Patients
Forty-nine children with ALL, previously treated
according to protocol ALL II of the Dutch
Child-hood Leukemia Study Group (Table 1), were
stud-ied. The vaccination history of each patient was
Immunity
to Diphtheria,
Pertussis,
Tetanus,
and
Poliomyelitis
in Children
with
Acute
Lymphocytic
Leukemia
After
Cessation
of
Chemotherapy
A. van der Does-van den Berg, J. Hermans, J. Nagel, and
C. van Steenis
From the Dutch Childhood Leukemia Study Group, The Hague, Department of Medical Statistics, University of Leiden, and Rijks lnstituut voor de Volksgezondheid, Bilthoven, The Netherlands
ABSTRACT. Antibody titers to diphtheria, pertussis,
tet-anus, and poliomyelitis (types I to III) were measured in previously vaccinated children with acute lymphocytic leukemia in remission after cessation of therapy. The
response to revaccination one year after therapy was
stopped was also studied. The patients’ antibody titers
were compared with those of healthy children, matched
for age and sex. Two groups of patients were studied: one
group (group A, N = 30) was given two drugs
(6-mercap-topunne, methotrexate); the other group (group B, N=
19) was given three drugs (6-mercaptopurine,
methotrex-ate, and cyclophosphamide) for maintenance treatment.
In general, the patients’ antibody titers were lower than those of healthy children, but in most patients they were stifi at leveLs considered to be protective. No significant
differences in antibody levels between the two patient
groups were found. A spontaneous rise in antibody titers in the first year after termination of therapy was not
observed. After revaccination the rise in antibody titers
was correlated with preexisting antibody titers in the same way in patients as in healthy children, and the antibody titers in patients and in healthy control subjects were on roughly the same level. Pediatrics 67:222-229, 1981; antibody levels, diphtheria, pertussis, tetanus,
po-liomyelitis, acute lymphocytic leukemia.
Intensive treatment of childhood acute
lympho-cytic leukemia (ALL) on the basis of “total therapy”
has resulted in prolonged leukemia-free survival
and potential cure in a large proportion of the
2 In The Netherlands, since 1973, children
with ALL have been treated according to protocols,
based on the principles of “total therapy.” Between
1973 and 1975, a prospective comparative study was
performed to evaluate the effect of the addition of
cyclophosphamide (Cyclo) to maintenance
treat-ment with 6-mercaptopurine (6-MP) and
metho-trexate (MTX). All patients received the same
in-duction treatment and CNS prophylaxis as well as
intermittent “pulse courses” during maintenance
treatment, which was given for 24 months.
It is important to identify (late) side effects of
disease and therapy and to institute-if
possible-adequate treatment.3
As long-term combination chemotherapy, given
together with cranial irradiation, may cause severe
immunosuppression,4 the immunity to diphtheria,
pertussis, tetanus, and poliomyelitis in both groups
of patients was investigated by comparing the
an-tibody titers of these patients with those of healthy
children. Patients and healthy children had been
previously immunized according to the Dutch
Na-tional Vaccination Scheme.
Immunity was studied in patients in continuous
complete remission at cessation of therapy and 3, 6,
and 12 months thereafter. In addition, the response
to revaccination with diphtheria, tetanus, and
po-liomyelitis vaccine was evaluated one year after
therapy was stopped.
PATIENTS AND METHODS
Received for publication Feb 1, 1980; accepted June 19, 1980. Reprint requests to (A.V-V.) Dutch Childhood Leukemia Study Group, Juliana Kinderziekenhuis, P0 Box 60604, 2506 LP The Hague, The Netherlands.
TETANUS N type I
P0 ItOPlYE I IT IS
typ, II type III
DIPHTHERIA PERTUSSIS
- r i
I
P
I :
d
#{163}
.
.
m
r
, ..
*
:
L
,
4-7 -P -1-4-3-2-10 1 ?
