Copyright 0 1993 by the Genetics Society of America
Genetic Studies of mei-1 Gene Activity During the Transition from
Meiosis
to
Mitosis in Caenorhabditis elegans
Thomas
R.
Clandinin' and Paul E. Mains'
Department of Medical Biochemistry, University of Calgary, Calgary, Alberta, T2N 4Nl Canada Manuscript received September 17, 1992
Accepted for publication January 1 1 , 1993
ABSTRACT
Genetic evidence suggests that the mei-1 locus of Caenorhabditis elegans encodes a maternal product required for female meiosis. However, a dominant gain-of-function allele, mei-l(ct46), can support normal meiosis but causes defects in subsequent mitotic spindles. Previously identified intragenic suppressors of ct46 lack functional mei-1 activity; null alleles suppress only in cis but other alleles arise frequently and suppress both in cis and in trans. Using a different screen for suppressors of the dominant ct46 defect, the present study describes another type of intragenic mutation that also arises at high frequency. These latter alleles appear to have reduced meiotic activity and retain a weakened dominant effect. Characterization of these alleles in trans-heterozygous combinations with previously identified mei-1 alleles has enabled us to define more clearly the role of the mei-1 gene product during normal embryogenesis. We propose that a certain level of mei-1 activity is required for meiosis but must be eliminated prior to mitosis. The dominant mutation causes mei-1 activity to function at mitosis; intragenic trans-suppressors act in an antimorphic manner to inactivate multimeric mei-1 complexes. We propose that inactivation of meiosis-specific functions may be an essential precondition of mitosis; failure to eliminate such functions may allow ectopic meiotic activity during mitosis and cause embryonic lethality.
C
O M P L E T I O N o f meiosis and initiation of mitosis in Caenorhabditis elegans requires extensive re- organization of the embryonic cytoplasm (STROME a n d WOOD 1983; ALBERTSON 1984; HILL a n d STROME1988, 1990). Meiotic and mitotic spindles are mor- phologically different but presumably contain com- ponents that perform similar functions. Certain com- ponents might be specific to the meiotic spindle and might have analogous but functionally noninter-
changeable mitosis-specific counterparts. Since female meiosis a n d t h e first mitotic cleavage occur within a common cytoplasm, mechanisms must ensure that
meiotic and mitotic components are used only in t h e appropriate spindle. What genetic pathways coordi- nate these two forms of division?
Prior to fertilization, the oocyte nucleus of C . ele- guns is arrested at diakinesis of meiosis 1. Upon fertil-
ization, it completes both meiotic divisions, migrates t o a n d fuses with the sperm pronucleus, and the first mitotic spindle is assembled (NEON, GUERRIER a n d MONIN 1960; HIRSH, OPPENHEIM a n d KLASS 1976; STROME a n d WOOD 1983; ALBERTSON 1984). These processes take place in a spatially distinct and tempo- rally restricted manner. For example, the meiotic spindle is positioned near the anterior pole of t h e embryo, pronuclear fusion occurs in the posterior
' Present address: Division of Biology, California Institute of Technology, ' To whom correspondence should be addressed.
Pasadena, California 91 125.
Genetics 1 3 4 199-210 (May, 1993)
region, and the mitotic spindle assembles along the anterior-posterior axis, slightly posterior of center. At 25" these steps are complete approximately 35 min after fertilization; given the invariant sequence of events, strict temporal control of t h e activities in- volved is implied.
Previous work (MAINS, SULSTON a n d WOOD 1990; MAINS et al. 1990) identified the mei-I locus as essen- tial for meiosis in the female germline. Embryos lack- ing maternal mei-I activity fail to form proper meiotic spindles, leading to defective polar body formation and variable incorporation of maternal chromosomes into the zygote nucleus; subsequent mitotic cleavages are morphologically normal. Such embryos presum- ably die due to chromosome aneuploidy. However, a dominant, temperature-sensitive (ts), gain-of-function
( g f ) allele a t t h e locus, mei-l(ct46), causes mitotic rather than meiotic defects. While the mitotic defects suggest that the gfallele is neomorphic (displaying an activity not seen in wild type), gene dosage studies revealed that wild-type activity competed with mu- tated product, making precise genetic classification of this allele problematic. Finally, this previous work also identified mutations at three loci, designated mei-2, mel-26 a n d zyg-9, that appear to interact genetically with mei-l(ct46); these loci may form part of the same genetic pathway.
200 T. R. Clandinin and P. E. Mains
as suppressors of the dominant mei-l(ct46) allele in a screen that identified two classes of intragenic pseudo- revertants. T h e expected group of alleles were capa- ble of suppressing only in cis; these were interpreted to be null alleles (designated class 1) that had con- verted the dominant,
gf
mutation into a recessive,y
allele. The second type of intragenic mutation, des- ignated class 2, arose at high frequency, lacked func- tional mei-1 activity, and suppressed the dominant gf allele both in cis and in trans. It was proposed that mei-l(+) gene product is required in meiosis and is normally inactivated prior to the first mitotic cleavage. T h e dominant mutation causes mei-I gene product to retain normal activity during meiosis but become re- sistant to postmeiotic inactivation. Trans-suppression by class2
alleles was thought to involve defective products that could complex with and promote the inactivation of the gf product, perhaps in an anti- morphic (dominant negative) manner. However, one might expect that trans-suppressors could also cause a reduction in mei-I(+) activity during meiosis and thereby create meiotic defects. As trans-suppressors caused no apparent meiotic defects in heterozygotes, one must postulate that either the inactivation of meiotic activity by trans-suppressors specifically occurs after meiosis or that a threshold of mei-1 activity exists; enoughgf
activity is eliminated by trans-suppressing product to correct the mitotic defects but sufficient activity remains to support meiosis. We have extended the genetic analysis of this locus and have distin- guished between these two models.In this paper we describe an alternate genetic screen for suppressors of the dominant maternal-effect lethal defect of mei-l(ct46). This identified a third type of intragenic suppressor that, like the other two classes of intragenic pseudo-revertants, arises at high fre- quency. Characterization of these alleles in trans-het- erozygous combinations with previously identified mei-1 alleles has provided evidence for a threshold level of mei-I activity; a certain level of activity is required for meiosis but must be eliminated prior to mitosis. Analysis of these strains also demonstrated that trans-suppressors do indeed act in an antimorphic manner, reducing mei-1 activity at both meiosis and mitosis. Finally, genetic evidence suggests that mei-1 gene product functions as a multimeric complex.
