Saccharomyces cerevisiae RAD52
Alleles Temperature-Sensitive for the
Repair of
DNA Double-Strand Breaks
Michael
D.
Kaytor
and Dennis
M.
LivingstonDepartment of Biochemistry, University of Minnesota, Minneapolis, Minnesota 55455 Manuscript received January 26, 1994
Accepted for publication April 10, 1994
ABSTRACT
We have screened for mutations of the Saccharomyces cereuisiae RAD52 gene which confer a temperature-sensitive (ts) phenotype with respect to either the repair of DNA lesions caused by methyl methanesulfonate (MMS) or the recombination of an intrachromosomal recombination reporter. We were readily able to isolate alleles ts for the repair of lesions caused by MMS but were unable to find alleles with a severe ts deficiency in intrachromosomal recombination. We extensively characterized four strains conferring ts growth on MMS agar. These strains also exhibit ts survival when exposed to y-radiation or when the HO endonuclease is constitutively expressed. Although none of the four alleles confers a severe ts defect in intrachromosomal recombination, two confer significant defects in tests of mitotic, inter- chromosomal recombination carried out in diploid strains. The mutant diploids sporulate, but the two
strains with defects in interchromosomal recombination have reduced spore viability. Meiotic recombi- nation is not depressed in the two diploids with reduced spore viability. Thus, in the two strains with reduced spore viability, defects in mitotic and meiotic recombination do not correlate. Sequence analysis revealed that in three of the four ts alleles the causative mutations are in the first one-third of the open reading frame while the fourth is in the Gterminal third.
T
HE Saccharomyces cerevisiae RAD52 gene plays a central role in the repair of double strand DNA breaks. Mutants are defective in the repair of DNA dam- age caused by agents that make double strand scissions such as X-rays (RESNICK 1969; GAME and MORTIMER 1974;STRIKE 1978) and the radiomimetic chemical methyl methanesulfonate (MMS) (PRAKASH and PRAKASH 1977; STRIKE 1978). In addition mutants are defective in mat- ing type switching, a process involving a double strand
break at MAT (MALONE and ESPOSITO 1980; WEIFFENBACH and HABER 1981; WHITE and HABER 1990). Mutants are
also defective in genetic recombination (STRIKE 1978; GAME et al. 1980; PRAKASH et al. 1980; HOEKSTRA et al.
1986), a process which can be initiated by double strand breakage (RESNICK 1976; SZOSTAK et al. 1983; KOLODKIN
et al. 1986; NICKOLOFF et al. 1989; MCGILL et al. 1993). Whether all RAD52-mediated recombination is initiated by double strand breaks and whether RAD52 plays the same role in double strand break repair as it does in recombination is not known.
We have begun to examine the latter issue by char- acterizing rad52 mutations which retain abilities miss- ing in null strains. For example, we have found that a mutant, rad52-327, bearing a nonsense mutation two- thirds into the open reading frame, retains the ability to undergo certain forms of mitotic recombination while faltering in its ability to repair MMSinduced lesions (BOUNDY-MILLS and LMNGSTON 1993). We noted the similarity between the truncation allele and the rad52-2 missense allele which had been characterized by MALONE
Genetics 137: 933-944 (August, 1994)
and colleagues (RESNICK et al. 1986; MALONE et al. 1988). Both alleles confer almost complete sensitivity to MMS while retaining significant abilities in carrying out in- tramolecular, plasmid recombination. The mutant alle- les differ in that the rad52-2 strain has a hyperrecom- binational phenotype for interchromosomal, mitotic recombination and fails to sporulate successfully, while the rad52-327 strain is depressed for interchro- mosomal, mitotic recombination but sporulates, al- beit with a reduced efficiency. The significant loss of repair abilities in these two mutants coupled with their facility in certain forms of recombination sug- gests that RAD52 plays different roles in DNA repair and in recombination.
To learn more about whether RAD52 plays the same role in both double strand break repair and in recom- bination, we have undertaken a screen for rad52 mu- tations conferring a temperature-sensitive
(ts)
pheno- type. We were interested in learning whether all mutations of this type would affect both repair and re- combination functions equally or whether some muta- tions would confer selective phenotypes with respect to repair and recombination. In this study we show that mutations which confer temperature-sensitive double strand break repair are not necessarily defective in car- rying out mitotic or meiotic recombination.MATERIALS AND METHODS
934 M. D. Kaytor and D. M. Livingston
TABLE 1 Strains
Strain Genotype Source or reference
SSL204 SSL204.4 SSL2 12 SSL2 12A SSL340 SSL341 SSL231 SSL30l SSL3OlA SSL350 SSL351 SSL352 SSL353 SSL354 SSL355 SSL356 RM 10-32D SSL370 SSL371 SSL372 SSL373 SSL374 SSL375 RM2826C SSL380 SSL38 1 SSL382 SSL383 SSL384 SSL385 SSL390
SSL391
SSL392
SSL393
SSL394
SSL395
SSL396
RAD52 MATa his?-A200 leu2 trpl ura3-52 ade2-101 RAD52 MATa his3-A200 leu2 trpl ura3-52 ade2-I01 rad52-AHS MAT@ his3-A200 leu2 trpl ura3-52 ade2-I01 rad52-AHS MATa his3d200 leu2 trpl ura3-52 ade2-I01 rad52-23A M A T a h i s 3 d 2 0 0 leu2 t r p l ura3-52 a d e 2 - I 0 1 rad52-76AB M A T a his3-AZ00 leu2 trpl ura3-52 ade2-102