I
N AU/I 2t, AU/mI
::
t
7,
‘ 0 01 AU/( AU/t
#{182}Q
:;
-L
0 23 S P 7 I 9‘011#{182}2
t OO2t,,, ,y,,
1J1
H
T
1;z.:,4
ht,, .80_2Io,-.n,
1 Y,S F-F-I
-7 -6 4#{149}-3-2-1 0 1 3 5
t
lU/mt U/mt
H1
,
;
i
0 01 lU/mt lU/mt
ri
r
iH
:i
;
dl titer T U
N 2to, lU/mt
,p
I fl
[
PL
dI titerl.4
r 21,9 lU/mt
tOE
rr
‘L
:
? ‘
diltiter T 4
210g lU/mI
n
:
d.t tte 142t, lU/mt
-i
p
dl t,t., 1
2t, U/mt
n
I
:;
dI t,te I
2Iog U/mI
P
001 AU/,L 2Lm AU/m BO2 tit 0.01 lU/mI 2109 lU/mt dt. tte, TI lU/mI dItter TI 2k,, lU/mI dl tte I 2109 lU/mt
known. Thirty children (group A) had received two-drug maintenance treatment (6-mercaptopurine
and methotrexate), and 19 children (group B)
three-drug maintenance treatment (6-mercaptopurine,
methotrexate, and cyclophosphamide). Group A
TABLE 1. Treatment Scheme Protocol ALL II
(SNWLK) (1973_1975)* Remission induction (6 wk):
Vincristine: 2 mg/sq m/wk, IV, six doses Prednisone: 40 mg/sq m daily, orally for 6 wks Preventive CNS therapy (2’/2 wk):
Age <1 yr: 1,500 rads cranial Age 1-2 yr: 2,000 rads cranial Age 3 yr: 2,400 rads cranial
in combination with
MTX: 12.5 mg/sq m, intrathecaily, five doses Diadreson-F: 12.5 mg/sq m, intrathecally, five doses Maintenance chemotherapy (24 mo):
Group A: 6-MP: 50 mg/sq m, daily, orally; MTX: 30 mg/sq m, weekly, orally
Group B: 6-MP and MTX as group A + cyclophospha-mide: 200 mg/sq m, every two wk, orally
Both groups received vincnstine-prednisone pulseS (for two weeks) intermittently every five weeks
Duration of therapy:
24 mo after first complete remission
* Abbreviations used are: IV, intravenous; MTX,
metho-trexate; 6-MP, 6-mercaptopurine.
Fig 1 . Antibody titers to diphtheria, pertussis, tetanus,
and poliomyelitis types I, II, and III in relation to age and
previous vaccinations in children with ALL, at the time of cessation of therapy and in healthy children. Group I: age < 4 years, vaccinated in infancy; II: age 4-8 years,
consisted of 16 boys and 14 girls, aged 3 to 13 years
(mean age 6 years); group B of 14 boys and five
girls, aged 3 to 12 years (mean age 6 years). The
difference in the number of patients in the two
groups is due to lethal infections during
mainte-nance treatment among group B patients in
com-plete remission.
During the 15 months of the present
investiga-tion, 15 patients relapsed: seven in group A and
eight in group B. These children were studied until
relapse.
Five venous blood samples were collected: just
before therapy was terminated, 3, 6, and 12 months
after termination, and 1 month after revaccination
with 1 ml of adsorbed diphtheria, tetanus,
macti-vated polio (DT-polio) vaccine, given at the time of
the fourth bleeding.
The patients had been previously immunized
ac-cording to the Dutch National Vaccination Scheme
(see “Control Subjects”). Some patients, however,
were inadequately vaccinated, due to interference
of the disease with the vaccination program (Fig 1
and Table 3).