MATERIALS AND METHODS
Culture conditions: C. elegans (var. Bristol) was grown as described by BRENNER (1974). All stocks were maintained at 15 O and all crosses between strains bearing ts mutations
were performed at this temperature; other crosses were performed at 20 O . Embryonic viability was determined by scoring embryos laid by 2- 12 self-fertilizing hermaphrodites (1 or 2 animals per plate) over 48 hr at 25" (this usually represented a complete brood). Animals were transferred to fresh plates after 24 hr and unhatched embryos were scored 12 hr after removal of the parent. Arrested larvae
H 7 d
FIGURE 1.-Genetic map of the central region of LC I. The
extent of the deficiency nDj23 and the duplication gaDpl are
displayed below.
(animals much smaller than their sibs) and males were noted the following day. For calculation purposes, arrested larvae are not considered viable progeny. The same shelf of the same incubator was used to minimize variation between experiments.
Genes, alleles and rearrangements: The nomenclature of HORVITZ et al. (1979) is followed. The following genes and alleles were used. Map positions of selected loci are shown in Figure 1.
LG I: dpy-5(e61), lin-lO(e1438), lin-1 l(n566), mei-l(b284, ct46ts, ct46ct82, ct46ct99, ct46ct100, ct46ct103, ct46sb8ts, ct46sb9ts, ct46sblOts, ct46sb16ts, ct46sbl7ts, ct46sb18ts, ct46sb19ts, ct46sb2lts, ct46sb22ts, ct46sb23ts and ct46sb24ts), mei-2(ct102), unc-l3(el091), unc-29(e193 and e1072), and unc-l20(st364ts). The deficiency nDf23 and the duplication gaDp1 (Z;J were also used.
LC X: him-8(e1489), lon-2(e678).
Isolation and characterization of suppressors of mei-
Z(ct46): mei-l(ct46) is a dominant, ts, maternal effect lethal mutation (MAINS, SUBTON and WOOD 1990); mei-l(ct46ct99) is a recessive maternal effect lethal allele lacking detectable mei-1 activity that suppresses ct46 only in cis. mei-l(ct46ct99) unc-29/unc-l3 mei-l(ct46) lin-11; lon-2 heterozygotes were mutagenized with ethyl methanesulfonate (EMS, Sigma, St. Louis, Missouri) under standard conditions (BRENNER 1974). Two mutagenized animals were placed on each plate and allowed to produce self progeny at 15" for 2 days. Approximately 45,500 F1 animals on 623 plates were shifted to 25 " ; plates bearing relatively large numbers of FP animals were saved. A total of 14 independent lines were retained,
13 of which were outcrossed at least five times to remove extraneous mutations and maintained as homozygous Unc- 13 stocks at 15 O . The remaining line (designated ct46sb24) was inviable as an Unc-13 stock due to a spurious larval lethal mutation and was maintained as a ct46ct99 unc-291 unc-13 ct46sb24 lin-11; lon-2 strain.
Mapping mei-1 mutations by their suppression of ct46:
Genetics of Meiosis in C. elegans
TABLE 1
Summary of the embryonic phenotypes of all known mei-1 alleles
20 1
Meiosis Mitosis
Mutation type” m/m m/+ m/m m/+
Trans-suppression
m fct46 Interpretation
1. Class 1 -b
+
+
+
-
Null; complete If2 . Class 2
-
+c+
+
+
If; antimorphic-can inactivate mei-1 complexes4. Class 3
+
+
+/-
-
-
Weakgf; causes mei-l(+) to act at mitosis5 . ct46
+
+
-
-
3 . ct46ct103 +/-
+
+d+/-
+
Antimorphic but can cause mei-l(+) to act at mitosis-
&, causes mei-1 product to disrupt mitosis“
Examples of alleles in each class used in this paper: class 1, ct46ct99; class 2 , ct46ct82, ct46ct100, b284; and class 3, ct46sb17, ct46sb18,ct46sb22, ct46sb23.
“+”
indicates a normal division, ”-” indicates an abnormal division.“+”
also indicates the ability to trans-suppress mea-l(ct46).ct46ct82/ct46ct82/+ shows meiotic defects.
ct46ctlO3 homozygotes have slight effects on mitotic cleavages (see text).
excised from gravid hermaphrodites into “egg salts” (EDGAR and MCGHEE 1986), transferred to fresh plates at 25”, and scored for hatching. Given that recombinants were identi- fied at 25”, animals which had lost the suppressor but retained ct46 would not be detected as these animals would produce only inviable eggs (so clones could not be estab- lished). In each case, recombinants either displayed a level of embryonic lethality consistent with the presence of both ct46 and the suppressor or they displayed no maternal-effect lethality [mei-l(+)sup(+)]. This observation is consistent with two explanations. Either both ct46 and the suppressor map within the lin-10 unc-120 interval and are tightly linked or the suppressor maps distal to ct46 and possesses no apparent phenotype in the absence of ct46. To exclude the second possibility, two independent Lin-non-Unc recombinants (one each from ct46sb22 and ct46sb24) displaying no embry- onic lethality were tested for their ability to suppress mei- l(ct46) in trans; such suppression was not observed.
Microscopy: Gravid hermaphrodites were dissected in egg salts and living embryos were observed on agar pads using a Zeiss Axioplan microscope equipped with Nomarski optics (SULSTON et al. 1983). The embryos were time-lapse videotaped with a Panasonic WV 1800 camera and a Pana- sonic AG6750 videorecorder or flash-photographed with Kodak (Rochester, New York) Tech Pan film developed at ASA 100. In all figures, embryos are oriented anterior-left, dorsal-up.
