawa, MD, PhD,‖ Masayuki Noguchi, MD, PhD,¶ Fumihiro Oshita, MD,# Shun-ichi Watanabe, MD,**
Ryu Nakajima, MD, PhD,†† Hirohito Tada, MD, PhD,†† and Kanji Nagai, MD, PhD‡‡
* Division of Thoracic Oncology, National Cancer Center Hospital East, Chiba, Japan; †Division of Thoracic Oncology, Shizuoka Cancer Center, Shizuoka, Japan; ‡Department of Medical Oncology, Graduate School of Medicine, Chiba University, Chiba, Japan; §Pathology Division, Research Center for Innovative Oncology, National Cancer Center Hospital East, Chiba, Japan; ‖Department of Pathology, Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo, Japan; ¶Department of Pathology, Institute of Basic Medical Sciences, University of Tsukuba, Tsukuba, Japan; #Department of Thoracic Oncology, Kanagawa Cancer Center, Yokohama, Japan; **Division of Thoracic Surgery, National Cancer Center Hospital, Tokyo, Japan; ††Department of General Thoracic Surgery, Osaka City General Hospital, Osaka, Japan; and ‡‡Division of Thoracic Surgery, National Cancer Center Hospital East, Chiba, Japan. Disclosure: The authors declare no conflict of interest.
This work was supported in part by a National Cancer Center Research and Development Fund (23-A-18), a Grant-in-Aid for Cancer Research (17S-2) from the Ministry of Health, Labour and Welfare and a Grant from the Ministry of Health, Labour and Welfare for the third-term Comprehensive Strategy for Cancer Control, Japan.
Address for correspondence: Seiji Niho, MD, PhD, Division of Thoracic Oncology, National Cancer Center Hospital East, Kashiwanoha 6-5-1, Kashiwa, Chiba 277–8577, Japan. E-mail: siniho@east.ncc.go.jp
Combination chemotherapy consisting of irinotecan (CPT) and cisplatin (CDDP) is active against both NSCLC and SCLC.8–12 Taking these rationales into consideration, we conducted a multicenter phase II study of CPT and CDDP in patients with advanced LCNEC.
PATIENTS AND METHODS Patient Population and Treatment Plan
Patients were required to have histologically confirmed advanced-stage LCNEC. Recurrences after surgical resection were permitted. Other criteria included an age of 20 to 75 years, an Eastern Cooperative Oncology Group performance status of 0 or 1, measurable disease, a PaO2 at room air 65 Torr or more, and adequate organ function. Key exclusion criteria included prior chemotherapy, interstitial pneumonia as deter-mined by chest radiograph, and symptomatic brain metasta-ses. All the patients were required to provide written informed consent, and the Institutional Review Board approved the protocol.
Patients received 60 mg/m2 of CDDP on day 1 and 60 mg/m2 of CPT on days 1, 8, and 15 every 4 weeks for up to four cycles if neither unacceptable toxicity nor tumor progres-sion were observed.
Study Evaluation
Imaging studies were scheduled to assess the objective response every 2 months. Diagnostic specimens including hematoxylin-eosin staining and immunohistochemistry for neuroendocrine markers were centrally reviewed by six expert pathologists (TK, MN, YI, KI, GI, and KT) who were blinded to the patients’ clinical information. The pathology panel members performed the pathology review independently, and a final diagnosis was established by mutual consent. Histologic diagnostic criteria for LCNEC included organoid nesting, trabecular growth, and rosette-like and palisading patterns, which suggest neuroendocrine differentiation. The neuroendocrine features need to be confirmed by immunohis-tochemical markers, such as chromogranin, synaptophysin, and CD56.
The Response Evaluation Criteria in Solid Tumors guidelines, version 1.0, was used to evaluate antitumor activ-ity.13 Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0.
Study Design and Statistical Analysis
This trial was designed as a multicenter, prospec-tive, single-arm phase II study. The primary endpoint of this study was the response rate (RR) in the eligible patients with advanced LCNEC diagnosed by institutional pathologists. The secondary endpoints included overall survival (OS), progres-sion-free survival (PFS), and toxicity. In accordance with the minimax two-stage phase II study design reported by Simon,14 the treatment program was designed to refuse RRs of 30% (P0) and to provide a significance level of 0.05 with a statisti-cal power of 80% for assessing the activity of the regimen as a 50% RR (P1). The upper limit for first-stage drug rejection was six responses in the 19 assessable patients; the upper limit for second-stage rejection was 16 responses in a cohort of 39 assessable patients.