Control Subjects
The antibody titers and the effect of
revaccina-tion in each patient were compared with those of
vaccinated in infancy and at preschool age; III: age 9
years, vaccinated in infancy, at preschool age, and at
school age. Open bars, control subjects; solid circles, group
A patients; solid triangles, group B patients; open circles,
(usually) two healthy children matched by sex and
age. Three venous blood samples were collected:
two samples were collected with an interval of six
months, and the third sample was collected one
month after revaccination with 1 ml of adsorbed
inactivated DT-polio vaccine, given at the time of
the second bleeding.
These children had been previously immunized
according to the Dutch National Vaccination
Scheme, which calls for vaccination with 1 ml of
adsorbed diphtheria, pertussis, tetanus, and
inacti-vated polio vaccine at the ages of 3, 4, 5, and 12
months, and with 1 ml of adsorbed DT-polio
vac-cine at the ages of 4 and 9 years.
On the basis of the number of previous
vaccina-tions, the children can be divided into three groups:
I: 3 to 4 years old, vaccinated in infancy; II: 4 to 8
years old, vaccinated in infancy and at the age of 4;
III: 9 years old, vaccinated in infancy and at the
ages of 4 and 9.
Methods
Antibody titers were determined in serum
sam-ples that had been stored at -20 C. All serum
samples of the patient and the matched control
subjects were tested in the same run except for the
titration of tetanus antitoxin, which was performed
independently. All titers are given as log2 values.
Diphtheria Antitoxin Titers. Diphtheria
anti-toxin titers were determined by the enzyme-linked
immunosorbent assay (ELISA) method7 and are
expressed in log2 antitoxin units (AU) per milliliter.
Titers were measured up to 4.00 AU/mi.
Antibody Titers to Bordetella pertussis.
Anti-body titers to B pertussis were measured by
mi-croagglutination tests8 with a freeze-dried
suspen-sion of strain 3838 (RIV).
Tetanus Antitoxin Titers. Tetanus antitoxin ti-ters were measured by toxin neutralization tests in
mice.9 Twofold serum dilutions were used. The tests
were performed at the L+ (limes tod)/1,000 level.
Titers were measured from 0.01 to 5.12 lU/mi. If
values above 5.12 lU/mi were found, the serum was
retitrated, starting from a dilution 1/10, which
re-sulted in a titration range from 0.1 to 51.20 lU/mi.
Titration of Polio Virus Antibodies. Polio virus
neutralizing antibody titers (types I, II, and III)
were determined by a microadaptation of the
met-abolic inhibition test, originally described by Salk
et al.’#{176}For types I, II, and III, the Brunenders,
Middle East forces (MEF) and Saukett strains,
respectively, were employed, and as cell substrate
the U cell line derived from human amnion. As
reference serum, a trivalent polio antiserum
(mon-key), calibrated against the International Standard
Serum, was included in each test.
Criteria for Protective Antibody Titers. For
diph-theria and tetanus, titers of 0.01 AU/mi and 0.01
lU/mi, respectively, are considered to be protective.
For pertussis, consensus has not been reached.
Ac-cording to the data of Wilkins et al,” we assume
this level to be 1:80, as determined in the
micro-technique. For poliomyelitis, any positive antibody
titer is consdered to be protective.
Statistical Analysis. For analysis of the data the
age, sex, and number of previous vaccinations of
the patients and the control subjects were taken
into account by matching each patient for sex and
age with usually two healthy control subjects and
by comparing the antibody titers of each patient
with the mean value of the titers of his/her matched
control subjects.
The antibody titers of each patient at the five
consecutive sampling times are denoted by Po, P3,
P6, P,2, and PB, the mean values of the titers of the
matched control subjects at the three sampling
times as Co,
C6,
and OB.The patients’ antibody titers at cessation of
ther-apy (Po) can now be studied by comparing these
values with the mean values of the matched control
subjects
(e#{176}),
so by investigation of Po - o.In the same way the time course of the patients’
antibody titers after cessation of therapy can be
assessed by P0 - Co, P3 -
e0,
p6 - 6, P2 C6, P,2-
e6,
and P, - eB.With respect to the number of previous
vaccina-tions healthy children can be classified into one of
the vaccination-status groups (I, II, and III) as
mentioned above. This classification is almost
equivalent to a classification into age groups.
