EFFECTS OF POPULATION SIZE AND SELECTION INTENSITY ON RESPONSES TO DISRUPTIVE SELECTION I N
DROSOPHILA MELANOGASTER
J. S. F. BARKER AND L. J. E. KARLSSON
Department of Animal Husbandry, Uniuersity of Sydney, Sydney, N.S.W. 2006, Australia
Manuscript received January 21, 1974
ABSTRACT
Disruptive selection f o r sternoplcural bristle number with opportunity f o r random mating was done in the four treatment combinations of two population sizes (40 pairs and 8 pairs of selected parents) and two selection intensities (1 in 40 and 1 in 2). In each generation, matings among selected parents were obsserved in a mating chamber, and progeny collected separately from each female parent. In the high number, high selection intensity treatment, diver- gence between the high and low parts ceased about generation 11. The isolation index increased rapidly to generatioa 3, but then fluctuated to termination ob the population at generation 17. The overall isolation index was significant, indicating a real tendency to assortative mating. The failure of the isolation index to increase after generatiomn 3 was attributed to lower average mating fitness osf high males (due to inbreeding) and reduced receptivity of law females (due to a homoeygoas lethal gene with a large effect on sternoleural bristle number in heterozygotes). In the two lolw number treiatments, isolation indices fluctuated from generation to generation with no obvious trends, and none of the overall isolation indices were significantly different fro” zero. The
high number, low selection intensity treatment shoswed very little divergence, and one of the replicates showed, in contrast with expectation and the high
number, high selection intensity treatment a significant tendency to disassor- tative mating. Intense disruptive selection may lead to assortative mating.
HODAY (1967) stated as one of a number of generalizations concerning dis- Truptive selection, that it “can split a population into two parts between which there is considerable reproductive isolation”. This generalization remains contro- versial because the majority of laboratory experiments on disruptive selection have not lead to reproductive isolation between the two parts of the population. A total of 22 separate experiments utilizing different foundation stocks (all but one D. melanogaster) were reviewed by THODAY and GIBSON (1970). Only two of these (replicate experiments from the same foundation population) showed strong evidence of isolation. Four more experiments have since been reported. GRANT and METTLER (1969) obtained little divergence and no evidence of isola- tion in selecting f o r induced vertical I-maze activity. COYNE and GRANT (1972) selected for the same character from the same foundation population, but used a modified experimental design. High and low females were placed in separate culture vials after mating, and possible migration between the two parts was
716 J .S. F. BARKER A N D L. J. E. KARLSSON
reduced (but not precluded as stated by COYNE and GRANT) by selecting high and low respectively from the two subpopulations. Significant isolation was found in one of two replicates. ROBERTSON (1970) and BEARDMORE and AL BALDAWI
(reported by THODAY 1972) both repeated the experiment of THODAY and GIBSON (1962), using the same character and the same selection method. ROBERTSON obtained no isolation, while the brief report of the experiment of BEARDMORE and AL BALDAWI indicated the development of reproductive isolation by generation 16. The probability of disruptive selection leading to isolation therefore would appear to be quite low, but the controversy centers on the nature of the founda- tion populations, on aspects of the experimental design to which the results may be sensitive, and on the frequency with which disruptive selection in wild popu- lations may lead to isolation (THODAY and GIBSON 1970, 1971; SCHARLOO 1971). For isolation to develop, there must be some relevant genetic variance present in the foundation population. Clearly the development of isolation would be more likely if there were a genetic correlation between the primary character under selection and isolation tendency (i.e., assortative mating). The possibility of such a correlation has been investigated by BARKER and CUMMINS (1969b), with negative results, and by GRANT and METTLER (1969), with positive results. Alternatively, isolation could develop through selection against hybridization- reducing the frequency or success of hybrid matings, o r reducing the fitness (via- bility or fertility) of the progeny of hybrid matings. The possibility of developing isolation in this way is likely to be enhanced (given that relevant genetic vari- ability exists) by utilizing large populations (ROBERTSON 1970)
,
and by intense selection for the primary character.The experiment reported here was done to investigate the effects of population size and selection intensity on responses to disruptive selection. W e have chosen to use the same foundation population and to select for the same primary charac- ter as previously (BARKER and CUMMINS 1969a)-that is, the Canberra cage population, in which we found no evidence for a genetic correlation between this primary character (sternopleural bristle number) and isolation tendency.
MATERIALS A N D METHODS
Experimental d e s i g n In comparison with previous studies of disruptive selection, two refine- ments of technique were used in this experiment. In previous studies, the selected high and selected low flies of both sexes were all placed in a mating bottle for periods of up to two days. The males then were discarded, and die groups of high and of low females put into separate bDttles. Thus information on the relative frequency of the four possible mating types and the progeny productivity of each, together with estimates of the degree of isolation between the high and low parts olf the line, hnd to be obtained in separate supplementary experiments.
