Heavy Metal Resistance and Antibiotic Susceptibility
Pattern of Bacteria Isolated from Selected Polluted
Soils in Lagos and Ota, Nigeria.
Owolabi, J. B.* and Hekeu, M. M.
Department of Biological Sciences, Microbiology Unit, School of Natural and Applied Sciences, College of Science and Technology, Covenant University, P.M.B. 1023 Ota, Ogun State, Nigeria.
* joshua.owolabi@covenantuniversity.edu.ng, Tel. +2348155636901
Abstract--
Heavy metal pollutants are commonly found in soils, sediments and waste water where they remain persistent, and become toxic to many species above certain threshold concentrations. Bacteria resistant to heavy metals can be used for detoxification and prevent further deterioration of contaminated sites. The incidence of bacteria resistant to heavy metals in soils collected from six contaminated sites in Lagos and Ota, Nigeria was assessed in this study. Heavy metal resistant bacteria were isolated from the soil samples following enrichment by growth on media amended with high concentrations of lead, cadmium and chromium, and identified based on cultural, morphological and biochemical characteristics. A total of 11 heavy metal resistant bacteria isolated from the soil samples were tentatively identified as Aeromonas sp (3), Arthrobacter sp. (1), Corynebacterium sp (4),
Pseudomonas sp (1) and Streptococcus sp (2). The maximum tolerated concentrations of metals (in basal medium supplemented with lead, cadmium and chromium) ranged from 2 to 10 mM for the Pseudomonas sp. and 2 to 4 mM for the
Corynebacterium and Streptococcus spp. The three selected isolates also exhibited resistance to amoxicillin, augmentin, cefuroxime, erythromycin and cloxacillin, and sensitivity to ofloxacin and ceftazidime. The organisms with antibiotic and heavy-metal resistance isolated and identified in this study have potential application in bioremediation of environments polluted with metals and may also help to overcome the inhibition that heavy metals exert on the biodegradation of organic pollutants. In addition, such dual resistant organisms would have the capacity to compete well with antibiotic-producing flora in the polluted environment.
Index Term-- Heavy metal, resistance, antibiotics, bacteria, soils.
I.
INTRODUCTIONThe increasing rate of industrialization in the world today is a major concern for the quality of the atmosphere, soil and water bodies [8]. Pharmaceutical, food, chemical, metallurgical and textile manufacturing industries discharge large quantities of waste containing toxic substances [40, 45] into the environment. Other human activities such as mining, use of chemical fertilizers and pesticides in agriculture [7], automobile mechanic activities [1, 35], car battery manufacturing plants [3, 17, 44] and the use of vehicles for transportation [22, 42] release high amounts of pollutants including heavy metals into the biosphere.
Heavy metals are natural chemical elements and components of the earth crust with a specific gravity at least five times greater than that of water [23]. Some well-known metallic elements with a specific gravity greater than or equal to 5 are arsenic (5.7), cadmium (8.65), iron (7.9), lead (11.34) and mercury (13.546) [21]. Since they are non-degradable in the environment they are constantly recycled from one earth compartment to the other under the influence of natural processes such as weathering, erosion or biological activity. However, given significant industrialization and urbanization with ascendance of indiscriminate dumping of untreated industrial wastewater and municipal sewage in to the environment, heavy metals have been reported to accumulate to dangerous or toxic concentrations [24, 25, 26, 34].
High levels of heavy metals in soils have been associated with poor plant growth and reduced water and nutrients uptake and various enzymatic disturbances [5, 16, 37, 54]. Food safety is greatly threatened by heavy metal pollution. In China, food safety forum year of 2009 revealed that one sixth of the cultivated land was contaminated by heavy metals and an area of more than 20 million hectares were threatened to be banned from agricultural practices because of the high level of heavy metal pollution [30, 57]. Several studies have also shown evidence of heavy metal uptake and accumulation in edible vegetables [2, 13, 14, 27, 39, 58], rice [28, 56], and fish [49]. Accumulation of heavy metals in human tissues and organs has caused cardiovascular, kidney, and nervous system disabilities and bone diseases [26, 51, 52, 34, and 8].