RAD52 MATa his3-A200 leu2 trpl ura3-52 ade2-I01 can1::BYAIlZ rad52-AHS M A T a his?-AZOO leu2 trpl ura3-52 a d e 2 - I 0 1 c a n l : : B Y A I I 2 rad52-AHS MATa his3-AZ00 leu2 trpl ura3-52 ade2-101 can1::BYAIIZ rad52-2 MATa his3-A200 leu2 trpl ura3-52 ade2-101 canl::BYAI12 rad52-22 MATa his3-A200 leu2 trpl u r d - 5 2 ade2-I01 can1::BYAllZ r a d 5 2 - 2 3 M A T a h i d A 2 0 0 leu2 trpl ura3-52 ade2-I01 can1::BYAllZ rad52-252 MATa his3-A200 leu2 trpl ura3-52 ade2-101 can1::BYAIlZ rad52-76 M A T a h i s 3 d 2 0 0 leu2 trpl ura3-52 ade2-I01 can1::BYAlIZ rad52-76ABGH M A T a h i s 3 d 2 0 0 leu2 trpl ura3-52 a d e 2 - I 0 1 can1::BYAIIZ rad52-76CDEFGH M A T a his3-A200 leu2 trpl ura3-52 ade2-I01 can1::BYAlIZ
RAD52 MATa lys2-2 tyrl-2 his7-1 CANl ura3-l metl3-c cyhZr trp5c leulc ade6 ade2-I rau52-2 MATa lys2-2 lyrl-2 his7-l CANl ura3-l metl3-c ~ y h 2 ~ tq5c h l c ade6 &2-I
rd52::URA3 MATa lys2-2 lyrl-2 his7-1 CANl ura3-l mtlk qhZr tq5c le u l c ade6 ade2-1
rad52-23A MATa lys2-2 tyrl-2 his7-l CANl ura3-1 metl3-c cyh2' trp5c leulc ade6 ade2-1 rad52-76 MATa lys2-2 tyrl-2 his7-1 CANl ura3-1 metl3-c cyh2' trp5c leulc adeb adr2-1 rad5276AB MATa lys2-2 tyrl-2 his7-l CAN1 ura3-1 metl3-c qh2' bp5c leulc ade6 ade2-1
rad52-252 MATa lys2-2 tyrl-2 his7-l CANl ura3-l metl3-c cyh2' trp5c leulc adeb ade2-1 RAD52 MATa lys2-I canl' ura3-1 hisl ade5 CYHP trp5-2 leul-12 ade2-l
rad52-2 M A T a lys2-I canl' ura3-1 hisl ade5 CYH2 trp5-2 leul-12 ade2-I rad52::URA3 MATa lys2-I c a n l r ura3-1 hisl ade5 CYH2 trp5-2 leul-12 ade2-I rad52-23A MATa lys2-1 canl' ura3-l hisl ade5 CYH2 trp5-2 lrul-I2 ade2-1 rad52-76 M A T a lys2-I c a n l r ura3-1 hisl ade5 CYH2 trp5-2 leul-12 ade2-1 rad52-76AB MATa lys2-1 canl' ura3-l hisl ade5 CYWP trp5-2 leul-12 ade2-1 rad52-252 MATa lys2-I c a n l r ura3-1 his1 ade5 CYHP trp5-2 Eeul-I2 ade2-1
RAD52 MATa lys2-2 tyrl-2 his7-l CANl ura3-l t ade2-1 t metl3c cyhl trp5-c leul-c ade6 RAD52 MATa lys2-1 t t canl'ura3-1 hisl ade2-1 ade5 t CYH2 trp5-2 leul-12
+
rad52::URA3 MATa lys2-2 tyrl-2 his7-l CAN1 ura3-l i ade2-1 i metl3c c y h l trp5-c leul-c ade6 rad52::lJRA3 MATa lys2-1 i+
canl' ura3-1 hirl a d d - 1 ade5+
CYHP trp5-2 leul-12 i rad52-2 MATa lys2-2 tyrl-2 his7-l CAN1 ura3-1 i ade2-1 t metl33c c y h l trp5-c leul-c ade6 rad52-2 MATa lys2-1 i i canl'ura3-1 hisl ade2-l ade5+
CYHZ trp5-2 6u1-12 trad52-23A MATa lys2-2 tyrl-2 his7-1 CANl ura3-I i ade2-1
+
metl3c cyhltrpj-c leul-c adeh rad52-23A MATa lys2-1 t+
can]' ura3-l h i s l ade2-1 ade5 t CYH2 trp5-2 leul-12+
rad52-252 MATa lys2-2 tyrl-2 his7-l CAN1 ura3-l+
ade2-1 + m e t 1 3 c y h l trp5-c leul-c ade6 rad52-252 MATa lys2-1 i i canl' ura3-l hisl add-1 ade5 i CYHP lrp5-2 Eeul-12 irad52-76 MATa lys2-2 tyrl-2 his7-1 C A N l ura3-1 t ade2-1 t metl3c cyhl lrp5-r leul-c ade6 rad52-76 MATa lys2-1 i t canl' ura3-I hisl ade2-I ade5 t CYHP trp5-2 leul-12 i
rad52-76AB MATa lys2-2 tyrl-2 his7-1 CANl ura3-1 i ade2-1 t mvtl3c cyhl trp5-c leul-c ade6 radj2-76AB MATa lys2-1
+
+
canl' ura3-l hisl a d d - l ode5 i CYH2 lrp5-2 leul-12 i"
"
~-
~-
"
DORNFELD and LMNGSTON (1991) DORNFELD and LIVINGSTON (1991) DORNFELD and LMNGSTON (1991) DORNFELD and LMNGSTON (1991) This study
This study
This study
This study
This study
This study
This study
This study
This study
This study
This study
This study
ROBERT MALONE
This study This study This study
This study
This study
This study ROBERT MALONE This study
This study
This study
This study This study This study
RM10-32D X RM2626C
SSL371 x SSL381
SSL370 x SSL380
SSL372 x SSL382
SSL375 x SSL385
SSL373 x SSL383
SSL374 x SSL384
RM26-26C. SSL204 and SSL204A have been described previ- ously (DORNFELD and LMNCSTON 1991). The deletion- disruption allele rad52::URA? is identical to rad52-AHS
(DORNFELD and LMNGSTON 1991) except that URA? was used instead of LEU2. RM10-32D and RM2626C were kindly pro- vided by ROBERT MALONE. The E . coli strain JA300 was used for the propagation and purification of plasmids (CLARKE and CARBON 1980).
Cell growth: Cells were grown on either YPD, SD or po- tassium acetate agar (SHERMAN 1991). When required, plates contained 0.03% MMS, 60 mg/liter canavanine or 3.5 mg/ liter cycloheximide. When it was necessary for cells to be grown in liquid media they were grown in YPD to late log phase.
Polymerase chain reaction (PCR) mutagenesis: A cloned copy of the RAD52 gene was randomly mutagenized using a PCR scheme (LEUNG et al. 1989; RICE et al. 1992). Briefly, the PCR conditions were altered by adding 0.2 mM MnC1, and 2.4
mM MgC1, to a reaction mix that contains 40 PM dATP and 200 PM of the other deoxynucleotides. After mutagenesis the
BstEII-SphI fragment (nucleotides 1110-2466) containing 449 of the 504 amino acid open reading frame (nucleotides 968-2480) (SCHILD et al. 1983; ADZUMA et al. 1984) was cloned into a YCp50 (JOHNSTON and DAVIS 1984) vector that contained 1 kb of upstream and 0.5 kb of downstream RAD52 flanking sequences.
Intrachromosomal recombination reporter: To create a chromosomal reporter for recombination, the large EcoRI
fragment of recombination plasmid pBYAll2 containing his?
canl his3 pBR322 his3 canl FIGURE 1 .-Chromosomal recombination reporter. The re- combination reporter contains his3 heteroalleles created by insertion of restriction site linkers, represented by triangles (open, CZaI; closed, S a d ) , at two different sites within the
HIS3 coding region and at four other sites outside the coding region ( A H N and LMNGSTON 1986). The heteroalleles are sepa- rated by pBR322 plasmid sequence represented by the saw- tooth line. The reporter construct was integrated at the CANl locus. Restriction sites are shown which were used to confirm its integration into the yeast chromosome as well as to analyze recombination events.
at the two temperatures. After 2 days the number of His+ pa- pillae was recorded.