RESULTS
T h e phenotypic properties of all known classes of mei-1 alleles are summarized in Table 1. Based on analysis of these mutations, we propose the following model. mei-l(ct46) product can function normally at meiosis but can also act ectopically at mitosis. Products of class 3 alleles identified in this paper can act ectop- ically like ct46 but have reduced activity; ectopic func- tion of these products results in mitotic defects that are less severe. Further, we suggest that class
2
(trans- suppressing) mei-1 alleles act as antimorphs, reducing mei-1 activity at both meiosis and mitosis. Implicit is a balance between a minimum level of mei-1 activity necessary for meiosis and a maximum level of ectopic activity tolerated at mitosis.If correct, this model predicts that a weak trans- suppressor might ameliorate the mitotic defects caused by a class 3 allele but leave enough residual
mei-I activity to support normal meiosis. Stronger trans-suppressors would not be expected to further improve mitosis but would reduce mei-1 activity pres- ent at meiosis to the point where meiotic abnormalities were seen. We will first describe the identification and characterization of class 3 mei-1 mutations and will then test these predictions directly.
Isolation of suppressors of mei-l(ct46): T h e dom- inant, ts, maternal-effect lethal mutation mei-l(ct46) retains normal meiotic function but appears to express this activity ectopically at mitosis, thereby “poisoning” the mitotic spindle. A previous screen for suppressors of mei-l(ct46) employed strains in which ct46 was het- erozygous with wild type and primarily isolated intra- genic Ifsuppressors (MAINS et al. 1990; Figure 2, left- hand column). Only two of fourteen suppressors iso- lated in this screen were extragenic; both were muta- tions at a linked locus, mei-2. We therefore designed an alternate screen biased against intragenic ct46 sup- pressors lacking all functional mei-1 activity by utiliz- ing a strain in which mei-l(ct46) provided the only functional mei-1 product (see MATERIALS AND METH- ODS and Figure
2,
right-hand columns). Animals bear- ing mei-l(ct46) in trans to a mei-1 null allele, ct46ct99, should acquire recessive maternal-effect lethality if additional mutational events eliminate the activity of the ct46 gene copy (as frequently occurred in the previous screen). Such mutational events would not, therefore, be detected. Approximately 45,500 F1 progeny of EMS-mutagenized ct46ct99 unc-29/unc-13 ct46 lin-11; lon-2 parents were screened for suppres- sion of the dominant maternal-effect lethality; a total of 14 independent lines were isolated.202 T. R. Clandinin and P. E. Mains
(ts)
- - ,~mei- l(ct46)
p
0
25’
viable
5
Isolates primarily dominant intragenic suppressors that are recessive maternal-
F
none viable2
Isolates extragenic and weak intragenic dominant suppressors effect lethals
somes), indicates that they do not represent unusual or rare alleles; estimates of
If
mutation rate at “aver- age” C . elegans loci after standard EMS mutagenesis are similar (BRENNER 1974; ANDERSON and BRENNER1984; PARK and HORVITZ 1986; KEMPHUES, KUSCH and WOLF 1988; ROGALSKI and RIDDLE 1988; JOHN- SEN and BAILLIE 1991). T h e remaining allele in this group displays no apparent phenotype, may represent a revertant to wild-type, and was not investigated further. T h e remaining three suppressors were un- linked to unc-I3 and may identify a single locus (T.
R.
CLANDININ, unpublished).Intragenic ct46 suppressors define a new class of dominant mutation: A number of observations sug- gest that the 10 suppressors may define a new class of
mei-I mutation. First, three of these alleles were mapped to the same interval as ct46, between lin-IO and unc-120, the two known loci that flank mei-I most closely (a 0.7-cM region approximately 200 kb in length, S. CLARK-MAGUIRE and P. E. MAINS, unpub- lished). Second, the complementation analysis de- scribed below revealed a pattern consistent with these mutations being new mei-I alleles. Third, gene dosage studies demonstrate that these alleles display proper- ties distinct from all previously identified mei-1 muta- tions (Tables 1 and 2). T h e original dominant muta- tion, ct46, was phenotypically most severe when homo- zygous; ct46/Df or ct46lct46ct99 strains displayed improved hatching; ct46/+ lines were even less severe (Table 2, lines 5 and 6). T h e new mutations generally showed improved hatching in trans to either Df or ct46ct99 compared to the homozygote but, in contrast to ct46, hatching frequencies decreased when these mutations were in trans to wild type by as much as sixfold (Table 2, lines 1-4). Furthermore, unlike the previously identified classes of intragenic suppressor, these mutations were viable as homozygotes and could not significantly suppress ct46 in trans (Table 2, lines
FIGURE 2.-Schematic illustra-
tion of the two alternative genetic
screens used to identify suppressors of mei-I(ct46). The method outlined
on the left was used to isolate class I
and class 2 suppressors (MAINS et af.
1990), the right-hand columns de-
scribe the procedure used in this
work to isolate class 3 alleles. The
cross-outs of “mei-I(ct46)” indicate a
new mutation induced in cis to ct46
that eliminates functional mei-I activ-
ity, as was seen for class 1 and class 2 mutations.
TABLE 2
Allele dosage effects on viability of class 3 embryos at 25”
Mutation’ M f + b M / M c M / c t 4 6 ~ 1 9 9 ~ M / D f d M / ~ t 4 6 ~
1. ct46sb22f 5.3 14 41 22 0
2. ct46sbI7f 13 26 62 49 0.10 3. ct46sb23f 24 86 77 67 0.48
4. ct46sb18f 46 86 92 76 4.3
5 . ct46g 1 0.2 0.3 0.3 -
6. ct46g (20”) 33 3 22 14 h
7. ct46ct99g (class 1) 97 0 0 0 0.3 8. ct46ct82g(class 2) 98 0.2 0 0 93
h
-
a Headings indicate maternal genotypes. All values are percent
of self-progeny that were viable. Between 350 and 1050 embryos of each genotype were scored.
All hermaphrodites were morphologically wild type and coiso-
genic for the heterozygous recessive markers unc-29 and unc-13.
These hermaphrodites were morphologically Unc-13. These hermaphrodites were morphologically wild type and
coisogenic for the heterozygous recessive marker unc-13. nDf23 was
used in these experiments and values were corrected for the 25% zygotic lethality caused by the deficiency.
CThese hermaphrodites were morphologically wild type and
coisogenic for the heterozygous recessive markers h i m 4 and Lon-2.
f Note that the deficiency failed to increase viability to the same
level as that achieved by ct46ct99. The deficiency used also uncovers
mel-26, a locus that interacts with mei-1 (MAINS, SUUTON and WOOD
1990); this may account for the difference.