Various strategies have been developed to address the problem of environmental heavy metal pollution. Physicochemical methods such as chemical precipitation or solvent extraction have been employed [15]. However, these methods have proved to be difficult to apply on large surfaces, financially prohibitive and environmentally non friendly as they sometimes require toxic reagents [50, 15] in the remediation process.
ability of the bacteria to cope with metal toxicity by means of intrinsic properties while bacteria resistance is the ability to survive in higher concentrations of the toxic metals by detoxifying mechanisms which are activated as a result of the presence of the specific metals [26, 34].
Heavy metal resistant bacteria represent a better alternative for heavy metal decontamination and have already been successfully applied for such purposes in the developed world [33, 42, 51]. However, in Nigeria, there are limited studies investigating the potential use of metal resistant bacteria for bioremediation purposes [39, 48]. The specific objectives of this study were to isolate and identify bacterial species with resistance to cadmium, chromium and lead, and determine the antimicrobial susceptibility pattern of selected isolates from selected polluted sites in Lagos and Ota.
II. MATERIALS AND METHODS Study areas
Lagos and Ota constituted the two broad sampling locations for this study. Lagos is a major commercial and industrial hub in Nigeria with a population density of about 2,758 persons / km2 and is responsible for about 70% of the country’s industries and commercial activities. The sampling areas in Lagos were at the Owode Onirin and the Ladipo Spare Part Markets. Owode-Onirin is a vast automobile village situated at Kosofe local government area along Ikorodu Road in Lagos. Built on a five-hectare land, the Owode Onirin auto market has been in existence since 1979 and spare parts from old cars are sold or dumped at particular sites. Ladipo Auto Spare Parts Market is situated along Oshodi Expressway at Mushin local government area of Lagos. It is composed of several trading points, mechanic workshops, dumpsites and a canal where old vehicle parts are dumped.
Ota is the capital of the Ado-Odo/Ota Local Government Area with an estimated population of 163,783 residents. Ota has the third largest concentration of industries in Nigeria. Sampling sites in Ota were at three automobile garages and an industrial paint company’s dumpsite along Idiroko road.
Sample collection
A total of ten soil samples were collected from the contaminated locations. Two soil samples were collected from two different car spare part dumpsites at the Owode-Onirin Market and three soil samples were collected from three different sites of the Ladipo Auto Spare Parts Market, Lagos. Five soil samples were collected from Ota; three samples were from three different automobile garages along Idiroko road and two samples were from an industrial paint company’s dumpsite also along Idiroko road. All the samples were collected using a sterilized (with 70% alcohol) hand trowel, kept in plasticcontainers and labelled accordingly. They were carried to the laboratory and stored in a refrigerator at 40C until they were analysed.
Enrichment, isolation and identification of metal resistant bacteria
Using an enrichment procedure, 1.5 g of each soil sample was added to 15 ml of nutrient broth and incubated for 24 h at 370C with shaking at 110 rpm. To isolate metal resistant bacteria, 5 ml of each enriched sample was transferred to 50 ml of MS medium [39] containing 0.5 mM of metals (cadmium, chromium and lead) and incubated at 370C for 7 days with shaking at 110 rpm. Each culture was plated on the 4th and 7th day on MS agar supplemented with 1.0 mM concentration of cadmium, chromium and lead (MS-1.0) and incubated at 370C for 48 h. Colonies obtained from the MS-1.0 plates were further streaked on MS agar [29] supplemented with 2 mM concentration of cadmium, chromium and lead (MS-2) and incubated at 370C for 48 h. The isolated and distinct colonies on MS-2 plates were purified by sub-culturing repeatedly on nutrient agar using the streak plate method. The purified isolates were put into nutrient agar slants for storage at 40C. The bacterial isolates were identified on the basis of their colonial and cultural morphology and biochemical characteristics following standard methods [29, 55].