The OS was defined as the interval between enrollment in this study and death or the final follow-up visit. The OS and PFS were estimated using the Kaplan–Meier analysis method. Exploratory subgroup analyses for RR, OS, and PFS were planned according to the results of the central pathological review. Survival data were compared among groups using a log-rank test. All the reported p values were two-sided. This study was registered with the UMIN Clinical Trials Registry (number UMIN000004796).
RESULTS
A total of 44 patients from 11 Japanese institutes were enrolled between January 2005 and November 2011. All 44 patients were eligible for inclusion. The patient characteris-tics are listed in Table 1. Thirty patients (68%) completed four cycles of chemotherapy, whereas three patients received three cycles, seven patients received two cycles, and four patients TABLE 1. Patient Characteristics
Characteristic No. of Patients
Sex Men 35 Women 9 Age (yr) Median 62.5 Range 28–74 PS 0 21 1 23 Clinical stagea IIIB 5 IV 28
Recurrence after surgery 11
aClinical stage was based on the 5th edition of the tumor, node, metastasis staging
system of the Union Internationale Centre le Cancer. PS, performance status.
FIGURE 1. Overall survival curve including all 44 eligible patients. MST, median survival time; CI, confidence interval.
received one cycle. Reasons for the discontinuation of the pro-tocol treatment for the four patients who received only one cycle included progressive disease (n = 2), deterioration of PS (n = 1), and liver dysfunction prolonged for more than 2 weeks (n = 1). The median of relative dose intensity for CDDP or CPT was 94.3% (range, 66.2%–102.1%) and 82.4% (range, 33.3%–100%), respectively.
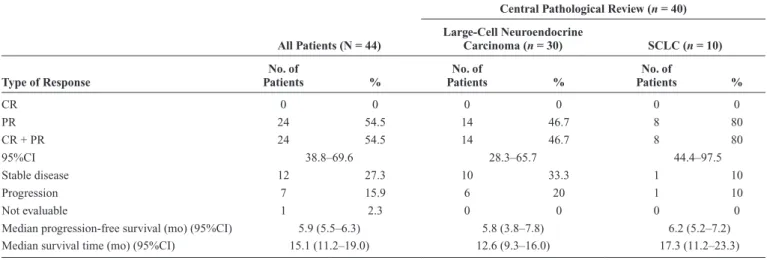
Twenty-four partial responses were seen among the 44 eligible patients, yielding an objective RR of 54.5% (95% confidence interval [CI], 38.8%–69.6%; Table 2). All 24 responders received four cycles of chemotherapy. Of them, eight patients received full planned dose of chemotherapy. The dose intensity of CDDP or CPT was not associated with RR (data not shown).
Figure 1 shows the OS curve for all 44 eligible patients. At 8 months after the last enrollment, the median survival time (MST) was 15.1 months (95% CI, 11.2–19.0 months). The 1-year and 2-year survival rates for all 44 eligible patients were 62.1% and 18.4%, respectively. The median PFS was 5.9 months (95% CI, 5.5–6.3 months; Fig. 2).
Toxicity was evaluated in all eligible patients. The most common toxicity was neutropenia (Table 3). Twenty-four patients (55%) experienced grade 3 or 4 neutropenia, but only three patients (7%) developed neutropenic fever. Three patients (7%) developed grade 3 infection with neutropenia, and another three patients (7%) developed grade 3 infection without neutropenia. Two patients (5%) developed grade 3 diarrhea. No treatment-related deaths occurred in this series. TABLE 2. Efficacy of Combination Chemotherapy with Irinotecan and Cisplatin
All Patients (N = 44)
Central Pathological Review (n = 40) Large-Cell Neuroendocrine
Carcinoma (n = 30) SCLC (n = 10)
Type of Response PatientsNo. of % PatientsNo. of % PatientsNo. of %
CR 0 0 0 0 0 0 PR 24 54.5 14 46.7 8 80 CR + PR 24 54.5 14 46.7 8 80 95%CI 38.8–69.6 28.3–65.7 44.4–97.5 Stable disease 12 27.3 10 33.3 1 10 Progression 7 15.9 6 20 1 10 Not evaluable 1 2.3 0 0 0 0
Median progression-free survival (mo) (95%CI) 5.9 (5.5–6.3) 5.8 (3.8–7.8) 6.2 (5.2–7.2)
Median survival time (mo) (95%CI) 15.1 (11.2–19.0) 12.6 (9.3–16.0) 17.3 (11.2–23.3)
Four patients of 14 responders in the large-cell neuroendocrine carcinoma group and four patients of eight responders in the SCLC group received full planned dose of chemotherapy. CR, complete response; PR, partial response; CI, confidence interval; SCLC, small-cell lung cancer.