Construction of temperature-sensitive rad52 strains: Plas- mid DNA from yeast transformants which showed ts growth on
MMS agar was recovered, and the mutant rad52 copy was placed into an integrating vector (YIp5). The vector was cut within the RAD52 sequence, and the linear DNA was trans formed into wild-type strains. Appropriate mutant strains were recovered using the two step transplacement procedure (SCHERER and DAWS 1979). Southern analysis was used to con- firm that the mutant rad52 allele was intact and in a single copy
(TSAO et al. 1983).
MMS survivd. MMS survival was determined as described (PRAKASH and PRAKASH 1977). Strains were treated at 30" in a solution containing 0.5% MMS. At appropriate times samples were removed and washed. Viability was determined by plating dilutions onto warmed YPD plates and incubating them at either 25" or 33".
y-Ray survivak Cells were washed and suspended in water to a concentration of 1 X lo6 cells/ml. The cell suspensions were exposed to y-rays in an irradiator with a 57Cs source for times yielding doses of 15 or 30 krad. Cells were kept on ice before and after irradiation. Survival was determined by plat- ing dilutions onto warmed YPD plates and incubating at 25" and 33".
HO endonuclease expression: Cells were transformed with a plasmid containing HO under the control of the GAL1 pro- moter (HERSKOWITZ and JENSEN 1991). Cells grown in broth containing glucose were harvested, washed and plated on agar containing either glucose or galactose as the carbon source and incubated at 25" or 33".
Mitotic recombination: The rates of intrachromosomal re- combination and intragenic and intergenic chromosomal re- combination were calculated by the method of the median using five or more fluctuation cultures ( L u u and DELBRUCK 1943; LEA and COULSON 1949; A H N and LIVINGSTON 1986). The use of the CYH2 locus on chromosome VI1 to measure inter- genic recombination required correction of the recombina- tion frequencies for the contribution made by chromosome loss. Because the chromosome VI1 homologs we used were heterozygous for both ade5 and ade6 (strains RM10-32D and RM26-26C, Table 1) , we scored Cy,' cells which were white on agar plates with limiting adenine as events arising by chromo- some loss and those which were red as bona fide recombinants.
Sporulation and spore viability: Patches of diploids cells were grown on YPD plates at 30" for 2 days. The patches were then replica plated to 1% potassium acetate plates supple- mented with required amino acids and nucleic acid bases and incubated at either 25" or 33". After 5 days cells were analyzed under the microscope to determine the extent of sporulation.
Tetrads were dissected from diploid cells sporulated at each temperature. The dissected spores were allowed to germinate at the same temperature as that at which they had undergone sporulation.
Random spore analyses: Sporulated cells were removed from the potassium acetate plates and suspended in 5 ml water containing 100 units of Zymolyase and 10 pl pmercaptc- ethanol. This cell suspension was allowed to incubate in a shak- ing 30" bath overnight. The spores were separated as described (TRECO and WINSTON 1990). Spores were plated to omission plates containing 60 mg/liter canavanine or 3.5 mg/liter cycloheximide.
DNA sequencing: rad52 alleles were recovered from the chromosome by transformation with a RAD52 plasmid con- taining a gap between the BstEII and SphI sites (ORR-WEAVER and SZOSTAK 1983). Double-strand sequencing using RAD52
primers and Sequenase (U.S. Biochemical Corp.) was per- formed as described by the manufacturer.
Identification of causative mutations: Using restriction sites within the RAD52 gene, we combined portions of the mutant sequences with the wild-type sequence in order to make new alleles with one or a few mutations. These were most often expressed from the YCp50 derivative describe above. Some alleles were integrated into the chromosome by the two step procedure, and the resulting strains were checked by se- quencing to ensure the presence of the desired mutations.
RESULTS
Mutagenesis, screening and strain construction: To introduce mutations into RAD52, a cloned copy of the gene was used as a template in a mutagenic PCR regimen (LEUNG et al. 1989; RICE et al. 1992). From the PCR products a large portion of the open reading frame was removed and introduced into a plasmid containing RAD52 flanking sequences including its promoter. Using only the portion of the PCR products which con- tained the open reading frame ensured that the muta- tions would not be in the promoter and would be in regions capable of yielding a temperature-sensitive phe- notype. After introduction of the plasmids into a strain with a deletion-disruption of RAD52, the transformants were given two independent screens. To screen for MMS sensitivity, the transformants were patched onto three agar plates containing MMS and incubated at 18", 25" and 33". To screen for recombination proficiency, the strains carried a chromosomal recombination reporter consisting of his3 heteroalleles oriented as an inverted repeat disrupting the CANl locus (Figure 1). Recom- bination between the repeats can produce a wild-type
HIS3 copy conferring prototrophic growth ( A H N and LMNGSTON 1986; DORNFELD and LMNGSTON 1992). Con- sequently, the recombination screen consisted of a patch test for His' papillae carried out at 25" and 33" (see MATERIALS AND METHODS).
936 M. D. Kaytor and D. M. Livingston
-8
'
25" 33"
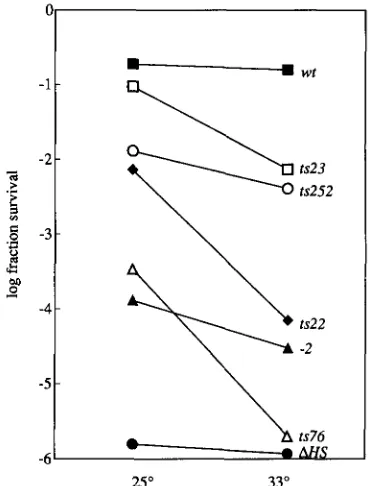
FIGURE 2.-Repair of MMS induced DNA lesions. Survival of haploid strains incubated in 0.5% MMS for 30 min was moni- tored at 25" and 33". A line connects the two points to illustrate the difference in survival. In general the values are the arith- metic mean of two or more determinations. The labels iden- tifylng the lines represent the RAD52 alleles present in the haploid strains. D, SSL204, RAD52; 0, SSL353, rad52-252;
+, SSL351, rad52-22; 0, SSL352, rad52-23; A, SSL354, rad52-
76; A, SSL350, rad52-2; 0 , SSL212, rad52-AHS.