Determination of maximum tolerated concentrations (MTCs) of heavy metals
The MTCs were determined for selected isolates on both PYE agar and MS medium in presence of each of the three metals (cadmium, chromium and lead) separately. The selected isolates were inoculated on PYE agar plates containing singly Cd, Cr and Pb at concentrations of 2, 4, 6, 8 and 10 mM. The selected isolates were also inoculated on MS agar plates supplemented singly with Cd, Cr and Pb at concentrations of 2, 4, 6 and 8 mM. The organisms were incubated at 370C for 72 h. The MTC was noted when the isolate failed to show growth on the plates after the three days of incubation. All experimental set-ups were prepared in duplicate.
Determination of antibiotic susceptibility
III. RESULTS
Samples of soil collected from selected contaminated sites in this study contained heavy metal resistant bacteria. A total of 11 different bacterial strains were isolated from the contaminated soil samples on MS agar – supplemented with 2 mM metal concentrations (cadmium, chromium and lead). The proportion of metal resistant bacteria from the study areas is as shown in Figure 1. The highest proportion of resistant bacteria were isolated from Ladipo spare parts market, Lagos (64%), followed by the automobile garages, Ota (18%) and the least were from Owode Onirin, Lagos and the paint company dump site, Ota (9%).
The heavy metal resistant bacterial isolates were identified on the basis of their cellular and colonial morphological characteristics and the results from biochemical tests. The results are as shown (Table 1). Bacterial isolate coded LA4B was found to be a Gram negative short rod, forming green colonies on nutrient agar with a characteristic pungent odour. It was oxidase positive with no indole production and tested negative for lactose fermentation, but positive for glucose without gas production. These results led to the tentative identification of LA4B as a Pseudomonas sp. Isolates coded LA4A, BS2, LA5B and LA6 were observed to be Gram positive short rods with whitish yellow colonies and undulated margins. They were all catalase positive, MR positive, VP positive and able to utilize citrate. These results led to their tentative identification as Corynebacterium spp. Isolates BS3, AS2 and LA2C were observed to be Gram negative short rods, yellowish colonies with undulated margins, catalase positive, indole positive, urease negative and glucose fermenters with no gas production. They were therefore tentatively identified as Aeromonas spp. Isolates LA1 and PC2B were observed to be Gram positive cocci with small, whitish yellow and translucent colonies. The two isolates were catalase positive and glucose fermenters with inability to hydrolyse starch nor grow on Mannitol salt agar. These results led to their tentative identification as Streptococcus spp. Isolate LA3 was observed to be a Gram positive short rod, catalase positive, MR positive and VP positive with yellow to white colonies and entire margins. The isolate was tentatively identified as Arthrobacter
sp.
When five bacterial isolates; Aeromonas spp. (AS2),
Arthrobacter spp. (LA3), Corynebacterium spp. (LA4A),
Pseudomonas spp. (LA4B) and Streptoccocus spp. (PC2B) were grown further at higher concentrations of cadmium, chromium and lead, three isolates; Corynebacterium spp.,
Pseudomonas spp. and Streptoccocus spp. were found to grow effectively at concentrations of 2 mM or more. Therefore, these highly resistant isolates were further examined to determine maximum tolerable concentration of each of the three metals (cadmium, chromium and lead) separately. The results of heavy metal tolerance tests, respectively, on MS agar and PYE agar are as shown (Table 2). The MTCs in MS agar plates for cadmium and chromium was 4 mM for all the three selected isolates and for lead 2 mM for Corynebacterium
and Streptococcus spp, and 8 mM for the Pseudomonas sp.
The MTCs in PYE agar plates for cadmium was 2 mM for
Pseudomonas sp. and 4 mM for Streptococcus and
Corynebacterium spp., for chromium was 2 mM for both
Pseudomonas and Corynebacterium spp and 4 mM for
Streptococcus sp., and for lead was 2 mM for
Corynebacterium sp., 4 mM for Streptococcus sp. and 10 mM for the Pseudomonas sp.