RESULTS
Data below have been divided into three
subdi-visions: (1) antibody titers at cessation of therapy;
(2) course of antibody titers after cessation of
ther-apy; and (3) response to revaccination one year
after cessation of therapy. The two groups of
pa-tients are compared with each other, and the
pa-tients are compared with the control subjects.
Antibody Titers at Cessation of Therapy
The differences in antibody titers in patients
during therapy and the mean titers of the matched
control subjects (Po - Co) are summarized in Table
2. No significant differences were found between
the patients in groups A and B for any of the six
antibody categories. When patients were compared
with the control subjects, the mean value for Po
-Co was negative for all antibody categories (Table
2). For adequately vaccinated patients (N = 42)
TABLE 2. Differences in Antibody Titers Between Patients at Cessation of Therap Control Subjects (Po - e0) Relation to Previous Vaccinations
y and Mean Values of Matched
All Patients Adequately Vaccinated
(N = 49) Patients (N = 42)
Inadequately Vaccinated
Patients (N = 7)
Diphtheria -0.78 ± 2.88 -0.77 ± 3.00
(log2 AU/mi)
B pertussis -2.82t ± 2.41 -2.15t ± 2.21
(-log2 titer)
Tetanus -2.40t ± 2.81 -2.28t ± 2.78
(log2 lU/mi)
Poliomyelitis I -1.18t ± 1.90 -0.96t ± 1.80 (log2 lU/mI)
Poliomyelitis II -0.88t ± 2.08 -0.83t ± 1.86
(log2 lU/mi)
Poliomyelitis III -0.62 ± 2.67 -0.38 ± 2.67 (log2 lU/mi)
-0.86 ± 2.28
-0.58 ± 3.23
-3.08t ± 3.15
-2.36t ± 2.10
-1.17 ± 3.18
-1.93 ± 2.43
AValues are means ± SD.
t P < .01, Student’s one-sample test.
TABLE 3. Patients with Antibody Titers Below “Normal” Range at Cessation of
Ther-apy* Patient Group Previous Vaccinations Age at Diagnosis (Group)
Diphtheria B pertussis Tetanus Poliomy I II elitis III A B + + + + + + + + -+ + + + + + + + -I II III III III II I II I II II II III I II III I III II In III I II x x xx x x x x x x x x xx xx xx xx xx xx xx xx xx x x x x xx x x x x x x x xx xx x xx x x x x x xx xx xx x
A Five patients (not shown) aLso had antibody titers to B pertussLs less than 1:80; these
values were, however, within the “normal” range. Symbols used are: +, adequately
vaccinated; -, inadequately vaccinated; x, lower than “normal,” but considered to be
protective; xx, lower than “normal” and lower than protective value.
tetanus, poliomyelitis type I and II; for the small
group of inadequately vaccinated patients mean
values were significantly negative for tetanus and
poliomyelitis type II.
The antibody titers are also shown in relation to
age and number of previous vaccinations in Fig 1.
In 16 patients (eight in group A and eight in
group B) who had been adequately vaccinated
ac-cording to age, antibody titers lying below the range
of healthy children were found (Table 3). In seven
other patients the occurrence of ALL and the
treat-ment of this disease had interfered with the
vacci-nation scheme, and these children were
made-quately vaccinated.
A few patients had one or more antibody titers
below values considered to be protective (Table 3);
in some of them, this was combined with low
IgG-levels and severe lymphopenia (data not shown).