MMS agar at any temperature and presented a dimin- ished level of recombination similar to that shown by the deletion strain. The remaining 15 isolates exhibited temperature-sensitive growth on MMS agar. Of these, 11 were heat-sensitive mutants, i . e . , they grew at 18" and 25" but not at 33", and 4 were cold-sensitive, i . e . , they grew at 33", weakly at 25" and not at 18". None of the temperature-sensitive isolates had an obvious tem- perature-dependent deficiency in recombination. Fur- thermore, none appeared to have a defect in recombi- nation as severe as that presented by the deletion strain. Next, four heat-sensitive alleles were selected for fur- ther examination, and their mutations were substituted for the chromosomal copy in a wild-type strain ( SCHERER and DAVIS 1979). Our ability to recover a ts strain for each of these four alleles demonstrates that our screen using plasmid-borne alleles did not exaggerate the ts phenotype. The four strains had no obvious growth de- fects at 25". As described below, we recovered the chro- mosomal alleles and sequenced them and their plasmid progenitors to insure that the same mutations were present in each.
rad52 ts alleles confer temperature-sensitive resis- tance to MMS: To evaluate the ability of the ts rad52
strains to repair MMS lesions, we incubated cells, which had been grown at 30°, in a phosphate buffer solution containing 0.5% MMS for 30 min, plated portions to two
0-
-1 -
-2 -
-3 -
-4 -
-5 -
-6-
25" 33"
FIGURE 3.-Repair of "irradiation induced DNA damage. Survival of haploid strains after exposure to 30 krad of -prays was monitored at 25" and 33". A line connects the two points
to illustrate the difference in survival. In general the values are the arithmetic mean of two or more determinations. The labels identifymg the lines represent the RAD52 alleles present in the haploid strains. D, SSL204, RAD52; 0, SSL353, rad52-252;
+
, SSL351, rad52-22; 0, SSL352, rad52-23; A, SSL354, rad52-76; A, SSL350, rad52-2; 0 , SSL212, r a d 5 2 d H S .
warmed agar dishes (lacking MMS) and incubated one at 25" and the other at 33". Figuie
2
shows the survival of the ts strains, as well as that of isogenic wild-type, deletion-disruption and rud52-2 strains. The wild type is resistant to MMS induced lesions retaining greater than 10% viability at both temperatures, while the deletion- disruption strain is very sensitive, retaining approxi- mately one in 1 Xlo6
viable cells at both temperatures. The slight resistance conferred by the rud52-2 allele at 25" is in accord with our previous observation (BOUNDY- MILIS and LMNGSTON 1993). At the permissive tempera- ture all the ts strains are slightly more sensitive than the wild type but exhibit four orders of magnitude greater survival than the deletion-disruption strain. The four ts strains all show a decrease in survival at 33". Although all the ts strains exhibit a drop in viability at the restrictive temperature, they differ with respect to the magnitude of the drop. Furthermore, even the tightest of the mu- tants, rud.52-76, is more resistant at 33" than the deletiondisruption strain. We note that from our ex- perience a strain, which exhibits a decrease in viability of three orders of magnitude after a 30 min incubation in 0.5% MMS, cannot form colonies on agar containing 0.03% MMS, while a strain, which is decreased by two orders of magnitude, can form colonies on MMS agar. rad52 ts strains are temperature-sensitive for therad52
25" 33"
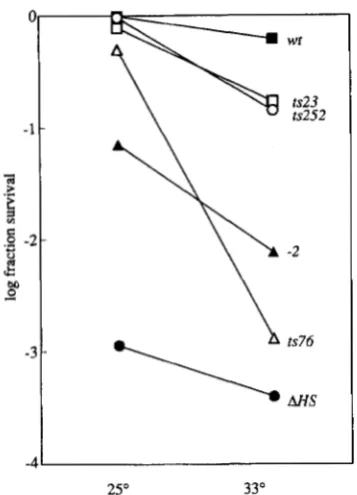
FIGURE 4.-Repair of HO endonucleolytic double-strand breaks. The fraction of cells, transformed with a plasmid bear- ing the H O gene under the control of the GALl promoter (HERSKOWITZ and JENSEN 1991), able to form colonies on agar containing galactose are plotted for the temperatures of 25" and 33". A line connects the two points to illustrate the dif- ference in survival. The values are the arithmetic mean of three determinations. The labels identifying the lines represent the RAD52 alleles present in the haploid strains. W, SSL204, RAD52; 0, SSL353, rad52-252; 0, SSL351, rad52-23
A,
SSL354, rad52-76; A, SSL350, rad52-2;
a,
SSL212, rad52- A HS.lyze the repair capabilities of these strains, we exposed them to yrays and monitored survival at both permissive and restrictive temperatures (Figure 3). All strains with the possible exception of the rad52-252 mutant are tem- perature sensitive. At the restrictive temperature three of the strains exhibit the same relative sensitivity to yirradiation as they do to MMS treatment with the
rad52- 76 mutant having the most profound defect. The exception is the rad52-22 mutant which is more sensi- tive to yray killing than the rad52-23 and rad52-252
mutants even though it was equally as sensitive to MMS killing. The rad52-2 strain, while sensitive to yirradia- tion at both temperatures, exhibits 1.5 orders of mag- nitude higher level of resistance than the deletion- disruption strain at 25". This correlates with its slight MMS resistance (Figure 2).
HO-induced breaks are repaired in a temperature- dependent manner: Next, we determined how well mutant strains were able to survive the continual action of the HO double strand endonuclease on MAT (STRATHERN et al. 1982). To measure this ability, we transformed three of the ts strains with a plasmid con- taining HO under the control of the GALl promoter (HERSKOWITZ andJENsEN 1991). Transformed cells were
TABLE 2
Mitotic intrachromosomal recombination rates
Rate
(x10"j evenu/cell/generation)
Strain RAD52 allele 33" 25"
SSL231 RAD52 23 (1.0) 51 (1.0) SSL3Ol rad52-AHS 0.66 (0.03) 1.8 (0.04) SSL350 rad52-2 21 (0.91) 26 (0.51) SSL351 rad52-22 9.4 (0.41) 8.6 (0.17) SSL352 rad52-23 8.7 (0.38) 10 (0.20) SSL354 rad52-76 4.9 (0.21) 8.8 (0.17) SSL353 rad52-252 1 1 (0.48) 35 (0.69)
These rates were derived by the method of the median (LEA and COWLSON 1949) using five or more cultures. Relative rates, the derived values divided by the wild-type values, are given in parentheses.
plated on agar containing either glucose or galactose as the carbon source and incubated at 25" or 33". The re- sults shown in Figure 4 reveal that at the permissive tem- perature all three strains, like the wild type, exhibit close to 100% viability. At the restrictive temperature they ex- hibit a range of sensitivity. The rad52-23 and rad52-252
strains are only slightly temperature sensitive. The
rad52-76 strain exhibits a 2.5 orders of magnitude dif- ference between the two temperatures, nearly wild type at 25" and nearly null at 33". Thus, at 33" the sensitivity of these three mutants to HO endonuclease is similar to their sensitivity to y-irradiation.
Intrachromosomal recombination: We next exam- ined the ability of the ts strains to carry out recombi- nation during mitotic growth. We first analyzed recom- bination of the intrachromosomal reporter (Figure 1) using the method of the median on five or more fluc- tuation cultures to measure the recombination rate (LUIUA and DELBRUCK 1943; LEA and COULSON 1949) (Table 2) (see Figure
7).