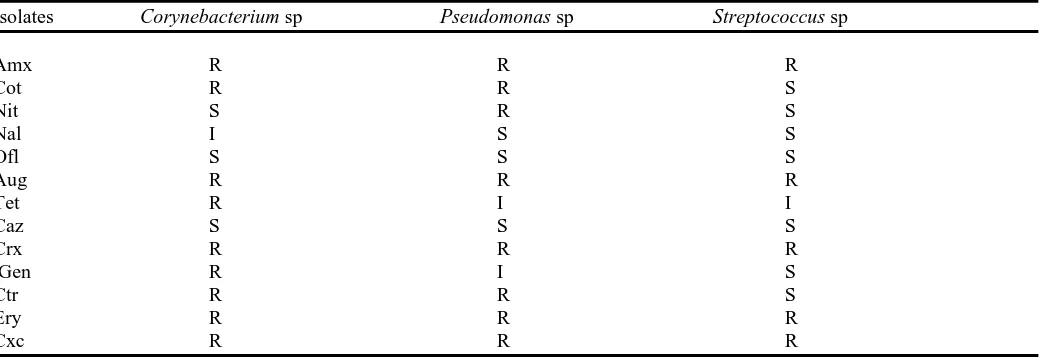
The results of antibiotic sensitivity tests as shown in Table 3 indicate that the three selected multiple heavy metal resistant isolates; Pseudomonas, Corynebacterium and Streptococcus
spp. exhibited multiple resistances to amoxicillin, augmentin, cefuroxime, erythromycin and cloxacillin, and sensitivities to ofloxacin and ceftazidime. The Corynebacterium sp. appeared to be resistant to most number of antibiotics (69%) and less sensitive to all the antibiotics. Similarly, the
Pseudomonas sp. was resistant to eight out of thirteen antibiotics (62%) and sensitive to two only. The Streptococcus
sp. showed the highest level of sensitivity (seven antibiotics) and was resistant only to five (38%).
IV. DISCUSSION
In this study, a total of eleven heavy metal resistant bacteria were isolated from soil samples collected from automobile mechanic garages, waste dumpsites of automobile spare part markets and paint manufacturing company in Lagos and Ota, Nigeria. The bacteria were selected in the presence of cadmium, chromium and lead at concentrations of up to 2 mM. The isolates were putatively identified as Pseudomonas
sp. (1), Aeromonas sp. (3), Corynebacterium sp. (4),
Streptococcus sp. (1) and Arthrobacter sp. (1) on the basis of their cultural, morphological and biochemical characteristics. Similar findings of occurrence of heavy metal resistant bacteria in contaminated soil samples have been reported [44, 20, 37, 47]. Olukoya and others [44] isolated Pseudomonas
and Streptococcus sp. showing resistance to Cr and Pb from a lagoon area. Oyetibo and his colleagues [47] found
Corynebacterium and Pseudomonas spp. with resistance to Cr and Cd from soil samples from an industrial estate. Nwaugo and collaborators [37] identified Pseudomonas sp. resistant to Pb, from soil samples collected from a mining pit.
The MTC value for Pb in this study (10 mM) is higher than that obtained by Fagade and Adetutu [20] and Sanuth and others [48] which ranged between 2 and 2.5 mM. However, the MTC values for Cr (4 mM) and Cd (4 mM) are much lower than those reported by Oyetibo and others [47] where the MTC for Cd and Cr were 14 mM and 17 mM, respectively. These studies suggest variability in the potency of bacteria towards heavy metals to which they are resistant.
In this study, the selected heavy metal bacterial isolates exhibited resistance to multiple antibiotics.
Pseudomonas sp. showed 62% resistance to the 13 antibiotics tested, Streptococcus sp. showed 38% while Corynebacterium
colleagues [31] found that heavy metal resistant bacterial isolates; Pseudomonas sp., Aeromonas sp., Bacillus sp.
Micrococcus sp., Proteus sp .and E. coli exhibited high tolerance to gentamycin (77.7%), rifampicin (66.0%), and ofloxacin (57.3%). In the study by Oyetibo and colleagues [47], five heavy metal resistant bacteria strains; Micrococcus
sp., Nocardia sp., Acinetobacter junni, Actinomyces turicensis
and P. aeruginosa, resisted all the 18 antibiotics tested. Nageswaran and collaborators, [32] in their study of the antibiotic sensitivity pattern of eight strains of metal resistant
Serratia marcescens reported finding resistance to eight out of 22 antibiotics tested.