FIGURE 2. PFS curve including all 44 eligible patients. CI, confidence interval; PFS, progression-free survival.
TABLE 3. Maximum Toxicity Grades
Toxicity Toxicity Grade % 3–4
1 2 3 4 Leukopenia 8 16 6 4 23 Neutropenia 3 9 11 13 55 Anemia 18 18 6 1 16 Thrombocytopenia 13 2 0 0 0 Febrile neutropenia — — 3 0 7 Bilirubin 6 2 0 1 2 AST 13 2 2 0 5 ALT 14 1 2 0 5 Creatinine 10 2 1 0 2 Hyponatremia 27 0 4 1 11 Hypokalemia 9 0 1 0 2 Hyperkalemia 10 1 1 0 2 Nausea 17 9 3 — 7 Vomiting 8 4 1 0 2 Appetite loss 21 12 3 0 7 Diarrhea 17 12 2 0 5 General fatigue 17 7 2 0 5 Constipation 3 2 0 0 0 Alopecia 17 9 0 0 0
Infection with neutropenia 1 0 3 0 7
Infection without neutropenia 1 1 3 0 7
Fever (noninfectious) 5 1 0 0 0
A total of 36 patients (82%) received second-line che-motherapy. Twenty-five patients received amrubicin, four patients received platinum-based chemotherapy (CDDP + CPT [n = 2], CDDP + etoposide [n = 1], and carboplatin + paclitaxel [n = 1]), and six patients received docetaxel.
Pathological specimens for central review were avail-able in 41 patients. Pathological specimens in three patients were not available because these specimens were returned to other institutions, where biopsy was conducted. Thirty patients were diagnosed as having LCNEC, whereas 10 patients were diagnosed as having SCLC, and one patient was diagnosed as having NSCLC with a neuroendocrine structure. The RR was 47% (95% CI, 28.3%–65.7%) for patients diagnosed as having LCNEC and 80% (95% CI, 44.4%–97.5%) for patients diag-nosed as having SCLC (p = 0.082). The median PFS was 5.8 months (95% CI, 3.8–7.8 months) in the LCNEC group and 6.2 months (95% CI, 5.2–7.2 months) in the SCLC group (p = 0.382) (Fig. 3A). The MST was 12.6 months (95% CI, 9.3–16.0 months) in the LCNEC group and 17.3 months (95% CI, 11.2– 23.3 months) in the SCLC group (p = 0.047) (Fig. 3B).
DISCUSSION
This trial is the first to evaluate the use of combination chemotherapy consisting of CPT and CDDP prospectively in patients with advanced LCNEC. The lower limit of the CI for the RR exceeded 30%, which was the predefined threshold. The MST was 15.1 months (95% CI, 11.2–19.0 months), and the median PFS was 5.9 months (95% CI, 5.5–6.3 months). LCNEC is currently categorized as a variant form of large-cell carcinoma, that is NSCLC, but LCNEC has neuroendocrine features, similar to SCLC. Combination chemotherapy consisting of CPT and CDDP seems to be promising as a first-line chemotherapy for patients with advanced LCNEC.
According to the central pathological review, which was blinded to all clinical information, 30 patients (73%) were diagnosed as having LCNEC, and 10 patients (24%) were diagnosed as having SCLC. The PFS was similar between the LCNEC and SCLC patients; however, the OS of the SCLC patients was significantly longer than that of the LCNEC patients. The RR was also superior in the SCLC patients. A previous retrospective study examined the clinical outcome of the patients with high-grade neuroendocrine carcinoma, prob-able LCNEC, which was diagnosed on the basis of biopsy results. The RR for second-line chemotherapy was 17% among the patients with HGNEC-probable LCNEC (2/12), and 43% among the patients with SCLC (45 of 102) (p = 0.12).15 The chemosensitivity of LCNEC is considered to be lower than that of SCLC, especially in response to second-line chemotherapy. Consequently, the OS of the LCNEC patients was inferior to that of the SCLC patients.