These values were obtained previously (MAINS et af. 1990).
Class 1 alleles suppress mei-I(ct46) only in czs and are putative null
alleles; class 2 mutations lack functional mei-1 activity but suppress
mei-l(ct46) both in cis and in trans. Same values as for column 3.
1-4; compare with 7 and 8). Extending the previous nomenclature (MAINS et al. 1990), these alleles have been designated class 3 suppressors of mei-l(ct46) (null mutations defining class 1 ; alleles with trans-suppress- ing activity representing class 2). In this paper, “class 3 embryos” or “mutant embryos” refer to embryos produced by class 3 mutant hermaphrodites.
Genetics of Meiosis in C. eleguns
A
C
E
B
D
F
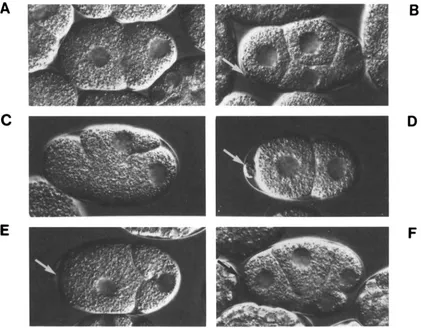
FIGURE 3.-Nomarski photomicrographs showing representative phenotvpes in self-progenv embryos produced by mei-l(lf), mei-l(gf) and wild-type hermaphrodites. (A) Wild type, two-cell; ( R ) wild tvpe, four-cell; (C) meGI(ct46). two-cell. Note the aberrant plane of mitotic cleavage;
(D) mei-l(cf46ctYY)). two-cell. Note the large polar body (arrow); (E) mei-I(ct46sbIR)/+, two-cell. Compare the plane of mitotic division with that seen in (C) and compare the size of the normal polar body (arrow) with the larger polar body in (D); (F) mei-l(ct46sbl8)/+, four-cell. Compare the relative sizes of blastomeres in this embryo with those in (B). Anterior is to the left, dorsal is upward.
totic defects similar to, but less severe than, defects produced by the original ct46 mutation (Figure 3). In wild-type embryos, maternal meiosis occurs near the anterior pole of the embryo and produces two small polar bodies. T h e subsequent mitotic spindle assem- bles along the anterior-posterior axis of the embryo: cleavage produces two unequally sized blastomeres, AB (anterior) and PI (posterior) (Figure 3A). In em- bryos produced by ct46 hermaphrodites, meiosis ap- pears to occur normally but the first mitotic spindle is often oriented perpendicular to the anterior-posterior axis of the embryo; the first mitotic cleavage usually occurs improperly and often incompletely (Figure 3C). In homozygous and heterozygous class 3 em- bryos, the first mitotic spindle is usually oriented correctly but division is often defective and results in abnormal distributions of cytoplasm between the AB and P I blastomeres (Figure 3E). In some embryos the first cleavage is apparently normal but cleavages to the four-cell stage occur improperly, producing un- usual blastomere sizes and positions (Figure 3F, com- pare with the wild-type embryo displayed in Figure 3B). Consistent with this observation, the tempera-
ture-sensitive period for ct46 extends for the first several cleavages, suggesting that mutant product can also disrupt later mitotic cleavages (MAINS, SULSTON and WOOD 1990). Weak class 3 suppressors display mitotic defects very similar to those produced by mei- l(ct46) (data not shown). Note that the embryonic phenotypes produced by class 3 alleles are quite dif- ferent from the recessive meiotic defects displayed by class 1 and class 2 mutations (Figure 3D).
mei-I(ct46ct103) is phenotypically enhanced by wild-type activity and can cause normal product to function inappropriately at mitosis: T h e earlier sup- pressor screen (MAINS et al. 1990) identified one homozygous viable mei-1 allele, ct46ct103, that caused weak, ts, meiotic defects as a homozygote (MAINS et
T. R. Clandinin and P. E. Mains 204
A
C
B
D
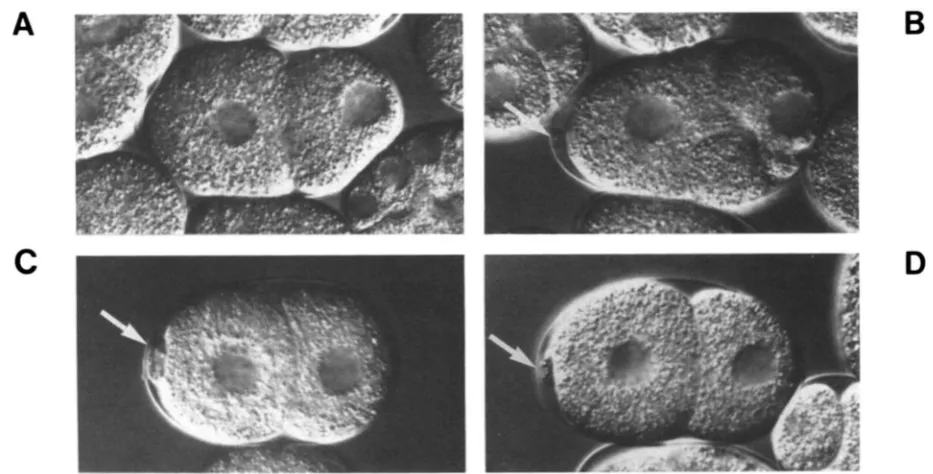
FIGURE 4.-Nomarski photomicrographs of self-progeny embryos produced by mei-l(ct46ct103) and wild-type hermaphrodites. (A) wild-
type, two-cell; (B) mei-l(ct46ct103)/+, two-cell; arrow, polar body; (C) mei-l(ct46cf103), two-cell; arrow, polar body; (D) mei-l(ct46ct103)/mei-
l(ct46ct99), two-cell; arrow, polar body. Compare the normal size of the polar body produced in (B) with the large ones seen in (C) and (D).
Also compare the abnormal mitotic division in (B) with the remaining plates. Anterior is to the left.
TABLE 3
Quantitation of meiotic and mitotic defects caused by mei-1 alleles at 25'
Meiotic defectsb Mitotic defectsb Viable embryosb
Genotypea N (o/.) ( W (%)
1. ct46ct103 34 44 6 70
2. ct46ct103/+ 55 4 24 73
3. cf46sb22/ct46ct99 42 0 83 24
4. ct46sb22/ct46ct100 67 4 30 72
5 . ct46sb221b284 76 26 4 91
6. ct46sb22/ct46ct82 47 49 0 81
a Maternal genotypes.