Unprotective antibody titers were observed in
LU /mt
Polio type I
A2
0-L
0
-1
-2
-3
Pertu ssis
0____
. oo
0
2Log U/mt
Polio type III
0
-1
-2
-3
-I. I
0.00
-too
-2.00
-3.00
-4.00
0
-
--L _---_1__
___-
I6 9 12 13
months after stopping therapy
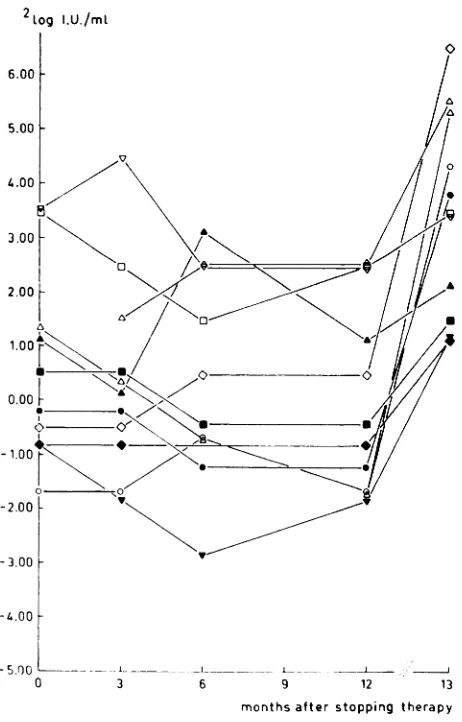
Fig 2. Time course of antibody titer to poliomyelitis type I in group B patients at cessation of therapy and 3, 6, and 12 months after cessation of therapy, as well as after revaccinat,ion, 12 months after cessation of therapy.
noticed that titers lower than 1:80 were also found
in a large proportion of the healthy children (23/
106). Only incidentally, no positive antibody titer to
poliomyelitis was found in healthy children (type
II: one child; type III: three children).
Course of Antibody Titers after Cessation of
Therapy
Because it would require too much space to show
the course of all six antibody categories for each
patient separately, only a representative case will
be given in detail. Fig 2 shows the course of polio
virus type I antibodies for the patients in group B,
who are currently in continuous remission. This
figure shows that the level of the antibodies in the
individual patients is fairly constant and mutually
quite similar. Therefore, the use of mean values to
study the time course seems justified; the time
courses of all antibody categories are shown,
ex-pressed as the mean differences between patients
2 .u/mL Diphtheria
c:i
-3
4
A2tog titer
U/mt
Tetanus
0
TN
1
E
0 3 6 12T3
Polio type 11
0
- 1
‘L
1T
months months
Fig 3. Time course of antibody titers, expressed as the
mean differences in antibody titers between patients and
matched control subjects, at cessation of therapy and 3,
6, and 12 months after cessation of therapy, as well as
after revaccination. Solid circles, group A patients in
complete remission; open circles, group B patients in
complete remission; solid diamonds, group A patients
with relapse; open diamonds, group B patients with re-lapse; arrow indicates revaccination.
and matched control subjects at various time points
(Fig 3 and Table 4).
During the first three months after cessation of
therapy, the values of patients tended to decrease.
Therefore, the differences in antibody titer between
patients and control subjects increased. Three to 12
months after the termination of therapy, the level
was fairly constant.
There was no significant difference between
pa-tients in the two groups. The course in patients who
relapsed could be studied only for the first six
months after cessation of therapy. In this
period
no
significant differences between the patients who
relapsed and those who remained in remission could
be established (Fig 3).