As
we had not previously char- acterized this reporter construct, we first tested the wild- type strain. At 25" its recombination rate is 100-fold lower than the rate at which these heteroalleles recom- bine when present on a plasmid (AHN and LMNGSTON1986). For unknown reasons the rate of recombination increases 2-fold at 33". The salient result is that the re- combination rate for this reporter is reduced 35- and 30-fold in the deletion-disruption strain relative to the wild type at the permissive and restrictive temperatures, respectively. This is similar to the decrease we observed using plasmids with his3 heteroallele pairs in the same isogenic pair of wild-type and null strains (DORNFELD and LMNCSTON 1992). Again, recombination in the deletion- disruption strain increases 2-fold between permissive and restrictive temperatures.
As
expected from the work of KLEIN (1988), molecular analyses of independentevents from both the wild-type and the deletion- disruption strains revealed that the majority occur by gene conversion (data not shown).
938 M. D. Kaytor and D. M. Livingston
TABLE 3
Mitotic recombination rates
Intragenic Intergenica
( ~ 1 0 " events/cell/generation) ( X 10"j events/cell/generation)
trp5-2 trp5-c -
Strain RAD52 alleles 25" 33"
SSL390 RAD52/RAD52 27 (1.0) 40 (1.0)
SSL391 rad52::URA3/rad52::URA3 1.2 (0.04) 1.1 (0.03) SSL394 rad52-252/rad52-252 148 (5.5) 17 (0.43) SSL395 rad52- 76/rad52- 76 34 (1.3) 9.5 (0.24) SSL396 rad52-76AB/rad52-76AB 14 (0.52) 38 (0.95)
leul-12 leu 1 -c 25" 33"
38 (1.0) 50 (1.0) 1.7 (0.04) 2.3 (0.05) 189 (5.0) 11 (0.22) 42 (1.1) 11 (0.22) 15 (0.39) 22 (0.44)
CYH2
cy h2
25" 33"
13 (1.0) 18 (1.0)
-
1.1 (0.08) 2.3 (0.13) 2.3 (0.18) 7.1 (0.39) 2.1 (0.16) 4.4 (0.24) 8.3 (0.64) 5.2 (0.29) These rates are derived by the method of the median (LEA and COWLSON 1949) on five or more fluctuation cultures. Relative rates, the derived
a These rates have been corrected for chromosome loss (see MATERIALS AND METHODS). Chromosome loss rates were comparable or lower than values divided by the wild-type values, are given in parentheses.
the recombination rates, and mutant loss rates did not surpass the wild-type value (except in the case of rad52-252 at 25").
temperature recombination rates of three of the strains (rad52-22, -23, and - 2 5 2 ) are reduced approximately %fold with respect to the wild type. The rad52- 76 strain has the most severe defect having a recombination rate 5-fold below the wild type (?fold above the null strain). At the restrictive temperature all the ts strains, except for the rad52-22 strain, show an increase compared to 25" in their rate of reporter recombination. This is true even for the rad52- 76 strain which has the most severe defect in the previous tests for repair of double strand breaks. Thus, even when normalized to the wild-type values, none of the strains, with the possible exception of rad52- 22, show a dramatic temperature-sensitive decrease in intrachromosomal recombination.
Mitotic interchromosomal recombination: To fur- ther explore the recombination phenotypes of the ts alleles both during mitotic and meiotic division, we made two additional sets of isogenic, haploid strains using two strains provided by ROBERT MALONE (Table 1). The diploids resulting from the mating of the two strains had markers suitable for measuring intergenic and in- tragenic recombination. In addition, they supported sporulation at 33".
Having diploid strains with multiple heterozygosities, we proceeded to measure both mitotic intragenic and intergenic recombination. Two heteroallele pairs, one at LEUl and the other at TRP5, were used to measure intragenic recombination, while the heterozygosity at CYH2 was used to measure intergenic recombination. Diploids homoallelic for the wild-type and deletion- disruption alleles were used as positive and negative con- trols, respectively. The results from analyses on two of the four ts mutations, rad52- 76 and rad52-252, given in Table 3 (see also Figure
7),
show that these two mutant diploids have defects in both intragenic and intergenic recombination. The rad52-76 diploid is partially ts for intragenic recombination. Its reduced rate of intragenic recombination at both LEUl and TRP5 is still well above the rate observed in the deletiondisruption diploid at33". In contrast, this mutant's rate of intergenic recom- bination is reduced at both temperatures to a value near that of the deletion-disruption diploid. The behavior of the rad52-252 homozygous diploid in tests of mitotic interchromosomal recombination departs from its be- havior in intrachromosomal recombination. This allele confers a hyperrecombinational phenotype at 25" and a partial defect at 33" making it appear temperature sen- sitive for intragenic recombination. These results con- trast with the intrachromosomal results which show neither a hyperrecombinational phenotype nor a tem- perature sensitive one. In contrast to its behavior in in- tragenic recombination, the rad52-252 mutant exhibits a severe deficiency in intergenic recombination at 25" and less of a deficiency at 33". Thus, both of these ts alleles give rise to defects in mitotic recombination between homologs, but their phenotypes with respect to intrachromosomal and interchromosomal events vary.
The ts strains complete sporulation at both tempera- tures:
As
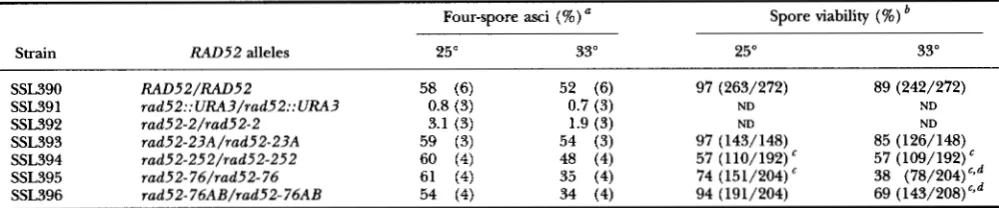
a final test, we examined whether the diploids could successfully complete sporulation. Null rad52 mutant diploids either do not make spores or make a low number of spores which prove inviable (GAME and MORTIMER 1974; STRIKE 1978; GAME et al. 1980; PRAKAsH et al. 1980; RESNICK et al. 1986).To measure sporulation proficiency, the diploids were patched onto WD plates, grown at 30°, replica-plated to potassium acetate plates and incubated at the permissive and restrictive temperatures. All of these homoallelic diploids sporulate at both 25" and 33" in contrast to the rad52-2 and rad52::URA3 diploids which produce very few asci at both temperatures (Table 4; see Figure 7). When spore viability was analyzed we found that the rad52-23A diploid yields viable spores with a proficiency comparable to that of the wild type at both temperatures
TABLE 4
Four-spore ascus formation and spore viability
Strain RAD52 alleles
SSL390 SSL391 SSL392 SSL393 SSL394 SSL395 SSL396
RAD52/RAD52
rad52::URA3/rad52::URA3 rad52-2/rad52-2
rad52-23A/rad52-23A rad52-252/rad52-252 rad52- 76/rad52- 76 rad52-76AB/rad52-76AB
Spore viability (%)
25" 33"
97 (263/272) 89 (242/272)
ND ND
ND ND
97 (143/148) 85 (126/148) 57 (110/192) 57 (109,492) 74 (151/204) 38 (78/204) ' j d 94 (191/204) 69 (143/208)c3d
a The number of independent sporulations is given in parentheses.