D I S R U P T I V E S E L E C T I O N IN DROSOPHILA 71 7
The practical implications of these pro'cedures determined to some extent the experimental design. Preliminary tests showed that the maximum number of flies that could be ob'served in a mating chamber was 40 pairs. Available data on progeny productivity per female, together with the recognition that this woiuld decreas2 as selection proceeded, indicated that a n average of 40 pairs of progeny per female parent should have been readily obtained over the 15 or SO
generations that the experiment was expxted to continue. The high selection intensity was set, therefore, at 1 in 40. A low population size of 8 pairs od parents and a low selection intensity of
50% also were used, so that the four treatments were:
1) High number, high selection intensity (Hr,Hs)-40 pairs selected each generation from 1600
2) High number, low selection intensity (HnLs)--iN) pairs selected each generation from 80
3) Low number, high selection intensity (LnHs)-8 pairs selected each generation from 320
4,) Low number, low selection intensity (LnLs)-8 pairs selected each generation fro,m 16 pairs
In all lines, the disruptive selection was symmetrical, so that one half of the selected parents osf each sex were those with the highest scores, and one half those with the lowest scores. This selection was from ail flies scored, i.e., regardless of the culture from which the flies came. When one or more ob several flies with the same bristle number had to be selected, random numbers were used t o ensure against bias toward choosing flies from particular cultures.
Initiation of the lines: A sample of 50 pairs of flies was taken from the cage population by egg sampling in 50 vials put into the cage for four hours, and collecting one male and one female virgin progeny from each vial. Fifty single pair matings were set up, using males and females from different egg sampling vials. Six pairs of virgin prcgeny were collected from each vial, and 30 bottles set up, each with 10 pairs of parents, again using males and females collected from different vials.
Progeny were collected as virgins fromm these bottles. The first 2COO pairs which emerged were allocated to HnHs. The next 2000 pairs to emerge were allocated to the other three treat- ments, and kept in storage vials at 20" for an additional week, so that HnHs and the other three treatments were scored in alternate weeks throughout the experiment.
Following scodng and selection in HnHs, HnLs and LnLs, either all the flies selected as high parents or all those selected as low parents had the tips of both wings lightly clipped to allow identification during the mating observations. This clipping was alternated between the two sets of selected parents in subsequent generations. In LnHs, each of the eight selected flies of each sex was uniquely identified by using a system of punching small holes in the wings in different positions. Detailed pedigree information therefore was kept for this line. After selection and wing marking, the selected parents were stored for 18-24 hours before the mating observations commenced.
Mating observations: Modified ELENS and WATTIAUX (1964) mating chambers were made of perspex with sloping walls to facilitate obmservation. For the small population size lines, chambers were cut i n 2.0-cm-thick clear perspsx, with an internal diameter of 1.5 cm at the bottom, and 8.0 cm at the top. The base (3.0 mm white perspex) and the tap (3.0 mm clear perspex) were screwed on to allow easy dismantling for cleaning between obesrvation runs. For the large popu- lation size lines similar chambers were made with intsrnal diameters of 9.0 cm at the bottom and 16.5 cm at the top, thus giving approximately the same surface area per fly during mating observations in all treatments.
During observation, the chambers were illuminated by a circular fluorescent tube. This surrounded a biconcave lens (12.7 cm diameter, 4.0 dioptre, 2'5.4 cm focal length) which facili- tated the recordings of matings, particularly for the large population size lines.
Flies were transferred to tlie mating chamber without etherization, with females introduced about 10 minutes before the males. At the end of the observation period, flies were transferred directly to a bottle for the additional mating period, again without etherization. In the HnHs
treatment, mating cbservations in every generation commenced at 4:080 p.m. to avoid po'ssible pairs scored (1 replicate only).
pairs scored (2 replicates).
pairs scored (2 replicates).
718 J .S. F. BARKER A N D L. J. E . KARLSSON
effects of diurnal variation in mating behavior (BARKER 1962a). For the eight lines of the other three treatments which ran concurrently in the alternate week to HnHs, this possibility had to be balanced against possible age effects. T h e d o r e in each generation, observations of these eight lines were done on the one day in random order, with the first starting at 8 : 0 a.m. Each treat- ment was to be obsa-ved for one hour or uniil such time that any co'pulation begun within this period had finished. However, for HnHs, the period of observation was increased from generation 5 to two hours.
Virgin collection and fitness of the lines: 111 the low selection intensity lines, where only two male and two female progeny were required from each parental female, all progeny were collected from one 12-hour emergence period on the ninth day after mating. Therefore in these treatments, selected parents in every generation were the same age, obviating any effects of age on mating behavior (BARKER 1962b, 1967).
For the high selection intensity lines, this was not possible, but in an attempt to minimize effects of age on mating behavior, the first two-thirds ob the collected virgins were stored at 20",
and the later collections at 25". Vials containing developing progeny were checked on the mom- ing of the eighth day after mating. Very rarely had progeny emerged at this time, but this check insured that all emergences twelve hours later could be collected as virgins. Virgin collection continued every 12 hours. To generation 3, sufficient total progeny were obtained in five collec- tions. The number of collections required steadily increased, and by generation 8, it was necessary t o collect until the morning of the thirteenth day after mating. The possible range of ages of selected parents at mating was then 3oL138 hours. In addition, selection could be confounded by parental age effects on the sternopleural bristle number of their progeny (PARSONS 1962) and by later-emerging flies having smaller body size and hence smaller bristle numbers (PARSONS 1961).
By generation (G.) 13 of HnHs, developmental time had increased such that the first virgin collection was 24 hours later than at G.O., so that virgin collection was continued until the morning of the fourteenth day after mating, and this treatment was then put on a 15-day cycle.