Under conditions of high levels of heavy metals in their environment, metal and antibiotic resistance in bacteria most likely help them to adapt faster by the spread of resistant factors. The organisms with antibiotic and heavy-metal resistance isolated and identified in this study have potential application in bioremediation of environments polluted with metals and may also help to overcome the inhibition that heavy metals exert on the biodegradation of organic pollutants. In addition, such dual resistant organisms would have the capacity to compete well with antibiotic-producing flora in the polluted environment. The studies assessing the potential ability of the selected isolates to remove heavy metals from contaminated industrial waste water and their plasmid profiles are currently underway.
ACKNOWLEDGEMENT
The corresponding author, Owolabi, J. B. would like to acknowledge the Management of Covenant University for providing a Seed Research Grant that funded this work. We thank Taiwo, O. S. for technical assistance.
REFERENCES
[1] Adewole, M. B. and Uchegbu, L. U. (2010). Properties of soils and plants uptake within the vicinity of selected automobile workshops in Ile-Ife Southwestern, Nigeria. Ethiopian J. Environ. Studies Manage. 3: 31-35.
[2] Adewuyi, G. O., Dawodu, F. A. and Jibiri, N. N. (2010). Studies of the concentration levels of heavy metals in vegetables
(Amaranthus caudatus) grown in dumpsites within Lagos
metropolis, Nigeria. Pacific Journal of Science and Technology 11: 616-621.
[3] Adie, G. U. and Osibanjo, O. (2009). Assessment of soil pollution by slag from an automobile battery manufacturing plant in Nigeria. African Journal of Environmental Science and
Technology3: 239-250.
[4] Adu, A. A., Aderinola, O. J. and Kusemiju, V. (2012). An assessment of soil heavy metal pollution by various allied artisans in automobile, welding workshop and petrol station in Lagos State, Nigeria. Science Journal of Environmental Engineering Research 2012: 1-8.
[5] Agarwal, S.K. (1999). Studies on the effect of the auto exhaust emission on the Mitragynapatri flora. Ajmeer, India: MDS University; Master Thesis.
[6] Ahemad, M. (2012). Implications of bacterial resistance against heavy metals in bioremediation: A Review. IIOAB Journal3: 39-46.
[7] Aikpokpodion, P. E., Lagide, L. and Aiyesonmi A. F. (2010). Heavy metal contamination in fungicide treated cocoa plantation in Cross River State, Nigeria. American Eurasian Journal of
Agriculture and Environmental Science8: 268-274.
[8] Ali, S., Sardar, K., Hameed, S., Afzal, S., Fatima, S., Shakoor, B. M., Bharwana, S. A. and Tauqeer, H. M. (2013). Heavy metals
contamination and what are the impacts on living organisms.
Greener Journal of Environmental Management and Public Safety 2: 172-179.
[9] Amaechi, J. N., Nwodu, J. A., and Ahaotu, E. O. (2014). Effect of different concentrations of metal ions on Bacillus and
Pseudomonas spp. isolated from industrial effluents of aluminum extrusion industry (ALEX) Inyishi, Imo State, Nigeria. Inter J Agric Biosci3: 55-60.
[10] Anyakora, C., Ehianeta, T. and Umukoro, O. (2013). Heavy metal levels in soil samples from highly industrialized Lagos environment. African Journal of Environmental Science and
Technology7: 919-924.
[11] Anyanwu, C.U. and Ugwu, C.E. (2010). Incidence of arsenic resistant bacteria isolated from a sewage treatment plant.
International Journal of Basic & Applied Sciences IJBAS-IJENS 10: 43-47.
[12] Arikpo, G.E., Eja, M.E., Enyi-Idoh, K.H., Etim, S.E. and Ikpeme, E.M. (2010). Heavy metal uptake potentials of Pseudomonas aeruginosa and Micrococcus luteus. African Journal Online8(1). [13] Atayese, M. O., Eigbadon, A. I., Oluwa, K. A. and Adesodun, J. K.
(2008). Heavy metal contamination of Amaranthus grown along major highways in Lagos, Nigeria. African Crop Science Journal 16: 225-235.