Developing embryos were analyzed by time-lapse video recording. Meiotic defects were indicated by abnormally large polar bodies as determined by measurements taken on the video screen. Mitotic abnormalities were scored at the one to four cell stages and included incomplete or misplaced cleavage furrows or unusual membrane blebs. Embryos were then incubated overnight and scored for hatching.
gotes to function aberrantly at mitosis. Individual embryos produced by either ct46ct103/+ heterozy- gotes or c46ct103 homozygotes were scored for ab- normal polar bodies and aberrant mitotic cleavages (Table 3). As predicted, defective embryos produced by ct46ct103 homozygotes primarily display defects in meiosis (Table 3, line 1); defective embryos produced by ct46ct103/+ animals primarily display mitotic de- fects (Table 3, line
2).
Together, these experiments demonstrate that wild-type activity potentiates the mitotic defects and reduces the meiotic defects caused by ct46ct103. T h e phenotypic enhancement of class 3 alleles by wild-type activity suggests that these muta- tions are likely to interact with wild type in a similar manner.trans-suppressors of meGl(ct46)are antimorphic: T o proceed, a quantitative assay specific for defects
Genetics of Meiosis in C. elegans 205
TABLE 4
m i - 1 trans-suppressors antagonize mei-I(+) activity at 25"
Viable rogeny Male
Maternal genotypea &) @)
1. ct46~t82/ct46ct82/+~ 9 32
2. ct46ct99/ct46ct99/ib 80 0.3
4. ct46ct82/V 96 0 . 2 4 3. + / + / + b 83 0.36
5 . ct44ct99/+c 98 0.07
Between 650 and 3 100 embryos of each genotype were scored.
The duplication used was gaDpZ(1;f.
These hermaphrodites were morphologically Unc and homo-
zygous for the recessive marker dpy-5 (covered by the duplication).
Hermaphrodites in line 1 were also heterozygous for the recessive
marker 1072-2.
These hermaphrodites were morphologically wild type and
coisogenic for the heterozygous recessive markers unc-29, unc-Zjr
and lin-11.
If, as we propose, trans-suppressors inactivate ct46 product during mitosis, perhaps they also reduce mei- I(ct46) and mei-I(+) activity during meiosis. Alterna- tively, perhaps trans-suppressors act only after meiosis is complete. These two possibilities are distinct; only the first predicts that trans-suppressor heterozygotes might cause meiotic defects (due to insufficient mei-I activity). We tested this prediction using the strongest trans-suppressor available, ct46ct82. Although we were unable to detect consistent effects in ct46ct82/+ animals (Table 4, line
4,
and data not shown), a slight increase in the relative proportion of ct46ct82 product to wild type (using ct46ct82/ct46ct82/+ strains) dra- matically reduced embryonic viability (Table 4, line 1 ; compare with lines 2-5). Consistent with this le- thality being caused by mei-I(+) activity being reduced by a dominant-negative effect, this strain produces large numbers of spontaneous males (Table 4, line 1). Neither lethality nor high rates of spontaneous males were observed in coisogenic strains bearing the null allele (Table4,
line2).
Given that a small difference in gene dosage has severe phenotypic effects, ct46ct82/+
animals probably produce just enough mei-I(+) ac- tivity to support normal meiosis.mei-1 protein activity must be eliminated after meiosis to allow normal mitosis: If class 3 mutations are weaker than ct46, they might be more easily suppressed by class
2
alleles. We examined trans- heterozygotes between class 3 alleles and a represent- ative series of other mei-I mutations that showed increasing ability to trans-suppress ct46 (and presum- ably had increasing antimorphic activity) in the order ct46ctY9, ct46ct100, b284 and ct46ct82 (Figure 5 ,MAINS et al. 1990 and data not shown). In these experiments, the percentage of inviable embryos is a measure of maternal defects that, as we will demon- strate, represent primarily meiotic or primarily mitotic abnormalities, depending on genetic background.
Meiotic defects were assayed by noting the frequency
of spontaneous males in hermaphrodite populations. Strikingly, all class 3 alleles displayed similar patterns of embryonic viability and spontaneous male fre- quency. As mentioned earlier, embryonic viability increased in heterozygotes when the wild-type copy was replaced by the null allele ct46ctW. Testing against a battery of trans-suppressors resulted in vari- able changes in viability, depending on the class 3 allele used. Most notable was that class 3 alleles (for example, ct46sb22) that hatched poorly when hetero- zygous with wild type displayed high viability when heterozygous with strong trans-suppressors (Figure 5, upper left). Conversely, class 3 alleles (for example ct46sb23) that displayed good viability in trans to wild type displayed poor viability in trans to the strongest trans-suppressor known, ct46ct82 (Figure 5, lower left). Seven of 1 1 class 3 alleles produced viabilities intermediate to those displayed by ct46sb22 and ct46sb23 in all trans-heterozygous combinations tested (for example, ct46sb17; Figure 5 , upper right). Two class 3 alleles, ct46sb18 and ct46ct103, were different, displaying relatively good viability in trans to both wild type and strong trans-suppressors (Figure 5, lower right, and data not shown). T h e significance of this latter observation is unclear; perhaps the suppress- ing mutations in these two alleles affect a second functional property of the mei-1 protein in addition to reducing its activity. For example, these mutants might interact inefficiently with trans-suppressing products, reducing the antimorphic effect.
206
ctMsb22
T. R. Clandinin and P. E. Mains
ct46sbl7
100 1 r 2 0 1001
ct46sb23 ct46sb18
14.5 5.0
L O 3.5
a0
g
2.5
g
2.0 1.5 1.0 0.5n
r
PD18 18 14 12
10
g
8 6 4 2 0a46682
FIGURE 5.-Bar graph plots of embry- onic viability and spontaneous male fre-
quency for a series of class 3 trans-heter-
ozygotes at 2 5 " . Hatched bars represent
the percentage of viable embryos, dark
bars represent the percentage of animals that survived to adulthood that were male. Note the different axis scales employed to represent the spontaneous male fre-
quency. Plots for mei-l(ct46sb22). mei-
l(ct46sbl7). mei-l(ct46sb23) and mei- l(ct46sb18) are as indicated. The relative strength of each trans-suppressor is shown at bottom right: this data is taken from
MAINS et al. (1 990).