Scorin,g and selection: For the high selection intensity lines where dams had progeny in excess of the required number, the first emerging 40 male and 40 female prosgeny were scored. For dams with less than 40 progeny of either or both sexes, a dam of the same mating type with excess progeny was randomly allocateJ to make up the numbers. The bristle sco'res of the two lots of progeny were kept separately, so that the mean bristle number of the progeny from each dam could be calculated. Where a dam was infertile, she was allocated to a mating type on the basis of the observed mating frequencies. Then a dam from the cho'sen mating type was randomly used to provide as many progeny as possible, then if necessary ano'ther of the same mating type, and so on.
All flies were reared on a dead yeast fortified medium (Medium F of CLARINGBOLD and BARKER 1961). The experiments (including mating observations) were done at 25" -C 0.5" and a relative humidity of 65-70% in a room lit for 12 hours per day (6:OO a.m. to 6:OO p.m.).
RESULTS
Selection responses: Changes in mean bristle number in the high and low parts
(i.e., progeny of high and low female parents) of H n H s are shown in Figure 1,
and of H n L s and LnHs in Figure 2. Selection in L n L s was essentially ineffective,
DISRUPTIVE SELECTION I N DROSOPHILA 719
L
0 2 4 6 8 10 12 14 16
G E N E R A T I O N S
FIGURE 1.-Response to disruptive selection in HnHs. Generatioa means are averages of male and female bristle numbers.
1969a) and random mating. The observed divergences in the two replicates of
L n H s of 1.51 and 1.54 bristles were, respectively, 6% and 4% more than pre- dicted, while those in H n L s of 0.14 and 0.20 bristles were, respectively, 81% and 71% less than predicted. In all lines, response in the first generation was asymmetric, with greater response for high bristle number. Near-linear responses with this asymmetry continued to G.4 in HnHs, but in L n H s there was essentially no further increase in bristle number in the high parts of the lines. Selection responses in the high part of H n H s became erratic from G.4, and in the low part from G.8 (Figure 1 ) . The average difference between the high and low parts (Figure 3) showed no effective increase from G . l l . This difference, averaged over 9.1 1-G.17 (the measure of total divergence achieved), was 10.62 bristles. In L n H s , the same high selection intensity was remarkably ineffective, and both replicates failed to diverge further from G.3 (Figure 4). The differences between the high and low parts averaged over G.3-G.10 were 2.03 bristles in Replicate 1 and 1.90 in Replicate 2. The low selection intensity in H n L s also was ineffective in increasing divergence after G.2 (Figure 4); the average differences (G.2-G.9) being 0.67 bristles in Replicate 1 and 0.74 in Replicate 2.
720 J .S. F. BARKER A N D L. J . E. KARLSSON
19
17
0
y
21z
I-
v)
-
a
m19
17
0
2 4 6 8 10GENERATIONS
FIGURE 2.-Response to disruptive selection in (a) HnLs and (b) LnHs. Generation means are averages of male and female bristle numbers.
--
Rerlicate 1, - - - - Replicate 2.0
High part, 0 Low part.tions (percentage of their total area that the distributions had in common) gives a convenient measure of their separation, and is given for each treatment in Figure
5.
For treatments other than HnHs, these results emphasize the lack of separation of the high and low parts. For HnHs, the percentage overlap decreased rapidly to G.5, but increased markedly to G.6. This was followed by a slow decrease to G.17, but this final degree of overlap was still greater than that at G.5.D I S R U P T I V E S E L E C T I O N IN D R O SO P H I L A 721
GENERATIONS
FIGURE 3.-Difference in mean bristle number between the high and low parts of HnHs.
Differences between the means of the high and low selected parents are plotted against the
generation from which they were selected.
part of the line for which they were selected as parents were classified as “native” (i.e., in the high part of a line, the progeny of high females), while those from the other part of a line were classified as “immigrant”. For LnHs, where all selected parents were individually identified, migration rates were expressed as the numbers of parents of selected flies in each part that had themselves been selected into the other part.
Considering each treatment separately:
i) HnHs: I n each of G.l and G.2, three immigrants were selected among low parents and one among high parents. By G.2, the divergence between the high and low parts was sufficient to allow specification of the mating type for each female parent from the mean bristle number of her progeny. In addition to the above immigrants in G.2, one selected individual in the high part was the progeny of a high female X low male ( H L ) , while similarly one in the low part was the
progeny of a low female x high male ( L H )
.
But from G.3, all selected parents in both parts were the progeny of homogametic matings only. Therefore from G.3 there was no gene exchange between the two parts of the population, and each part was essentially a directional selection line for bristle number with imposed selection against heterogametic matings.722 J .S. F. BARKER A N D L. J. E. KARLSSON
15
c
c I /--
a
m5
PROGENY
1 I
0
2
4 6 8 10GENERATIONS
FIGURE 4.-Difference in mean bristle number between the high and low parts of (a) HnLs and (b) LnHs. Differences for parents plotted as in Figure 3 and replicate d eas in Figure 2.