Revaccination
The effect of revaccination in patients in
contin-uous complete remission one year after cessation of
therapy and in matched control subjects can be
seen in Table 5. The increase of the antibody titers
varied widely. The age of the child had no influence
on the intensity of the booster effect. In healthy
children, as well as in the patients, a negative
revaccin-TABLE 4. Time Course of Antibody Titers an Patients and Matched Control Subjects*
d Response of Revaccination, Expresse d as Mean Duff erences Between
Patient At Cessation
Group of Therapy
Time After Cessation of Therapy 1 mo After
Revaccination
3mo 6mo l2mo
A (N = 24) (in continuous
remission)
Diphtheria (log2 AU/mi) -0.77 ± 3.04 -1.38t ± 1.93 -1.54t ± 1.97 -0.92 ± 1.84 -0.40 ± 1.07
B pertussis (-log2 titer) -0.76 ± 2.52 -1.32 ± 2.48 -1.92t ± 2.03 -1.69f ± 2.32
Tetanus (iog2 lU/mi) -2.63t ± 2.87 -3.58t ± 3.04 -3.50k ± 2.59 -3.51t ± 2.00 -0.28 ± 1.07
Poliomyelitis type I (log2 -1.144 ± 1.98 -1.37t ± 2.09 -0.99k ± 1.99 -1.22t ± 1.87 -0.38 ± 1.86
lU/mi)
Poliomyelitis type II (log2 -0.81 ± 2.26 -1.36t ± 2.23 -1.48t ± 2.20 -1.20k ± 2.06 +0.18 ± 1.90
lU/mi)
Poliomyeiitis type III -0.42 ± 2.86 -1.29t ± 2.81 -1.02 ± 2.62 -0.70 ± 2.60 +0.69 ± 2.29
(log2 lU/mi)
B (N = 11) (in continuous
remission)
Diphtheria (iog2 AU/ml) -0.72 ± 3.18 -1.82j ± 2.23 -0.87 ± 2.53 -2.23 ± 2.51 +0.03 ± 0.83
B pertussis (-iog2 titer) -0.12 ± 2.38 -0.56 ± 2.32 -0.64 ± 2.45 -1.04 ± 2.55
Tetanus (iog2 lU/mI) -2.96t ± 2.42 -3.80f ± 2.72 -2.68 ± 3.76 -3.45 ± 3.56 -0.08 ± 0.77
Poiiomyelitis type I (log2 -1.52 ± 2.14 -1.70j ± 2.56 -1.52 ± 2.46 -1.724 ± 2.40 -0.04 ± 2.10
lU/mi)
Poliomyelitis type II (log2 -1.30 ± 2.22 -1.32 ± 2.20 -1.07 ± 2.46 -1.47 ± 2.33 -0.07 ± 2.25
lU/mi)
Poliomyelitis type III -1.30 ± 2.81 -0.68 ± 3.14 -0.13 ± 3.02 -0.53 ± 3.40 +0.22 ± 3.17
(iog2 lU/mi)
AValues are means ± SD.
tP < .01 ,Student’s one-sample test.
:1:P < .05, Student’s one-sample test.
§These values may be influenced by the upper limit for the measured antibody titer.
TABLE 5. Rise in Antibody Titers to Poliomyelitis After Revaccination in Two Treatment Groups of Children with
ALL in Continuous Remission One Year After Cessation of Therapy and in Matched Control Subjects
Poliomyeiitis Type Group A (N = 24) Group B (N = 11) Control Subjects (N = 83)
Rise Range Rise Range Rise Range
I (log2 lU/mi) 3.48 ± 1.86 (0.00-7.00) 3.36 ± 2.24 (1.00-7.00) 2.53 ± 1.94 (-2.00-7.00) II (log2 lU/mi) 3.52 ± 1.93 (0.00-7.00) 3.18 ± 2.22 (0.00-6.00) 2.15 ± 1.77 (-3.00-7.00) III (log2 lU/mi) 3.54 ± 2.26 (0.00-9.00) 3.63 ± 2.66 (0.00-8.00) 2.58 ± 2.11 (-1.00-8.00)
* Values are means ± SD.
ation and the previous antibody titer for
poliomy-elitis of types I, II, and III (Fig 4 and Table 6). This
tendency was also observed for tetanus and
diph-theria, but calculation of a correlation coefficient
was not possible, because there was a maximum in
the determination of antibody titers to tetanus and
diptheria.
The differences in antibody titers between the
patients and matched control subjects tended to
decrease after revaccination. (Fig3 and Table 5).