The present study had several limitations. First, only 30 patients (73%) were diagnosed as having LCNEC, based on a central review. The RR in these 30 patients was 47% (95% CI, 28.3%– 65.7%). Thus, the lower limit of the 95%CI for the RR did not exceed the predefined threshold of 30%. Second, data regarding the efficacy of second-line chemotherapy were not available. We could not confirm the chemoresistance of LCNEC in second-line chemotherapy, compared with that of SCLC. Third, the number of patients included in the study was small. The enrollment period exceeded 6 years despite the multicenter design. The rela-tively low incidence of LCNEC and the difficulty of diag-nosing LCNEC pathologically using small biopsy specimens was responsible for the slow accrual.
ACKNOWLEDGMENTS
We thank Ms Fumiko Koh and Ms Eriko Imai for data management, Dr. Toru Kameya (Shizuoka Cancer Center Hospital, Shizuoka), Dr. Ken Inoue (Osaka City General Hospital, Osaka), and Dr. Koji Tsuta (National Cancer Center Hospital, Tokyo) for the central pathological review, and Dr. Yoshihiro Matsuno (Hokkaido University, Sapporo) for his contribution to this study.
REFERENCES
1. Marchevsky AM, Gal AA, Shah S, et al. Morphometry confirms the presence of considerable nuclear size overlap between “small cells” and “large cells” in high-grade pulmonary neuroendocrine neoplasms. Am J
Clin Pathol 2001;116:466–472.
A
B
FIGURE 3. A, PFS and (B) overall survival curves for 30 patients diagnosed as having LCNEC and 10 patients diag-nosed as having SCLC based on a central review. MST, median survival time; CI, confidence interval; PFS, progression-free survival; LCNEC, large-cell neuroendocrine carcinoma; SCLC, small-cell lung cancer; MST, median survival time.
2. Cerilli LA, Ritter JH, Mills SE, Wick MR. Neuroendocrine neoplasms of the lung. Am J Clin Pathol 2001;116 Suppl:S65–S96.
3. Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann
Thorac Surg 2006;82:1802–1807.
4. Yamazaki S, Sekine I, Matsuno Y, et al. Clinical responses of large cell neuroendocrine carcinoma of the lung to cisplatin-based chemotherapy.
Lung Cancer 2005;49:217–223.
5. Jiang SX, Kameya T, Shoji M, Dobashi Y, Shinada J, Yoshimura H. Large cell neuroendocrine carcinoma of the lung: a histologic and immunohisto-chemical study of 22 cases. Am J Surg Pathol 1998;22:526–537.
6. Takei H, Asamura H, Maeshima A, et al. Large cell neuroendocrine car-cinoma of the lung: a clinicopathologic study of eighty-seven cases. J
Thorac Cardiovasc Surg 2002;124:285–292.
7. Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol 2005;23:8774–8785.
8. Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cispla-tin compared with etoposide/cisplairinotecan/cispla-tin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin
Oncol 2009;27:2530–2535.
9. Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study of cis-platin plus irinotecan versus carbocis-platin plus paclitaxel, ciscis-platin plus
gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol 2007;18:317–323.
10. Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial com-paring irinotecan/cisplatin with etoposide/cisplatin in patients with pre-viously untreated extensive-stage disease small-cell lung cancer. J Clin
Oncol 2006;24:2038–2043.
11. Negoro S, Masuda N, Takada Y, et al.; CPT-11 Lung Cancer Study Group West. Randomised phase III trial of irinotecan combined with cisplatin for advanced non-small-cell lung cancer. Br J Cancer 2003;88: 335–341.
12. Noda K, Nishiwaki Y, Kawahara M, et al.; Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85–91. 13. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to
evalu-ate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216.
14. Simon R. Optimal two-stage designs for phase II clinical trials. Control
Clin Trials 1989;10:1–10.
15. Shimada Y, Niho S, Ishii G, et al. Clinical features of unresectable high-grade lung neuroendocrine carcinoma diagnosed using biopsy specimens.