[14] Chang, P., Kim, J., and Kim, K. W. (2005). Concentrations of arsenic and heavy metals in vegetation at two abandoned mine tailings in South Korea. Environmental Geochemistry and Health 27: 109-119.
[15] Dhankhar, R. and Guriyan, B. R. (2011). Strategies for management of metal contaminated soils. International Journal of
Environmental Sciences1: 1884-1898.
[16] Ebbs, S.D., Lasat, M.M., Brandy, D.J., Cornish, J., Gordon, R. and Kochian, L.V. (1997). Heavy metals in the environment: Phytoextraction of cadmium and zinc from a contaminated soil.
Journal of Environmental Quality26: 1424-1430.
[17] Ekundayo E. O. and Killham K. (2001). Lead solubilization and accumulation by two strains of Pseudomonas obtained from a contaminated Alfisol's effluent in Southwestern Nigeria.
Environmental Monitoring and Assessment71: 243-254.
[18] Ezaka, E and Anyanwu, C. U. (2011). Chromium (VI) tolerance of bacterial strains isolated from sewage oxidation ditch.
International Journal of Environmental Sciences1: 1725 – 1734.
[19] Eze E, Eze, U., Eze, C. and Ugwu, K. (2009). Association of metal tolerance with multi-drug resistance among bacteria isolated from sewage. Journal of Rural and Tropical Public Health8: 25-29. [20] Fagade, O. E. and Adetutu E. M. (1999). Lead solubilization and
accumulation by two strains of Pseudomonas species obtained from a battery manufacturing factory effluent. Nigeria Journal of
Miccrobiology13: 39-46.
[21] Galadima, A., Muhammad, N. U. and Garba, Z. N. (2010). Spectroscopic investigation of heavy metals in waste water from University students’ halls of residence. International Journal of Chemistry. 20: 239-244.
[22] Galadima, A., Okoronkwo, M. U., Mustapha, D. G., and Leke, L.. (2012). Petrol in Nigeria: a fuel or a killer? ―Is shift to hydroisomerisation not overdue?‖ Elixir International Journal43: 6893-6897.
[23] International Union of Pure and Applied Sciences (IUPAC) (2002). ―Heavy metals‖- a meaningless term? Pure Applied
Chemistry74: 793-807.
[24] Iwegbue, C. M. A., Nwajei, G. E., Ogala, J. E. and Overah, C. L. (2010). Deterermination of trace metal concentrations in soil profiles of municipal waste dumps in Nigeria. Environmental
Geochemistry and Health32: 415-430.
[25] Jarup, L., Berglund, M., Elinder, C. G., Nordberg, G., Vahter, M., (1998). Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand. J. Work Environ. Health24: 1–52.
[26] Kumar, A., Bisht, B. S. and Joshi, V. D. (2011). Bioremediation potential of three acclimated bacteria with reference to heavy metal removal from waste. International Journal of Environmental
[27] Li, J., Xie, Z. M., Xu, J. M., and Sun, Y. F. (2006). Risk assessment for safety of soils and vegetables around a lead/zinc mine. Environmental Geochemistry and Health28: 37–44. [28] Lin, H., Wong, S. and Li, G. (2004). Heavy metal content of rice
and shellfish in Taiwan. Journal of Food and Drug Analysis. 12: 167-174.
[29] Madigan, M. T., Martinko, J. M., Dunlap, P. V. and Clark, D. P. (2009). Brock Biology of Microorganisms (12th ed.). Pearson Benjamin Cummings. ISBN 0-132-32460-1 pp 27-28.
[30] Ministry of Environmental Protection of the People’s Republic of China (MEPPRC). (2006). Report on the state of the environment in China. Beijing, China: MEPPRC (in Chinese).
[31] Mgbemena, C. I., Nnokwe, J. C., Adjeroh L.A. and Onyemekara N. N. (2012). Resistance of bacteria isolated from Otamiri River to heavy metals and some selected antibiotics. Current Research
Journal of Biological Sciences4: 551-556.
[32] Nageswaran, N., Ramteke, P. W., Verma O. P. amd Pandey A. (2012). Antibiotic susceptibility and heavy metal tolerance pattern of Serratia marcesens isolated from soil and water. Bioremediation
and Biodegradation3: 158-167.