'This value is significantly different from the wild-type value at the corresponding temperature ( P < 0.01). The ratio of viable spores to total spores at each temperature is given in parentheses. ND, not determined.
The value at 33" is significantly different from the value at 25" ( P < 0.01).
TABLE 5
Meiotic recombination
Prototroph frequency ( X Exchange frequency'
trp5-2 leul-12
trp5-c leu 1 -c ADE5-ADE6' MET13-CYH2
-
Strain RAD52 alleles 25" 33" 25" 33" 25" 33" 25" 33"
SSL390 RAD52/RAD52 76 75 39 36 0.26 (0.24) 0.28 (0.28) 0.15 (0.18) 0.15 (0.08)
SSL393 rad52-23A/rad52-23A 71 55 35 32 0.25 (0.23) 0.26 (0.29) 0.17 (0.12) 0.16 (0.13) SSL394 rad52-252/rad52-252 49 47 31 24 0.26 (0.25) 0.28 (0.22) 0.16 (0.09) 0.13 (0.11) SSL395 rad52-76/rad52-76 76 51 44 23 0.23 (0.24) 0.27 (0.28) 0.17 (0.11) 0.16 (0.15) SSL396 rad52-76AB/rad52-76AB 56 53 35 23 0.27 (0.22) 0.26 (0.27) 0.16 (0.12) 0.16 (0.10)
Three independent diploids for each strain were sporulated and their prototroph frequencies were measured. Each value represents the geometric mean.
The values in parentheses are the frequencies determined from dissected spore colonies. The values represent the frequency of red spore colonies.
peratures ( P
<
0.01). The rad52-76 diploid also has a partial germination defect, but in this case there is a significant decrease in viability with temperature ( P<
0.01). Thus, both the rad52-252 and rad52-76 strains have partial germination defects, but only in the case of rad52-76 is the defect temperature sensitive.
Meiotic recombhation: Next, to ascertain the cause for the modest reduction in spore viability effected by the rad52-252 and rad52-76 mutations but not by the rad52-23 allele, we looked for a defect in meiotic re- combination. This was accomplished using random spore analyses to measure the formation of prototrophs at the two sets of heteroalleles within the diploids which we used to measure intragenic mitotic recombination. We also measured reciprocal exchanges in two ways. First, we determined the number of crossovers in the METl3-CYH2 interval on chromosome VII. Second, utilizing the ade5 and ade6 mutations in the strains, we measured exchanges which occurred between these dis- tantly linked markers along chromosome VII. The re- sults (Table 5; see Figure
7)
show that neither of the twots mutations, rad52-76 and rad52-252, yield drastic re- ductions in prototroph formation or reciprocal ex-
change at either 25" or 33". For instance, the frequency of crossovers between MET13 and CYH2 on chromo- some VIIis similar among spores from the wild type and the mutant diploids. To confirm this result and to ex- clude any problem associated with contaminating veg- etative cells and adherent spores, we scored dissected spores. Although there is greater scatter among the val- ues owing to the limited number of dissected spores hav- ing undergone exchange, recombination frequencies among the dissected spores from the mutant diploids neither depart drastically from the wild-type frequencies at either temperature, nor do they show any large change with temperature. These results indicate that the reduction in spore viability observed for rad52-76 and rad52-252 diploids cannot be ascribed to a reduction in meiotic recombination.
940
allele
raa52-23 raa52-23A
rad52-23BCDE
raa52-22 raa52-22B rad52-22AB
raa52-22BC
raa52-22A rads2-22C
raa52-76
rads2-76A raa52-76AB
rads2 - 76ABGH
raa52 -76B
M. D. Kaytor and D. M. Livingston
Chromosomal (C) Plasmid (P) or MMS encoded
P,C P,C K44aR
-
T *
-V128I W61G R362C E4893
-
-T T T
NWS V162A V322A
raa52-76CDEFGH
-
:=
raa52-252
-
D3hG raa52-I
AdoV
raa52-2
-
PA1 .
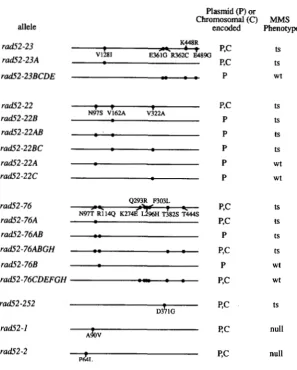
we were interested to know whether the mutations giv- ing rise to ts alleles might cluster within a portion of the open reading frame. rad52-252 has a single mutation at amino acid residue 371 (Figure
5).
We found that the other alleles have multiple mutations. rad52-22 has three mutations, rad52-23 has five mutations, and rad52-76 has eight mutations (Figure5).
The complex- ity of this result was easily reduced by constructing alleles with one or a few of the changes. We found that in each case we could isolate a single mutation which by itself gives rise to temperature-sensitive growth on MMS plates (rad52-23A, rad52-22B, rad52- 76A; Figure5).
These three mutations are located within the N-terminal one- third of the protein. Furthermore, we could show that the remaining mutations in rad52-22, rad52-23, and rad52-76, most of which are in the last two-thirds of the open reading frame, do not give rise to a defect in MMS repair. For example, in the case of rad52-23 the four most C-terminal mutations (rad52-23BCDE) together yield a wild-type phenotype (Figure 5). This is also true for the two other mutations in rad52-22 (rad52-22AC) as well as for the six most C-terminal mutations in rad52-76 (rad52-76CDEFGH) (Figure 5). These ex- periments were carried out using plasmid-borne alleles. We also wanted to know whether these silent muta-Phenotype
ts
t S
W t
tS
tS
tS
FIGURE 5.-Location of mutations. Chimeric al-
wt the location of the causative mutation(s). These
wt
alleles were expressed either from a plasmid in a null (rad5SPdHS) strain or from the chromo- some in an isogenic strain. Each horizontal line represents the RAD52 open reading frame from amino acid 1-504 (ADZUMA et al. 1984). The mu- allele of each group.
tS leles were constructed, and tested to determine
tS
tS tational changes are identified for the original
tS
tS
W t
wt
ts
null
null
tions alter the MMS repair capabilities conferred by the single causative mutation. We began by comparing the MMS susceptibility of the original rad52-23 strain car- rying all five changes with a strain carrying allele rad52- 23A, the allele having only the single change giving rise to the ts phenotype (Figure 6). The resistance of each at the permissive temperature and their sensitivities at the restrictive temperature are very similar. This result indicates that the MMS phenotype conferred by the single causative mutation is not attenuated by the four other mutations.