only one produced progeny (Table 1 ) . But of 12 observed
H L
matings, 9 pro- duced progeny, with these latter progeny largely determining the mean bristle number of the high part in G.5. All selected parents in the high part in G.5 came from the one H H mating, so that the full-sib matings among them would have restricted the genetic variability available f o r further response. Separate data for each mating type are given in Figure 6, which shows mean bristle number, phenotypic variance and coefficient of variation for male progeny in each genera- tion. Data for females were similar except for a smaller variance in LL. After the restriction at G.5, response in H H continued at a slower rate to G.9, but from G.9 to G.17, no further response occurred. The large fluctuations in mean bristle number of progeny of high females in later generations (Figure 1) also were largely due to reduced numbers of fertile H H matings in some generations (Table I ) , probably a result of accumulated inbreeding. As the numbers of selected offspring from each mating were known, the effective population size of each part in each generation was estimated, and used to estimate the average coefficient of inbreeding (Table 2 ) . The effective population size ( N e in genera- tion t ) was estimated as:DISRUPTIVE SELECTION I N DROSOPHILA 723
8
O ' ' ~ ' 1 ' 1 1 ' ' ' ' 1 1 ' 1 'LnHs
80
1
HnHsLnLs
1
HnLswhere
N t
= number of individuals in generation t,and V k = mean and variance of number of gametes contributed per
parent in generation (t-1)
(CROW and KIMURA 1970, p. 351), while the average coefficient of inbreeding i n generation t ( f t ) was estimated as:
(CROW and KIMURA 1970, p. 102). The coefficient of inbreeding increased far more rapidly in the high part than in the low part. Even in G.0 of the high part, the effective population size was restricted, as one mating contributed 21 of the 40 selected individuals in G. 1. Then, apart from the restriction to one pair at G.4, in G.7 only
4
H H matings contributed selected progeny (with 18 selected from one mating) ; in G.12, only 2 H H matings contributed (27 from one mating) ; in G.14, only 4H H
matings contributed (25 from one mating) ; and in G.15, only one H H mating contributed all 40 selected high parents.Although response in the low part was linear to G.8 (Figure l ) , the mean bristle number of the progeny of LL matings showed a marked decrease from G.5 to G.6, and the variance in all three mating types including low parents dramatically increased in the same generation (Figure 6 ) . One of the low males selected in G.4 had only six sternopleural bristles, the previo'us lowest score being 12 bristles. Progeny scoring in G.5 showed that this male had mated with a low female-the progeny of this female segregating into two distinct phenotypic
724 J .S. F. BARKER A N D L. J. E. KARLSSON
TABLE 1
Number of observed mutings of each type (Obs.), number of fertile dams (Fert.), and number of these dams whose progeny were selected as parents in the next generation
(Select.) for each generation of HnHs
Mating type ( ? X d)
N L LH LL
~
IIH
Generation Obs. Fert. Select. Obs. Fert. Select. Obs. Fert. Select. O h . Fen. Select.
0 11 - - 8 - - 14 - - 5 -
-1 9 8 8 11 10 4 8 9 2 10 11 11
2 9 7 : 10 8 4 7 8 12 12
3 13 11 IO 6 6 6 6 13 14 7
4 8 1 1 12 9 7 3 13 I 4 4
5 9 9 8 9 6 6 9 8 11 10
6 12 11 9 7 7 7 9 9 11 9
7 9 4 4 8 7 6 5 9 14 10
8 12 8 7 7 6 10 8 7 8 7
9 11 7 5 7 7 8 7 10 12 10
10 14 8 8 8 8 5 4 11 13 I 2
11 13 4 3 2 4 8 5 16 15 13
12 12 2 2 7 10 8 4 8 9 9
13 - 10 8 - 5 - 5 - 13 9
14 8 4 4 11 9 5 3 13 13 10
15 8 2 1 13 9 5 1 10 12 10
16 14 12 6 6 5 5 4 13 14 13
17 17
-
- 9 - 9 - 10 - -Average 11.1 6.8 5.7 8.3 7.3 7.0 5.6 10.2 12.3 9.8
groups for bristle number, with approximately equal numbers in each. One group resembled their extreme low sire; the other had average bristle numbers similar to those for the progeny of other LL matings at this generation. All selected parents in G.5 (except for three females-one each from three different dams) were the progeny of this extreme low sire. Therefore, there was a substantial increase in the average coefficient of inbreeding in the low part in this generation (Table 2)
,
but the number of fertile LL matings did not decrease in later genera- tions (Table l ). The fluctuations in mean bristle number in the low part in later generations (Figure 1 ) were due to the contribution of the progeny of LH matings -mean bristle number being lowest in those generations where the number of festile LH matings in the previous generation was reduced (Table 1 ) . The fact that it was variation among mating types in number of fertile matings each generation, and in average number of progeny per mating that led to the fluctu- ations in mean bristle number in later generations in both parts of the line, is demonstrated by Figure 6. This shows that the changes in mean bristle number of progeny in each of the four mating types were relatively regular.DISRUPTIVE SELECTION I N DROSOPHILA 725
HnHs :
Males
i
FIGURE 6.-Mean bristle number, phenotypic variance and coefficient of variation for the male progeny of each of the four mating types HnHs.
A HH,
A
H L , L H , H LL.gene is homozygous lethal, and exhibits a variety of pleiottxophic effects-viz., high proportion of missing macrochaetae on head and thorax, microchaetae
(sternopleural, coxal and abdominal) reduced in number and in size, reduction in number of branches on the arista, terminal interruption of the fourth and fifth longitudinal wing veins, and a light straw body color.
Because of the continued segregation of this gene, the phenotypic variance of the LL mating type (Figure 6) increased rapidly to G.9, more slowly to G.13, and then remained stable at about 35.0 in males and 23.5 in females.