[33] Nakamura K., Hagimine M., Sakai M. and Furukawa K. (1999). Removal of mercury from mercury-contaminated sediments using a combined method of chemical leaching and volatilization of mercury by bacteria. Biodegradation10: 443-447.
[34] Nies, D. H., (1999). Microbial heavy-metal resistance. Applied
Microbiology and Biotechnology 51: 730-750.
[35] Nordberg, G. F. and Nordberg, M. (2001). Biological monitoring of cadmium. In: Clarkson TW, Friberg L, Nordberg GF, Sager PR, eds. Biological monitoring of toxic metals. New York: Plenum Press. pp. 151-168.
[36] Nwachukwu, M. A., Feng, H. and Alinnor J (2010). Assessment of heavy metal pollution in soil and their implications within and around mechanic villages. International Journal of Environmental
Science and Technology7: 347-358.
[37] Nwaugo V. O., Onyeagba, R. A., Akubugwo, E. I. and Ugbogu, O. (2008). Soil bacterial flora and enzymatic activities in zinc and lead contaminated soil. Biokemstri 20: 77-84.
[38] Nwinyi, C. O., Picardal, F. W., An, T. T. and Amund, O. O. (2013). Aerobic degradation of naphthalene, fluoranthene, pyrene and chrysene using indigenous strains of bacteria isolated from a former industrial site. Canadian Journal of Pure and Applied Sciences7: 2303-2314.
[39] Odokuma, L. O. and Akponah, E. (2010). Effect of nutrient supplementation on biodegradation and metal uptake by three bacteria in crude oil impacted fresh and brackish waters of the Niger Delta. Journal of Cell and Animal Biology 4: 001-018. [40] Oluwatosin, G. A., Adeoyolanu, O. D., Ojo, A. O., Are, K. S.,
Dauda, T. O. and Aduramigba-Modupe, V. O. (2010). Heavy metal uptake and accumulation by edible leafy vegetable (Amaranthus hybridus L.) grown on urban valley bottom soils in southwestern Nigeria. Soil and Sediment Contamination: 19: 1–20. [41] Oketola, A. and Osibanjo, O. (2011). Assessment of industrial pollution load in Lagos, Nigeria by industrial pollution project system (IPPS) versus effluent analysis, environmental management in practice. Dr Elzbieta Broniewicz (Ed), ISBN: 978-953-307- 358-3.
[42] Okino, S., Iwasaki, K., Yagi, O. and Tanaka, H. (2002). Removal of mercuric chloride by immobilized cells of genetically engineered mercury- volatilizing bacterium Pseudomonas putida
Pp Y101/pS134. Bill. Environ. Contam. Toxicol. 68: 712-719. [43] Olukanmi, D. O. and Adeoye, D. O. (2012). Heavy metal
concentrations in road side soils from selected locations in the
Lagos metropolis, Nigeria. International Journal of Engineering
and Technology2: 1743-1752.
[44] Olukoya D. K., Smith S. I. and M. O. Ilori (1997). Isolation and characterization of heavy metals resistant bacteria from Lagos Lagoon. Folia Microbiol (Praha). 42: 441-444.
[45] Orisakwe, O. E., Asomuqua, R., Afonne, O. J., Anisi, S. N., Obi, E. and Dioka, C. E. (2004). Impact of effluents from a car battery manufacturing plant in Nigeria on water, soil, and food qualities.
Arch. Environ. Health59: 31-36
.
[46] Osho, A., Mabekoje, O. O. and Bello, O. O. (2010). Preliminary evaluation of wastewater effluents from two food companies in Nigeria. African Journal of Microbiology Research4: 1395-1399. [47] Oyetibo, G. O., Ilori, M. O., Adebusoye, S. A., Obayori, O. S. and
Amund O. O. (2010). Bacteria with dual resistance to elevated concentrations of heavy metals and antibiotics in Nigeria in contaminated systems. Environmental Monitoring Assessment168: 305-314.