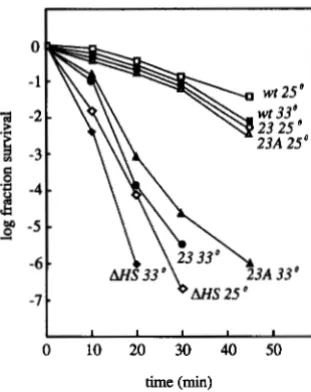
0 10 20 30 40 50
time (min)
FIGURE 6.Purvival of haploid strains in 0.5% MMS at 25" and 33". Strains were incubated in a MMS solution for the amount of time indicated. The MMS was inactivated and the cells were washed prior to plating as described in MATERIALS AND METHODS. The numbers identifymg the lines represent the RAD52 alleles present in the haploids. 0 (25"), W (33"),
A (33"), SSL340, rad52-23A; 0 (25"), (33"), SSL212, rad52-A HS.
SSL204, RAD52; 0 (25"), 0 (33"), SSL352, r ~ d 5 2 - 2 3 ; A (25"),
cantly different from rud52-76 spores at 33"
( P
<
0.01) (Table 4). The removal of the six Gterminal mutations also alters the mitotic intragenic and intergenic recombination phenotypes (Table 3).DISCUSSION
We have isolated ts rad52 alleles and characterized them in tests of DNA repair, mitotic recombination and meiotic capabilities. Our basic finding, summarized in Figure
7,
is that alleles conferring a temperature- sensitive ability to repair double strand DNA breaks do not necessarily confer similar levels of deficiency for mi- totic recombination, sporulation or meiotic recombina- tion. The phenotypes of these alleles suggest that dif- ferent functions require both quantitatively different amounts of action by the RAD52 gene product, Rad52p, and qualitatively different capabilities of that product.Although we screened independently for growth on MMS agar and for proficiency in intrachromosomal re- combination, we found alleles conferring ts growth on MMS agar without finding alleles which give rise to a ts deficiency in intrachromosomal recombination. One factor contributing to our inability to find alleles con- ferring a ts deficiency in recombination might be the difficulty in mutating RAD52 to produce such a phe- notype. Although a conditional recombination pheno- type may be difficult to achieve, severe deficits must not be because among the 41 independent isolates were 8 which had the null phenotype with respect to intra- chromosomal recombination. A potential problem
which might have limited the range of mutant pheno-
types which we recovered could have resulted from the mutagenesis procedure used. Although the number of mutational events per gene copy is greater than one, the majority of changes are A to G transitions as expected from the mutagenic regimen (LEUNG et al. 1989).
An-
other contributing factor is that by using only the BstEII-SphI fragment of the mutagenized gene we ex- cluded mutations in the first 49 residues of the open reading frame and the final 6 amino acids (SCHILD
et al. 1983; ADZUMA et al. 1984). We note that both the start of transcription and translation are not well de- fined (ADZUMA et al. 1984). Finally, in characterizing our alleles conferring ts growth on MMS agar we found that their phenotypes with respect to intra- chromosomal recombination did not always correlate with their phenotypes in other assays of mitotic and meiotic recombination. Thus, by using only intra- chromosomal recombination in our screen, we might have overlooked alleles conferring conditional profi- ciency in interchromosomal recombination. Indeed, the ts phenotype of the rud52-252 allele in intragenic recombi- nation (Table 3) is a case in point. We will now go on to explore why alleles giving rise to striking ts phenotypes in double strand DNA break repair do not necessarily give rise to conditional phenotypes in mitotic and meiotic recombination.
One issue which must be addressed in understanding the capabilities of the mutants is that of retained capacity or leakiness. None of the four alleles scrutinized confers perfect conditional growth in being completelywild type at 25" and completely null at 33". The mutants are able to survive exposure to MMS and expression of the HO endonuclease at 33" better than the null strain, and all but the rad52-76 strain are able to survive exposure to y-rays better than the null strain at 33". These results indicate that the gene products of these mutant copies are not completely and irrevocably denatured at the re- strictive temperature, or else the mutant strains would behave as the deletiondisruption strain at the restrictive temperature. That the ts mutants are not completely inactive at the restrictive temperature is not surprising considering our demand of wild-type like function at a temperature 8" cooler. Indeed, to some extent our abil- ity to recognize the leakiness results from the five to six orders of magnitude window of killing measurable in assays of y-irradiation and MMS exposure, respectively. If our only assessment had been conditional growth on MMS agar, none of the mutants would be considered leaky. Nevertheless, the retention of function at 33" raises questions as to whether different assays for repair and recombination require different quantities of the gene product.
942 M. D. Kavtor and D. M. Livingston
- 1
"t
25'' 33" 25" . . 7 7 '
rad52-252 rad52-76
we note that the amount of MMS exposure we used pro- duces approximately 85 double strand DNA breaks per cell (CHI.ERO"I<;% andJACHW.IC7.W 1979), the amount of y-irradiation produces approximately 20 double strand breaks per cell (RESNICK and MARTIN 1976), expression of the HO endonuclease should make a single double strand break per cell (STR~TI-IERN ct nl. 1982), and the frequency of mitotic intrachromosomal recombination is no more than a few events per 1 X 10' cells. Thus, given the leakiness of the mutants, it is not surprising to find that the most infrequent event, spontaneous intra- chromosomal mitotic recombination, is the one which does not exhibit a ts phenotype.
We point out a number of incongruities with the explanation that quantitative differences alone can ex- plain the various phenotypes. First, although none of the ts mutants has a null phenotype for intrachromosomal recombination at either temperature, most have a par- tial defect, even at 25" (Table 2). We would expect the mutants to be capable of a recombination rate compa- rable to wild-type cells, at least at 25", because the amount of Rad52p function should be more than suf- ficient to meet the infrequent occurrence of such events. Second, the capability of strains to recover from
yirradiation (Figure 3) does not always correlate with the number of double strand DNA breaks they experi- ence. For example, the m d 5 2 - 7 6 mutant is more resis- tant to both MMS exposure and HO endonuclease ex- pression at 33" than the deletion-disruption strain but is equally as sensitive to yirradiation as the null strain. A third anomaly is that there are varied defects among tests of mitotic intrachromosomal, intergenic and intragenic recombination. Although the occurrence of each at any one locus is rarer than one event per 10,000 cells per generation, the mutants display differences in their abilities to contend with each form of recombination. Most noticeable is the deficiency of the rad52-76 allele in the test of intergenic recombination at both tempera- tures (Table 3). The m d 5 2 - 2 5 2 allele also confers a marked deficiency in this test. A fourth inconsistency in the correlation between the amount of leaky Rad52p and the number of events requiring Rad52p action is the
1
phenotypes among FIGLIRE 7.-Summary mutants. The bar comparison of graphs represent five different pheno- types (MMS survival, intrachromosomal recombination, mitotic intergenic recom- bination, spore viability and meiotic crossing over) for three of the ts mutants and the deletion-disnlption strain( m d 5 2 - A H S ) at 2.5" and 33". The appro- priate strains may be found in the legend
to Figure 2 and Tables 2-5. Note that the MMS survival is represented on an abso- lute logarithmic scale, whereas the other phenotypes are plotted as values relative
to the wild-type values on a linear scale. nd, not determined
lack of a defect in meiotic recombination in mutant d i p loids. The frequency of meiotic recombination events in sporulating cells is comparable to the number of DNA lesions introduced in our mitotically dividing cells by MMS. We presume that even though the amount of Rad52p should increase in meiosis (COLE et al. 1989), the demand of meiotic recombination on Rad52p would be too great for a leaky protein product, especially at 33".