/!gj
"RogenyStrength
of
transsuppressionof
d46 :B
FIGURE 6.-Non1arski photomicrographs of representative embryonic phenotypes of ct46sb23 in trans to (A) wild type or (B) ct46cf82 (a
strong class 2 trans-suppressor). Note the large polar body in (B) and compare the plane of mitotic cleavage in (A) relative to (B). Anterior is
to the left.
strongest trans-suppressor only display defects in meiosis (Table 3, line 6). Emphasizing the balance between sufficient mei-1 activity to support meiosis and excess ectopic activity causing defects in mitosis, embryos produced by animals bearing ct46s622 het- erozygous with trans-suppressors of intermediate strength can display defects in either meiosis or mi- tosis, but show primarily one or the other defect for a given genotype (Table 3, lines 4 and 5). Consistent
with earlier observations (MAINS et al., 1990), neither abnormal polar body formation nor aberrant mitotic cleavage necessarily result in embryonic lethality. (None of the tested embryos displayed defects in both meiosis and mitosis; but given the relative frequencies of each type of defect and the number of embryos observed, we cannot exclude the possibility that rare embryos might display both phenotypes.)
Genetics of Meiosis i n C . eleguns 207
TABLE 5
Synergistic interactions between mei-1 and mei-2 at 25"
Viable progeny Male Maternal genotype' (%) (%)
60 4.7
1. ct102+/+cf46ct82
2. ct102+/+ct46cf99 97 0.19
4. ct46ct82/+ 96 0.24
5 . ct46cf99/+ 98 0.07
_ _ _ _ ~ _ ~
3. ct102/+ 97 0.08
a Hermaphrodites in lines 1 and 2 were morphologically Unc-29
and coisogenic for the recessive marker Ion-2. those in 3 were
morphologically Lon, those in 4 and 5 were wild type and coisogenic
for the heterozygous recessive markers unc-29, unc-13, and lzn-11.
Between 1500 and 3500 embryos of each genotype were scored.
genesis: Given the dominant negative properties of mei-l(ct46ct82), we tested for interactions between this mutant and a
lf
mutation, mei-Z(ctlUZ), in the same genetic pathway. Previous work (MAINS et al. 1990) demonstrated that mutations at mei-2 could trans- suppress mei-l(ct46). We examined the viability of embryos produced by+
mei-l(ct46ct82)/mei-2(ctlU2)+
animals (Table 5 , line 1) and although only weak synthetic maternal-effect lethality was observed, the frequency of spontaneous males was high, suggesting meiotic defects. Heterozygotes of each mutation alone display wild-type levels of hatching and produce few spontaneous males (Table 5 , lines 3 and 4); a null allele of mei-1 did not interact with mei-2(ctlU2) (Table5 , lines 2 and 5 ) . This supports the idea that ct46ct821
+
animals produce barely enough mei-l(+) activity to carry out meiosis;lf
mutations in the same genetic pathway reduce this activity below a critical level.DISCUSSION
In
C.
elegans, the meiotic spindles in the oocyte and the mitotic spindles in the zygote are structurally distinct but must fulfil somewhat similar functions. It seems reasonable to suggest that these spindles share certain analogous but functionally noninterchangea- ble components. Furthermore, these two processes occur in rapid succession within a common cytoplasm; some system must therefore ensure that division-spe- cific components are targeted to the correct spindle. Since meiosis and mitosis occur sequentially, perhaps the simplest such system would ensure that analogous components are never present at the same time. After meiosis, all meiosis specific structures could be inacti- vated or eliminated; the only active components pres- ent at the time of mitosis are then mitosis specific. We postulate that the mei-1 gene product is a meiotic spindle component, or a regulator of such compo- nents, that might have a functional analog in the mitotic spindle.Previous work demonstrated that mei-l(+) activity was only required maternally for meiosis in the oocyte (MAINS, SULSTON and WOOD 1990; MAINS et al. 1990).
A
mel- 1 (U46)
mei- I(+)
MEI- 1
\
ACTIVITY
B
mei- 1 (+)
-
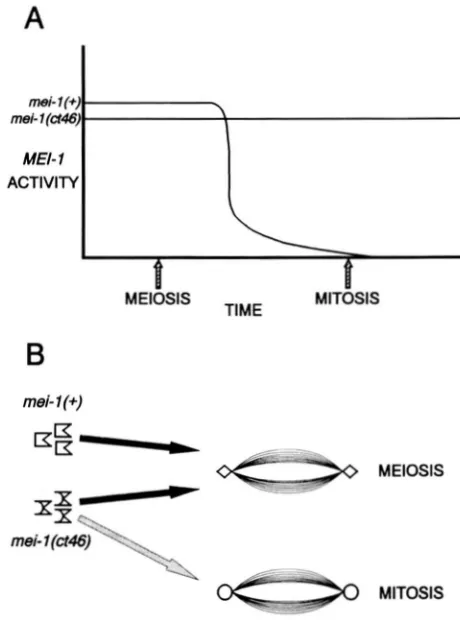
FIGURE 7."Two alternative models of the nature of the mei-
l(ct46) mutation. (A) "Hyper-stability"-mei-1 activity is normally
eliminated immediately after meiosis; mei-l(ct46) product is refrac-
tory to inactivation. (B) 'Specificity"-mei-1 activity is normally only compatible with the meiotic spindle; mei-l(ct46) product can incor- porate into both meiotic and mitotic spindles.
‘LO8 T. R. Clandinin and P. E. Mains
sors produce a defective product capable of complex- ing with ct46 or wild-type protein; such complexes may retain some activity but are inactivated prior to mitosis. Class 3 alleles reported in this work represent modifications of ct46 that have reduced activity at both meiosis and mitosis but have retained ectopic mitotic expression of this reduced activity. T h e class 3 products can complex with wild-type protein and appear to cause ectopic expression of wild-type activity at mitosis.