[48] Sanuth H. A., Ogunjobi, A. A. and Fagade, O. E. (2010). The growth and survival of lead solubilizing strains of Pseudomonas in the presence of carbon and nitrogen supplements in a lead culture medium. Au J T.14: 88 - 96.
[49] Sarma, B., Acharya, C. and Joshi, S. R. (2010). Pseudomonads: A versatile bacterial group exhibiting dual resistance to metals and antibiotics. African Journal of Micorbiology Research 4: 2828-2835.
[50] Schmitt, C. J., Brumbaugh, W. G., and May, T. W. (2007). Accumulation of metals in fish from lead–zinc mining areas of southeastern Missouri, USA. Ecotoxicology and Environmental Safety67: 14–30.
[51] Sinha, K. R., Valani, D., Sinha, S., Singh, S. and Herat, S. (2009). Bioremediation of contaminated sites: A low-cost nature biotechnology for environmental clean-up by versatile microbes, plants and earthworms. In: Solid waste management and environmental remediation. Timo Faerber and Johann Herzog. ISBN: 978-1-60741-761-3.
[52] Staessen, J., Roels, H. A., Emelianov, D., Kuznetsova, T., Thijs, L. and Vangronsveld, J. (1999). Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Lancet353: 1140-1144.
[53] Steenland K. and Boffetta P., (2000). Lead and cancer in humans: where are we now? American Journal of Industrial Medicine38: 295-299.
[54] Tamtam, F.,Van Oort, F., Lebot, B., Dinh, T., Mompelat, S., Chevreuil, M., Lamy, L.and Thiry, M. (2011). Assessing the fate of antibiotic contaminants in metal contaminated soils four years after cessation of long term waste water irrigation. Science of the
Total Environment405: 540-547.
[55] Yadav, S. K. (2010). Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African Journal of Botany 76: 167–179.
[56] Yang, Q. W., Lan, C. Y., Wang, H. B., Zhuang, P., and Shu, W. S. (2006). Cadmium in soil–rice system and health risk associated with the use of untreated mining wastewater for irrigation in Lechang, China. Agricultural Water Management84: 147-152. [57] Yao, Z. T., Li, J. H., Xie, H. H., and Yu, C. H. (2012). Review on
remediation technologies of soil contaminated by heavy metals.
Proc. Environ. Sci.16: 722–729.
Fig. 1. Occurrence of metal resistant bacterial isolates in the selected contaminated soil samples.
LA: Ladipo spare part market, Lagos; OO: Owode Onirin market, Lagos; PC: Paint company dump site, Ota; and AG: Automobile garages, Ota.
Table I
Heavy Metal Resistant Bacterial Isolates from Selected Contaminated Soils
Bacterial isolate code Identification Source of isolate
AS2 Aeromonas spp Owode-Onirin, Lagos
BS2 Corynebacterium spp Mechanic workshop 1, Ota
BS3 Aeromonas spp Mechanic workshop 2, Ota
PC2B Streptococcus spp Paint Company dumpsite, Ota
LA 1 Streptococcus spp Ladipo market, Lagos
LA2C Aeromonas spp Ladipomarket, Lagos
LA 3 Arthrobacter sp Ladipomarket, Lagos
LA4A Corynebacterium spp Ladipomarket, Lagos
LA4B Pseudomonas sp Ladipomarket, Lagos
LA5B Corynebacterium spp Ladipomarket, Lagos
LA6 Corynebacterium spp Ladipomarket, Lagos
Table II
MTCs of cadmium, chromium and lead on MS agar or PYE agar for the selected bacteria isolates
MTC (mM)
Bacterial isolates MS agar PYE agar
Cd Cr Pb Cd Cr Pb
Corynebacterium sp 4 4 2 4 2 2
Pseudomonas sp 4 4 8 2 2 10
Table III
Antibiotic sensitivity pattern of selected heavy metal resistant bacterial isolates
Isolates Corynebacterium sp Pseudomonas sp Streptococcus sp
Amx R R R
Cot R R S
Nit S R S
Nal I S S
Ofl S S S
Aug R R R
Tet R I I
Caz S S S
Crx R R R
Gen R I S
Ctr R R S
Ery R R R
Cxc R R R