726 J . S . F. BARKER A N D L. J. E. KARLSSON
TABLE 2
Effectiue population size (Ne) and average inbreeding coefficient (f) in each generation of the high and low parts of HnHs
High LOW
Genera tion
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
6.66 0
13.47 .075
9.68 .lo7
15.40 ,151
1.89 ,177
14.86 .388
11.86 .w2
6.46 ,426
10.38 .469
7.73 ,492
11.34 ,524
5.55 ,546
3.50 ,583
10.01 .637
4.41 ,652
1.89 ,690
12.26 .762 .769
20.72 0
27.47 .024 22.63 ,091 13.47 .m12
2.26 ,096
12.56 .288 11.49 ,309
15.56 .338 15.08 .359 17.79 ,379 19.89 .396 20.41 .411 19.34 .425 11.87 .439 20.72 . a 2 20.19 ,475 21.91 .487 .499
failed to produce the required two pairs of progeny throughout the 9 generations. Chance and inbreeding were probably unimportant in this treatment, but migra- tion between the two parts remained high in all generations, with no decreasing trend. The proportions of immigrants were very similar in the two. replicates,
TABLE 3
Numbers of parents of selecied flies in each of the high and low parts in each generation that had themselues been selected into the other part-for each replicate of LnHs
Replicate 1 Replicate 2 -
Generation High Low High L a v
1 5 1
2
3 1 6
4 4 2 5 2
5 7 3 5
6 1 1 5
7 3 8 8
8 3 3
9 4 2
10 1 2 8 2
Average 1.9 1.9 2.1 3.3
DISRUPTIVE SELECTION I N DROSOPHILA 72 7 and averaged 0.35 into the low part, and 0.39 into the high part in each genera- tion. Even though the migration rate into the high part was slightly greater, there was a steady increase in mean bristle number in the total population of both replicates (Figure 2), with total gains over 9 generations of 1.64 bristles in Replicate 1, and 0.89 bristles in Replicate 2.
iii) LnHs: Even though selection intensity was high, migration between the two parts continued through the 10 generations. As the eight selected flies in each part were individually identified, migration rate was measured as the number of parents (out of 16) in each generation that had themselves been selected into the other part (Table 3 ) , The average migration rate was higher in Replicate 2, and within this replicate, higher into the low than into the high part. Although this migration would be expected to be antagonistic to selection, selection was most effective in the low part of Replicate 2 (Figure 2). Also, migration between the two parts cannot explain the complete lack of selection response in the high parts of both replicates after G.l, as there was no migration into the high part of Replicate 2 until G.4, and there was no response even i n the progeny of
H H
matings.
The number of fertile matings per mating type covered the range from zero to four, thus contributing (as i n H n H s ) to the fluctuations in bristle number from generation to generation. But on average, there were no differences among mating types in fertility or average progeny numbers, and no tendency for these to deteriorate in any mating type over the 10 generations.
I n this treatment, with only four pairs of selected parents in each part in each generation, inbreeding might be expected to accumulate rapidly. As the flies were fully pedigreed, inbreeding coefficients were calculated for all selected individuals (Table 4). In Replicate 1, the inbreeding coefficient was higher in the low part to G.6, then higher in the high part, while in Replicate 2, the reverse occurred. The rate of inbreeding was much higher in the first few gener- ations in Replicate 2, but the average coefficient of inbreeding in G.9 and G.10 was higher in Replicate 1. Neither replicate showed the steady increase in inbreeding observed in HnHs, a1 though average inbreeding coefficients in G.10 were higher than at G.10 in HnHs. The fluctuations in inbreeding generally are closely related to migration-the average coefficient of inbreeding decreasing in the progeny when a proportion of their parents came from the other part.
Mating observations and sexual isolation: The isolation index of MALOGOLOW- KIN-COHEN, SIMMONS and LEVENE (1965) was calculated for each generation of each line (Figure 7). For HnHs, the index was based on the maximum number of matings of each type, determined from mating observations or progeny testing (Table 1 ) . Because of low fertility in some generations, particularly of
HH
728 J .S. F. BARKER A N D L. J . E. KARLSSON
TABLE 4
Mean and standard deviation of the coefficients of inbreeding of selected parents in each generation of the two replicates of LnHs
Generation Hieh Low
Replicate 1
1 0 0
2 0 .031 -C .088
3 .008 f .E22 .I56 f .058
4 ,137 t .I21 .273 f .131
5 .I31 f ,049 .345 t .I50
6 .lo1 f ,010 .351 t ,216
7 .276 t .071 .273 t .052
8 ,446 f 0 .260 f
.ow
9 .558 f 0 .420 t ,112
10 .630 f .029 . 4 8 5 f .M
Replicate 2
1 0 0
2 .250 t 0 ,250 0
3 .375 k 0 .094f .I74
4 .289 t ,145 .219 t .058
5 .356 f .a33 .264 t ,127
6 ,433 t .030 ,261 f .059
7 ,295 f .I354 .243 k ,037
8 .357 f .026 .W5 -t- .059
9 ,396 f 0 .485 t ,073
10 .384 t ,036 ,563 t .I31
G
EN E RAT IONS FIGURE 7.-Isolation index in each generation for each line.DISRUPTIVE SELECTION I N DROSOPHILA 729
TABLE 5
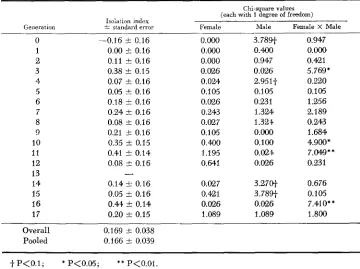
The isolation indices and mule and feinale mating ratio chi-squares for each generation of HnHs
~~ ~~~ ~~
Chi-square values (each with 1 degree of freedom) Isolation indcx
Generation i standard error Female Male Female X Male
0 -0.16 k 0.16 0.000 3.7891- 0.947
1 0.00 I 0.16 0 . 0 0.400 0.000
2 0.11 k 0.16 0.000 0.947 0.421
3 0.38 I 0.15 0.026 0.M6 5.769*
44 0.07 k 0.16 0.024 2.951-f 0.220
5 0.05 k 0.16 0.105 0.1015 0.105
6 0.18 k 0.16 0.026 0.231 1.256
7 0.24 I 0.16 0.243 1.324 2.189
8 0.08 I 0.16 0.027 1.324 0.243
9 0.21 k 0.16 0.105 0.mo 1.684
10 0.35 k 0.15 0.400 0.100 4.9001*
11 0.41 1 0 . 1 4 1.195 0.024 7 . w * *
12 0.08 I 0.16 0.Wl 0.026 0.231
13
-
14 0.14 k 0.16 0.027 3.2701- 0.676
15 0.05 t 0.16 0.421 3.789-f 0.105
16 0.44 5 0.14 0. a26 0.026 7.4101**
17 0.20 1 0 . 1 5 1.089 1 .O89 1.800
Overall 0.169 t 0.038
Pooled 0.166 & 0.039
i P<0.1; * P<O.05; * * P<O.Ol.