DISCUSSION
The immunity to diphtheria, pertussis, tetanus,
and poliomyelitis was studied in previously
vacci-nated children with ALL in remission, after
long-term chemotherapy. The results show that after
cessation of therapy most patients stifi have distinct
antibody levels, but on the average the antibody
titers are lower than those of healthy control
sub-jects. In general there was no difference between
patients and control children in protective titers;
however, in some patients antibody titers were
be-low values considered to be protective.
The addition of cyclophosphamide to
mainte-nance treatment with 6-MP and MTX proved to
have no further influence on the level.
During the first six months after cessation of
therapy, no significant difference was found
be-tween the antibody titers of patients who relapsed
and those who remained in remission.
After cessation of therapy no spontaneous rise of
the antibody titers to diphtheria, pertussis, tetanus,
and poliomyelitis such as that described by Borella
et a!’2 for the Hong Kong influenza virus was
ob-served in this study. In patients the rise in antibody
corre-0
0
H
0
0 A
0
.
E
:
cit 0
a)
>,
0
.0
C (0
C
In
x
a)
a)0
0
9.
c. 0
0
EnJ:c
0
9
0
DA
LA
0.00
- 5.00
0
r
. 0
&
A0.00 3.00 6.00
- 3.00
Rise in antibody titer ( 21.og U/mi.
Fig 4. Correlation between the effect of revaccination and preexisting antibody titers to
poliomyelitis type II in patients with ALL in remission and healthy control subjects. Open
squares, control subjects; solid circles, group A patients (complete remission); solid triangles
group B patients (complete remission).
5.00
-TABLE 6. Correlation Between Preexisting Antibody Titers and Rise in These Titers After Revaccination in
Two Treatment Groups of Children with ALL in
Remis-sion and in Matched Control Subjects
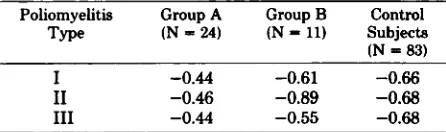
Poliomyelitis Group A Group B Control
Type (N=24) (N=11) Subjects
(N = 83)
I -0.44 -0.61 -0.66
II -0.46 -0.89 -0.68
III -0.44 -0.55 -0.68
AValues are correlation coefficients. All correlations are significantly less than zero.
lation with preexisting antibody titers just as in
healthy children.’3 As a result most of the
prevac-cination differences between patients and healthy
control subjects disappeared after revaccination.
Antibody-forming lymphocytes may be more
sen-sitive to chemotherapy than memory cells.’4
How-ever, the results of this study show that
revaccina-tion with DT-polio vaccine one year after cessation
of therapy induces adequate antibody response. In
view of the present results, an inherent defect in the
immune system of children with ALL, as suggested
by Bosu et al,’4 seems unlikely. This conclusion is,
however, restricted to the selected group of children
who successfully completed treatment according to
the above-mentioned protocol.
A practical implication of the present findings
concerns especially those children who had been
vaccinated inadequately or not at all before ALL
was diagnosed. Such children should, in any case,
receive adequate vaccination after cessation of
ther-apy. For those children who have previously
re-ceived adequate vaccination, revaccination with
DT-polio vaccine is recommended. Bpertussms
vac-cine, which is potentially neurotoxic, is not
admin-istered to children younger than 1 year.
The optimal time for revaccination was not
in-vestigated in this study. Gross et al’5 have shown
that children with malignancies have a good
im-mime response to influenza vaccine when they have
been off therapy for one month and have peripheral
white blood cell counts higher than 1,000/cu mm.
In the present study a good response to
revaccina-tion for diphtheria, tetanus, and poliomyelitis was
observed 12 months after cessation of therapy. In
view of the findings of Gross et al, it seems likely
that revaccination can be successfully performed
sooner after therapy termination.