Our evidence demonstrates that class 2 intragenic mei-1 alleles capable of trans-suppressing ct46 inhibit gfmei-1 gene products in a dominant-negative man- ner. Furthermore, these antimorphic alleles similarly affect mei-I(+) activity and do so prior to the comple- tion of meiosis; the strongest trans-suppressor avail- able, ct46ct82, can produce defects in meiosis by re- ducing mei-l(+) activity. We propose that the domi- nant negative effect of mei-1 trans-suppressors results from interactions between active and inactive gene products forming an inactive complex. Similar domi- nant-negative interactions have previously been de- scribed in C. elegans for certain loci involved in cuticle formation and muscle structure (KUSCH and EDGAR 1986; KRAMER et al. 1988; BEJSOVEC and ANDERSON 1988).
Since trans-suppressors appear to act early in meiosis, why are meiotic defects not observed in em- bryos produced by ct46ct82/+ or ct46ct82lct46 her- maphrodites? One possibility is that these animals produce just enough mei-l(+) activity to support nor- mal meiosis; further reductions cause significant phe- notypic effects. Two observations support this expla- nation. First, embryos produced by ct46ct82/ct46ct82/
+
parents display meiotic defects; a relatively small change in gene dosage reduces mei-1 activity below the critical threshold (Table 4). Second, an additionalIf
mutation at a second locus in the same genetic pathway, mei-2, causes meiotic defects in ct46ct82 trans-heterozygotes (Table 5). Since the mei-I(nul1) phenotype can cause defects in meiosis I , mei-1 gene product may assemble early in meiosis; trans-suppres- sors may co-assemble and inactivate these complexes as they form.T h e observation that class 3 alleles are phenotypi- cally enhanced by wild-type activity and can cause wild-type activity to function inappropriately at mito- sis is striking (Table 2). T h e classical interpretation (MULLER 1932) of phenotypic enhancement by wild type is that class 3 alleles represent hypermorphic mutations that increase gene activity. In this interpre- tation, additional activity enhances phenotypic sever- ity by exaggerating the initial excess. However, gene dosage experiments show that ct46 does not behave like a hypermorphic mutation (MAINS, SULSTON and WOOD 1990; P. E. MAINS, unpublished observations)
and since class 3 alleles arise frequently, we prefer an alternate explanation.
One plausible model is based on the idea that, like ct46, the products of class 3 alleles are unusually stable or refractory to postmeiotic inactivation. T h e pheno- typic enhancement of class 3 alleles by wild-type activ- ity could then result from the dominant product arti- ficially stabilizing wild-type product in a complex (i. e., conferring its unusual stability in trans). One apparent anomaly in this interpretation is that wild-type activity did not enhance the mei-l(ct46) phenotype. If ct46 produces a protein that is unusually stable but, unlike class 3 mutations, retains normal activity, complexes between ct46 and wild-type product might be unusu- ally stable but would possess the same activity as complexes containing only ct46 product; as a result, no phenotypic enhancement by wild type would occur. Instead, when wild-type product interacts with ct46, a different effect is revealed: wild-type product com- petes with ct46 product to relieve the dominant de- fects in a dose-dependent manner. Perhaps wild-type product competes with mutant product for assembly into mei-1 multimers; unincorporated ct46 product might then be incapable of acting at mitosis.
While models in which dominant mei-1 mutations cause mei-l(+) activity to become unusually stable seem plausible, alternative frameworks are also possi- ble (Figure 7B). For example, perhaps mei-1 protein activity normally persists after meiosis; mitotic mis- incorporation might be prevented because the mitotic spindle can distinguish between mei-1 and its mitotic analog; mei-l(ct46) might be altered such that this discrimination becomes more difficult. In this model, the postulated mechanism of trans-suppression can remain unaffected; incorporation of defective product into a multimeric complex inactivates the assembly.
Genetics of Meiosis in C. elegans 209
of conditions are fulfilled (for examples see WEINERT
and HARTWELL 1988; HOYT, TOTIS and ROBERTS
199 1 ; LI and MURRAY 199 1 ; SCHIMKE et al. 199 1). Unlike “adult” somatic cell division, embryonic cell cycles appear to have few checkpoint controls (re- viewed in MURRAY and KIRSHNER 1989). Our obser- vations are consistent with this idea; the switch from meiosis to mitosis during C . elegans embryogenesis may not be checkpoint dependent. Embryos lacking maternal mei-1 activity fail to assemble a meiotic spin- dle and segregate maternal chromosomes aberrantly. However, polar body formation, albeit aberrant, is often attempted and subsequent mitotic divisions in these embryos are normal and occur at appropriate times.
I n conclusion, a certain level of mei-I activity is required to support meiosis but ectopic activity can cause mitotic defects. Dominant mei-I alleles may sta- bilize the activity of a multimeric complex of mei-1 product, causing mitotic cleavage defects. Trans-sup- pressing alleles of mei-I produce defective products that can incorporate into these multimers, inactivate the complex and block the dominant effects. If mei-I dominant mutations do indeed cause mei-I protein to become unusually stable, an additional hypothesis can be made. If specific mechanisms are necessary to eliminate postmeiotic “debris,” these systems must rapidly inactivate meiosis-specific components and re- place them with mitotic counterparts. In this manner, meiosis may constrain the embryonic cell cycle: mitosis cannot occur properly until meiotic products have been eliminated. Genes known to interact genetically with mei-1, namely mei-2, mel-26 and ryg-9, may iden- tify additional components of this system (MAINS et al. 1990). Molecular characterization of the mei-I gene product should clarify the underlying mechanisms behind these genetic observations.
We would like to thank K. KEMPHUES, M. BENTLEY, J. MCGHEE,
P. SVENDSEN, G. SCHULTZ and anonymous reviewers for critical
comments on this manuscript, S. CLARK-MAGUIRE and C. H. HSIEH
for technical assistance and all of the members of the Calgary worm
group for helpful discussions. This work was supported by an
Alberta Heritage Foundation for Medical Research Studentship
award to T.R.C. and grants from the Medical Research Council of
Canada and the Alberta Heritage Foundation for Medical Research
to P.E.M. Some of the nematode strains used in this work were
obtained from the Caenorhabditis Genetics Center, which is sup-
ported by Contract No. N01-AG-9-2113 between the National
Institutes of Health and the Curator of the University of Missouri.