and transferred to a bottle. This problem was probably a result of ether fumes in the chamber. Normally the mating chamber was cleaned with ether the evening before use, to remove any olfactory stimuli that might influence mating behavior, but in this generation it was not cleaned w t i l three hours before it was used.
The isolation indices for H n H s in each generation, together with chi-squares for deviations of numbers of mated females and males from the expected 1:l
ratio, and for male x female interaction, are given in Table
5.
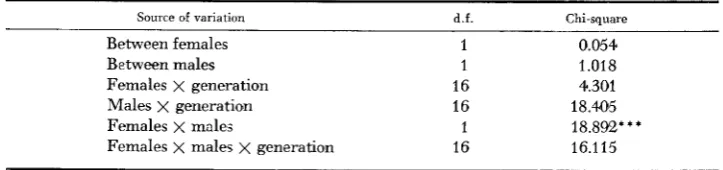
Isolation indices were generally positive, significantly so in 4 generations, but the regression of isolation index on generation number was not significant ( b = 0.013 f 0.007).An analysis of chi-square (Table 6) showed that overall, only the female X male interaction was significant, indicating a real tendency to assortative mating. As the female x male x generation interaction chi-square was not significant, the overall isolation index and its theoretical standard error were calculated from the total numbers in each mating type over all generations. This is given in Table 5 ,
together with the pooled estimate (arithmetic mean of individual generation indices). These two agree closely, and both are highly significant.
73 0 J .S. F. BARKER A N D L. J. E. KARLSSON
TABLE 6
Chi-squar~ analysis of the mating frequencies for HnHs
Source of variation d.f. Chi-square
Between females 1 0.054
Between males 1 1.018
Females x generation 16 4.301
Males x generation 16 18.44%
Females x males 1 18.892***
Females x males x generation 16 16.115
* * * P<O.OOl.
that Drosophila females are very unreceptive to males once they have mated (MANNING 1962), double matings by females, even during the short observation period, were not rare. Among high females, there were at least two in G.lO, one in G.15, and six in G.17, while among low females there were four in G . l l . At least one of the two matings by each of these females probably was partially abortive, because in each of the above generations, the mating records show a n equal number of matings with short copulation duration (5-10 minutes). Mat- ings of such unusually short copulation duration occurred in other generations, but possible double matings cannot be identified when the total number of mated females observed was less than or equal to 40.
The male mating ratio approached significance in four generations-with a deficiency of low males in G.O. and of high males in G.4, 14 and 15. Over all generations, the fitness of high males was lower, with a ratio of high to low males among known matings of 18.6 : 20.2, and among fertile matings of 12.3 : 19.5. The isolation index for H n H s increased rapidly to G.3, but decreased to little more than zero in G.4, and it was in this generation that poor mating ability of high males was first observed. Of the first 22 matings, 18 involved low males. For these 22 matings. the isolation index was 0.36. Although 11 more high males subsequently mated, the superior fitness of low males resulted in a high pro- portion of HP x L8 matings, and a non-significant isolation index. From G.5, with the appearance of the low bristle number gene in the low part. a proportion of the selected low females was very unreceptive, and some did not mate during the observation period (compare observed and fertile LL matings in Table 1 )
.
The numbers of matings of each type up to the time of break-up of the first copulating pair were recorded. For LL matings in G.1-4, a n average of 66% of the total occurred in this period. For G.5-10, this average dropped to 54%, but recovered to 66% in G.11-16. This refractory mating behavior in G.5-10 might be related to imperfect arista (BURNET, CONNOLLY and DENNIS 1971)-noted previously as one of the pleiotrophic effects of the low bristle number gene-with natural selection against its effects leading to improvement in the later genera- tions.
DI SRUPT IVE SE LE CTI ON IN DROSOPHILA 731
four generations in which the high and low males were noted during the obser- vations as approximately equivalent in mating fitness, and/or where low females showed unusually high receptivity, these indices were less than the indices given
in Table 5. There was, therefore, a general trend to a higher frequency of hetero- gametic matings (particularly H 0 X L 8 ) among the matings early in the mating period.