In children with ALL, immunity to measles,
mumps, German measles, and chickenpox should
also be investigated at cessation of therapy. If
im-munity cannot be established, vaccination should
be considered. This is only justified after recovery
of the immunologic system as attenuated live
vac-cines have to be used.
ACKNOWLEDGMENTS
This study was supported in part by the Netherlands
Organization For Cancer Research (Koningin Wilhelmina
Fonda, Nederlandse Organisatie voor de
Kankerbestrij-ding).
de Volksgezondheid (RIV), Bilthoven, The Netherlands.
The authors are indebted to Dr H. Cohen and V. M. Sekhuis (Rijks Instituut voor de Volksgezondheid, Bil-thoven) for tdheir generous cooperation, to R. Oei and E.
H. Schuiing (Port Health Office, Rotterdam) for
organiz-ing the study, and to Miss A. Lykiema (Department of
Medical Statistics, University ofLeiden) for the computer analysis.
REFERENCES
1. Pinkei D: Treatment of acute leukemia. Pediatr Clin North
Am 23:117, 1976
2. George SL, Aur RhJA, Mauer AM, et al: A reappraisal of
the results of stopping therapy in childhood leukemia. N
Engi J Med 300:269, 1979
3. Editorial: The price of survival of childhood leukaemia. Br
Med J 1:321, 1978
4. Borella L, Webster RG: The immunosuppressive effects of long-term combination chemotherapy in children with acute
leukemia in remission. Cancer Res 31:420, 1971
5. Campbell AC, Hersey P, MacLennon 1CM, et a!: The
Med-ical Research Council’s Working Party on Leukaemia in
Childhood: Immunosuppressive consequences of radiother-apy and chemotherapy in patients with acute lymphoblastic ieukaemia. Br Med J 2:385, 1973
6. Hitzig WH, Pi#{252}ssHJ, Joller P, et ai: Studies on the immune
status of children with acute iymphocytic ieukaemia. II. In
remission with and without cytostatic treatment. Clin Exp
Immun.ol 26:414, 1976
7. Hagenaars AM, Nagei J: Bepaling van difterie antitoxinen in mensensera met behuip van ELISA. Verslagen, adviezen,
rapporten van Ministerie van Volksgezondheid.
Milieuhy-giene 17:195, 1976
8. Manclark CR: Serological response to Bordetella pertussis,
in Rose NR, Friedman H (eds): Manual of Clinical Immu-nology. Washington, DC, American Society for Microbioiogy
1976, p 312
9. Ipsen J: Systemic and change sources of error in measure-ment of small antitoxine levels. Immun. Forschung Exp
Titer 102:347, 1942
10. Salk JE, Youngner JS, Ward EN: Use of color change of
phenoired as indicator in titrating poliomyelitis virus or its
antibody in tissue culture system. Am J Hyg 60:214, 1954 11. Wilkins J, Williams FF, Wehrie PF, et al: Aggiutinin
re-sponse to pertussis vaccine. J Pediatr 79:197, 1971 12. Borella L, Green AA, Webster RG: Immunologic rebound
after cessation oflong-term chemotherapy in acute leukemia.
Blood 40:42, 1972
13. Svehag SE, Mandel B: The formation and properties of polio
virus neutralizing antibody. J Exp Med 119:21, 1964 14. Bosu SK, Ciudad H, Sinks LF, et a!: Antibody response to
polio virus immunization in childhood leukemia. Med
Pe-diatr Oncol 1:217, 1975
15. Gross PA, Lee H, Wolff JA et al: Influenza immunization in immunosuppressed children. J Pediatr 92:30, 1978
INTERNATIONAL MEETING
VI Congress of Pediatrics and XIII Panamericano and Peruvian Congress of
Pediatrics will be held in Lima Peru in October 1981. For information, please
contact:
Congresos Internacionales De Pediatria Lima, 1981
Washington 1807-Of. 401
Apartado 1786
Lima 1, Peru