LITERATURE CITED
ALBERTSON, D. G., 1984 Formation of the first cleavage spindle
in nematode embryos. Dev. Biol. 101: 61-72
heavy chain mutants in the nematode Caenorhabditis elegans.
Proc. Natl. Acad. Sci. USA 81: 4470-4474.
ANDERSON, P., and S. BRENNER, 1984 A selection for myosin
BEJSOVEC, A,, and P. ANDERSON, 1988 Myosin heavy chain mu-
tations that disrupt Caenorhabditis elegans thick filament assem-
bly. Genes Dev. 2: 1307-1317.
BRENNER, S . , 1 9 7 4 T h e genetics of Caenorhabditis elegans. Ge-
netics 77: 71-94.
EDGAR, L. G., and J. D. MCGHEE, 1986 Embryonic expression of
a gut-specific esterase in Caenorhabditis elegans. Dev. Biol. 114:
109-1 18.
HARTWELL, L. H . , and T. A. WEINERT, 1989 Checkpoints: con-
trols that ensure the order of cell cycle events. Science 246:
629-634.
HARTWELL, L. H., J. CULOTTI, J. R. PRINGLE and B. J. REID,
1974 Genetic control of the cell division cycle in yeast. Sci-
ence 183: 46-5 1.
HILL, D. P., and S. STROME, 1988 An analysis of the role of
microfilaments in the establishment and maintenance of asym-
metry in Caenorhabditis elegans. Dev. Biol. 125: 75-84.
HILL, D. P . , and S. STROME, 1990 Brief cytochalasin-induced
disruption of microfilaments during a critical interval in 1-cell C. elegans embryos alters the partitioning of developmental
instructions to the 2-cell embryo. Development 108: 159-172
HIRSH, D., D. OPPENHEIM and M. KLASS, 1976 Development of
the reproductive system of Caenorhabditis eleguns. Dev. Biol.
49: 200-219.
HODCKIN, J., H. R. HORVITZ and S. BRENNER, 1979 Non-disjunc-
tion mutants of the nematode Caenorhabditis eleguns. Genetics
91: 67-94.
HORVITZ, H. R., S. BRENNER, J. HODGKIN and R. K. HERMAN,
1979 A uniform genetic nomenclature for the nematode
Caenorhabditis elegans. Mol. Gen. Genet. 175: 129-133
HOYT, M. A,, L. TOTIS and B. T. ROBERTS, 199 1 S. cermisiae
genes required for cell cycle arrest in response to loss of
microtubule function. Cell 66: 507-51 7
JOHNSEN, R. C., and D. L. BAILLIE, 1991 Genetic analysis of a
major segment [LG V(left)] of the genome of Caenorhabditis
elegans. Genetics 129: 735-752
KEMPHUES, K. J., M. KUSCH and N. WOLF, 1988 Maternal-effect
lethal mutations on linkage group 11 of Caenorhabditis elegans.
Genetics 1 2 0 977-986.
KRAMER, J. M . , J . J . JOHNSON, R. S. EDGAR, C. BASCH and S.
ROBERTS, 1 9 8 8 T h e sqt-I gene of C. elegans encodes a colla-
gen critical for organismal morphogenesis. Cell 55: 555-565.
KUSCH, M., and R. S. EDGAR, 1986 Genetic studies of unusual loci
that affect body shape of the nematode Caenorhabditis elegans
and may code for cuticle structural proteins. Genetics 113:
LI, R., and A. W. MURRAY, 1991 Feedback control of mitosis in
budding yeast. Cell 6 6 5 19-53 1
MAINS, P. E., I . A. SULSTON and W . B . WOOD, 1990 Dominant
maternal-effect lethal mutations causing embryonic lethality in
Caenorhabditis elegans. Genetics 125: 35 1-369.
MAINS, P. E., K. J. KEMPHUES, S. A . SPRUNGER, 1. A. SULSTON and
W. B. WOOD, 1990 Mutations affecting the meiotic and mi-
totic divisions of the early Caenorhabditis eleguns embryo. Ge-
netics 126: 593-605.
MCINTOSH, J. R., 1991 Structural and mechanical control of mi-
totic progression. Cold Spring Harbor Symp. Quant. Biol. 56:
613-619.
MULLER, H. J.. 1932 Further studies on the nature and causes of
gene mutations. Proc. 6th Int. Congr. Genet. 1: 213-255.
MURRAY, A. W., 199 1 Coordinating cell cycle events. Cold Spring
Harbor Symp. Quant. Biol. 56: 399-408.
MURRAY, A . W., and M. W. KIRSCHNER, 1989 Dominoes and
clocks: the union of two views of the cell cycle. Science 246:
NIGON, V., P. GUERRIER and H. MONIN, 1960 L’Architecture
polaire d e I’oeuf et movements des constituants cellulaires au
cour des premikres &tapes du dPveloppement che7 quelque
nimatodes. Bull. Biol. Fr. Belg. 94: 132-20 1.
6 2 1-639.
210 T. R. Clandinin and P. E. Mains
PARK, E.-C., and H . R . HORVITZ, 1986 Mutations with dominant
effects on the behavior and morphology of the nematode
Caenorhabditis elegans. Genetics 113: 821-852.
ROGALSKI, T. M., and D. L. RIDDLE, 1988 A Caenorhabditis elegans R N A polymerase I1 gene, ama-Z IV, and nearby essential genes.
Genetics 118: 6 1-74.
SCHIMKE, R. T., A. L. KUNG, D. F. RUSH and S. W. SHERWOOD, 1991 Differences in mitotic control among mammalian cells.
Cold Spring Harbor Symp. Quant. Biol. 56: 417-425. STROME, S., and W. B. WOOD, 1983 Generation of asymmetry
and segregation of germ-line granules in early C. elegans em-
bryos. Cell 35: 15-25.
SULSTON, J. E., E. SCHIERENBERG, J. G. WHITE, and J. N. THOMSON, 1983 The embryonic cell lineage of the nematode Caenorhab- ditis elegans. Dev. Biol. 100: 64-1 19.
WEINERT, T. A , , and L. H. HARTWELL, 1988 The RAD9 gene
controls the cell cycle response to DNA damage in Saccharo-
myces cerevisiae. Science 241: 3 17-322.