As the low females showed a strong tendency to homogametic matings, except for those intermediate generations where receptivity was low (Table I ) , it is clear that the failure of the isolation index to increase after G.3 was largely due to the lower average mating fitness of high males, compounded in the intermedi- ate generations by reduced receptivity of low females.
Although sexual isolation between the high and low parts of HnHs increased to G.3, and although from G.3 all selected parents in both parts were the progeny of homogametic matings only, the intense selection did not lead to an increase in hybrid sterility or inviability. I n fact, the reverse was true, undoubtedly the result of accumulated inbreeding in the HH and LL matings. Average fertility of low females cannot be assessed accurately, but L H matings probably showed lower fertility than did LL, due to the low fitness of high males. Similarly,
H H
matings were less fertile than H L (Table 1). Progeny numbers of all dams were recorded from G.8. Up to this generation, average progeny numbers per dam were in excess of requirements, but as it was obvious that progeny productivity was decreasing, all progeny were collected from every dam in this and subse- quent generations. From G.8, progeny numbers of high females fluctuated from generation to generation, but for low females, numbers generally decreased from G.8 to G.ll, and then increased to G.16-parallel to the changes in mating behavior previously noted. Average progeny numbers per dam for each mating type over G.8-G.16 were: HH-59.0; HL-82.5; LH-86.8; L L 5 1 . 4 . Also, the average developmental period of progeny of homogametic matings was nearly one day longer than that of the progeny of heterogametic matings.
For the three other treatments, isolation indices calculated from the numbers of each mating type observed in the mating chambers are shown in Figure 7. For HnLs, although none of the indices were significantly different from zero, most were negative, and for Replicate 1, the regression of isolation index on generations was significant ( b = -0.01 7k0.005, P<O.Ol). The overall isolation indices were -0.092*0.051 and -0.096t-0.051 in Replicates 1 and 2, with both types of heterogametic matings equally in excess. For the two low number (Ln) treatments, with only eight pairs of selected parents in each generation, the isola- tion indices fluctuated widely from generation to generation. No trends were obvious and none of the overall isolation indices were significantly different from zero. As the flies in LnHs were individually identified, all double matings by females were readily recorded. Of 163 females observed to mate during the observation period, three mated a second time. All were high females, one in G.l with observed durations of copulation of 17 minutes and 4 minutes, one in G.6
732 J .S. F. BARKER A N D L. J. E. KARLSSON DISCUSSION
The major focus of the experiment was on the high selection treatments, particularly HnHs. The other three treatments provided useful comparisons, although in retrospect they may have been more valuable if the selection had been more intense (20-30%) and population size larger.
The conditions required for disruptive selection to lead to isolation were reviewed by THODAY and GIBSON (1970). They and ROBERTSON (1970) empha- sized two basic weaknesses of the experimental design used in their own and in similar studies that would reduce the probability of obtaining isolation. First, the number of parents was very small, and thus the variance around the expected frequencies of fertile matings is expected to be large. Second, groups of selected high and of selected low females were placed in culture bottles to produce progeny. Thus, the numbers of females that did produce progeny and the relative numbers of their progeny were unknown. ROBERTSON (1970) has argued that the critical sampling element is the individual female insemination. and that differences between the high and low graups of males in mating ability, resulting from chance effects and inbreeding, would reduce the probability of obtaining stable divergence between the two parts of the population. The high population number treatments were used here to minimize chance effects and inbreeding, while the mating observations i n each generation and collection of progeny from single females allowed their effects to be assessed. However, placing each female in a separate vial for progeny production imposes a treatment different from previous experiments that could affect the results. THODAY and GIBSON (1970) pointed out that the rearing of all progeny types in one culture would accentuate any fitness difference between progeny of homogametic and of heterogametic mating. If the latter were less fit, divergence in the primary character and the probability of reproductive isolation would be increased. But if the homogametic matings were less fit due to inbreeding effects (as in the present experiment), divergence and the probability of reproductive isolation would be decreased. Rearing the progeny of each female separately therefore may have increased the probability of detecting divergence or isolation.
MAYNARD SMITH (1966) showed that in a heterogeneous environment divided into two niches, a stable polymorphism could exist between two alleles each con- ferring a selective advantage in one of the niches. This was true even when adults formed a single random-mating population, provided the population size was separately regulated in the two niches and the selective advantages were large. Further, rapid initial response to selection of the primary character was suggested by WADDINGTON and ROBERTSON (1966) as likely to facilitate isolation. The high selection intensity treatments therefore were used, with the expectation cjf
rapid divergence and, in the high number treatment ( H n H s )
,
possibly a stable divergence and maximum opportunity for the development of isolation.DISRUPTIVE SELECTION I N DROSOPHILA 733
of high males decreased drastically in G.4, with a male mating ratio of 15:26
(H
: L), and low fertility of high males ( 4 fertile matings of 15 observed, Table 1). The mating fitness of high males remained generally low i n subsequent generations, and the isolation index did not increase after G.3. Thus this high number population, designed to minimize chance effects and inbreeding, has in fact demonstrated their importance and validated this aspect of ROBERTSON’S(1970) hypothesis. In addition, the loss of genetic variability, particularly in the high part, must have contributed to the failure of the isolation index to increase after G.3, because from this generation there was direct selection for assortative mating, with all hybrids eliminated by the artificial selection. But there was further divergence in bristle number between the high and low parts from G.6 (after the appearance of the low bristle number gene), and the percentage overlap of the distributions showed a continued decrease from G.6 to G.16. It is probably doubtful whether appropriate genetic variability remained, but further selection after G.17 may have led to an increase in isolation, as the mating ability of high males improved remarkably in the last two generations, following the bottleneck in the high part at G.15.
