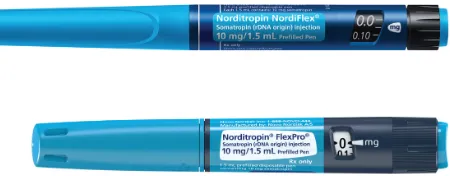
Developments in administration of growth hormone treatment: focus on Norditropin<sup>®</sup> Flexpro<sup>®</sup>
Full text
Figure
Related documents
The temporal dimensions of water availability in relation to demand and supply of the water resources for India, indicate a pressing problem of water
A multi-objective function having different performance requirements of minimum magnitude error and minimum pass band and stop band ripples is created to obtain the
Top panels: Bird 4’s average spatial and colour task performance across five sessions pre-and post lesion.. The first five blocks represent the acquisition phase, the break in
Our research is focused on the analysis of driver data, specifically looking at driver behaviour based on in-vehicle signals using extracted statistical features and also
applied which results are shown as under. The both confirmatory test and calculated results are shown in Table.7.. 581 | P a g e 1) Metal removal rate of the work piece increases
Currently she is pursuing M.Tech in Embedded System Design from VIF College of Engineering and Technology, Himayathnagar, RR (DT), Telangana, India. Her research
K, Fault-Tolerant Voltage Source Inverter for Permanent Magnet synchronous motor, International Journal of Advanced Research in Electrical, Electronics and
Synthesis and Spectral Characterization of Silver and Iron Nanoparticles and Their Antimicrobial Activity of Amaranthus Viridis.. Noha Inam