734 J .S. F. B A R K E R A N D L. J. E. K A R L S S O N
not be hybrids, but progeny of the opposite homogametic mating-i.e. progeny of H H matings selected into the low part, and uice uersa. But whatever proportion were hybrids with a dam from the opposite part, the same proportion would be expected to be hybrid-with dams from the same part, but sires from the other part. Therefore it is likely that more than 50% of selected flies were hybrid, sug- gesting preferential selection of hybrid flies. However, the isolation indices for this treatment were generally negative, so that more than 50% of progeny pro- duced were hybrid, and the probable excess of hybrids among selected parents might be expected because the selection applied was weak.
The negative isolation indices themselves provide the most interesting and unexpected result from this treatment. Although the overall isolation indices for both replicates were not significant, the significant negative regression coefficient of isolation index on generations for Replicate 1 would indicate selection favoring disassortative mating. Further, when isolation indices were calculated only on matings initiated in the first five minutes, the overall isolation indices for both replicates were negative and significantly different from zero (-0.14+ 0.06 and -0.15+0.07 in Replicates 1 and 2, respectively), so that there was an even stronger tendency to disassortative mating among the initial matings. This was not due to refractory mating behavior of one type of male (as in H n H s ) , as all male mating ratios were not significantly different from unity. Such a tendency to disassortative mating certainly would oppose the mild disruptive selection, and warrants further investigation to determine its generality. Perhaps disassortative mating contributes to the maintenance of heterozygosity and stabilization of phenotypic variance in populations exposed to mild disruptive selection.
This study was suppwted by a University of Sydney Research Grant to J.S.F.B.
L I T E R A T U R E C I T E D
BARKER, J. S. F., 1962a Studies of selective mating using the yellow mutant of Drosophila rnelanogaster. Genetics 47: 623-64.0. ~ , 1962b Sexual isolation between Drosophila melanogaster and Drosophila simulans. Am, Naturalist 96: 105-116. -- , 1967 Factors affecting sexual isolatioii between Drosophila melanogaster and Drosophila simulans. Am. Naturalist 101 : 277-287.
Disruptive selection f o r sternopleural bristle number in Drosophila melanogaster. Genetics 61: 697-712. ---, 1969b The effect of selection for sternopleural bristle number on mating behaviour in Drosophila rnelanogaster. Genetics 61: 713-719.
BURNET, B., K. CONNOLLY and L. DENNIS, 1971 The function and processing of auditory i n f o - mation in the courtship behaviour of Drosophila melanogaster. h i m . Behav. 19: 44X-415.
CLARINGBOLD, P. J. and J. S. F. BARKER, 1961 The estimation of relative fitness of Drosophila populations. J. Theoret. Diol. 1 : 19e203.
COYNE, J. A. and B. GRANT, 1972 Disruptive selection on I-maze activity 111 Drosophila melano-
gaster. Genetics 71: 185-188.
CROW, J. F. and M. KIMURA, 1970 A n Introduction to Population Genetics Theory. Harper and Row, New York.
ELENS, A. A. and J. M. WATTIAUX, 1964 Direct observation of sexual isolation. Drosophila Inform. Serv. 39: 118-119.
D I S R U P T I V E S E L E C T I O N I N D RO S O PH I L A
735
GRANT, B. and L. E. METTLER, 1969 Disruptive and stabilizing selection o n the “escape” behaviour of Drosophila melanogaster. Genetics 62 : 625-6337.
LATTER, B. D. H., 1965 The response to artificial selection due to autosomal genes of large affect. I. Changes in gene frequency at an additive locus. Aug. J. Biol. Sci. 18: 585-598.
MALOGOLOWKIN-&HEN, C. H , A. SOLIMA SIMMONS and H. LEVENE, 1965 A study of sexual isolation between certain strains of Drosophila paulistorum. Evolution 19 : 95-1 03.
MANNING, A., 1962 A sperm factor affecting the receptivity of Drosophila melanogaster females. Nature 194: 252-253.
MAYNARD SMITH, J., 1966
PARSONS, P. A., 1961
Sympatric speciation. Am. Naturalist 100: 637-650.
Fly size, emergence time and sternopleural chaeta number in Drosophila. Heredity 16: 455-473. -- , 1962 Maternal age and developmental variability. J. Exptl. Biol. 39: 251-260.
ROBERTSON, A., 1970 A note on disraptive selection experiments in Drosophila. Am. Naturalist 104: 561-569.
SCHARLOO, W., 1971 Reproductive isolation by disruptive selection: Did it occur? Am. Naturalist 105: 83-86.
THODAY, J. M., 1967 The general importance of disruptive selection. Genet. Res. 9: 119-120. Disruptive selection. Proc. R. Soc. Lond. B. 182: 1019-143.
THODAY, J. M. and J. B. GIBSON, 1962 Isolation by disruptive selection. Nature 193: 1164-1166. The probability of isolation by disruptive selection. Am. Naturalist 104:
-
, 1972-,
219-230. -, 1971 Reply to Scharloo. Am. Naturalist 105: 86-88.
Res. 7: 303-312. 1970
WADDINGTON, C. H. and E. ROBERTSON, 1966 Selection for developmental canalisation. Genet.