These anomalies suggest that quantity is not the only factor and that the capability of each mutant protein to repair DNA double strand breaks and to participate in mitotic and meiotic recombination events is different. The behavior of the complex md52-76 allele in mi- totic recombination also is suggestive that Rad52p could play different roles in the repair of double strand DNA breaks and in mitotic recombination. Noteworthy is the recombination deficiency of the md52-76 mutant at 25" in the assay for intrachromosomal recombination
(Table 2). This is the only strain we found in our screen which is able to grow on MMS agar yet exhibits a decided defect in intrachromosomal recombination. Indeed, this allele was chosen for study because it exhibited a noticeable recombination defect in the His' papillae screen. O u r analysis of the multiple mutations in this allele revealed that the recombination defect at 25" could be restored by removing four of the internal mu- tations to create md52-76ABGH (Figure 5, data not shown). This subset of mutations does not alleviate the recombination defect at 33" which means that different mutations within the m d 5 2 - 7 6 allele are responsible for its recombination defects at the two temperatures. Al- though the complexity of this allele with eight mutations makes definitive conclusions difficult to make, the cre- ation of a mutant strain which handles repair of MMS lesions better than recombination of an intrachromo- soma1 reporter suggests that Rad52p's roles are not nec- essarily the same during the two processes.
The behavior of the mutant diploids in sporulation is not in keeping with their other deficiencies (Figure
7).
the Gterminal third of the open reading frame, either do not sporulate (rad52-2) (STRIKE 1978; GAME et al. 1980; RESNICK et al. 1986) or sporulate poorly (rad52- 3 2 7 ) (BOUNDY-MILLS and LMNGSTON 1993). We noted that the sporulation capacity of mutants correlated with their sensitivity to MMS exposure (BOUNDY-MILLS and LMNGSTON 1993). The sporulation proficiencies of the ts alleles do not follow this correlation.
Some of the mutants do have modest defects in spore germination (Table 4). The rad52-252 diploid has ap- proximately half the spore viability of the wild type at both temperatures, while the rad52-76 diploid exhibits a mild ts defect in spore survival. In the case of the rad52- 252 allele the defect in spore viability must be conferred by its single mutational change. In the case of the rad52-76 allele much of the viability can be restored by removal of six mutations (Table 4, rad52-76AB). A pru- dent, but untested, hypothesis is that null strains fail to sporulate due to an inability to recombine chromo- somes during meiosis, a process in which double strand breakage has been implicated (SUN et al. 1989;
CAo
et al.1990; PADMORE et al. 1991; SUN et al. 1991). For both the rad52-252 and rad52-76 diploids, measurements of meiotic recombination (Table 5) did not reveal defi- ciencies which might have explained the problems in spore viability. (A caveat is that the inviable spores died because they underwent fewer exchange events than did the viable spores.) The two mutant strains exhibited de- fects in intragenic and intergenic mitotic chromosomal recombination rather than any measurable defect in meiotic recombination. The absence of a correlation be- tween the mitotic and meiotic recombination pheno- types of the ts mutants is another argument in favor of the hypothesis that these two forms of recombination are different (ESPOSITO and WAGSTAFF 1981; ROEDER and STEWART 1988). The contrast of the spore inviability, al- beit subtle, without a noticeable reduction in meiotic recombination implicates Rad52p in the completion of meiotic events other than recombination as suggested by MALONE and ESPOSITO (1981).
Our overall conclusion about the behavior of the mu- tants is that in putting Rad52p through many tests we are asking the protein to carry out qualitatively different ac- tions. For example, in repairing double strand breaks the protein might encounter different overhangs, i e . ,
5’-,
3’- and flush. Possibly, the DNA substrates it encoun-ters during spontaneous acts of recombination are not double strand breaks but DNA lesions which require similar enzymatic alteration. Another possibility is that Rad52p interacts with different sets of proteins in car- rying out different tasks (SHINOHARA et al. 1992). What- ever these differences, they are reflected in the behavior of mutants which perform some assays better than they do others. Although we cannot conclude that Rad52p has domains, we can be certain that some of its residues are not always involved in the same way in each assay.
Our analysis of the mutational sites once again shows the importance of the N-terminal portion of the gene product in carrying out double strand DNA break repair (BOUNDY-MILLS and LMNGSTON 1993). Three of the four causative mutations are within the first third of the open reading frame; one, rad52-252, is approximately two- thirds the way in. Furthermore, because of the heavy mutagenesis we could show that many mutations appear- ing in the Gterminal third do not confer a defect in MMS damage repair. The Gterminal third of Rad52p is a very basic region which may be important for inter- action with DNA or with other proteins (BOUNDY-MILLS and LMNGSTON 1993; MILNE and WEAVER 1993). A com- parison of the causative mutations with an alignment of the RAD52 homologs from Saccharomyces cerevisiae, Kleuveromyces lactis, Schirosaccharomyces pornbe, and chicken reveals that the rad52-23A mutation changes an amino acid residue invariant among the four while the other three causative mutations change a residue conserved among three of the four species (MILNE and WEAVER 1993; OSTERMANN et al. 1993; BEZZUBOVA et al. 1993). Furthermore, of the 13 ancillary mutations found in our four alleles, none is in invariant residues, three are in residues shared among three of the four species and the remaining nine change residues which are less conserved or absent in some species.
The ts alleles which we have procured will be useful in future studies aimed at understanding whether other rad52 phenotypes result from a singular activity. The ts alleles will also be useful for selecting suppressors which either bypass or interact with the gene product of RAD52.
We thank ROBERT MALONE for providing strains and ROBERT ROON and K m BOUNDY-MIU for reviewing the manuscript. This work was supported by National Science Foundation grant MCB-9304937.
LITERATURE CITED
ADzum, K., T. OCAWA and H. OGAWA, 1984 Primary structure of
the RAD52 gene in Saccharomyces cerevisiae. Mol. Cell. Biol. 4
A H N , B.-Y, and D. M. LMNGSTON, 1986 Mitotic gene conversion
lengths, coconversion patterns, and the incidence of reciprocal recombination in a Saccharomyces cerevisiae plasmid system. Mol. Cell. Biol. 6: 3685-3693.
BEZZUBOVA, 0. Y., H . SCHMIDT, K OSTEWN, W.-D. HEYER and J.-M. BUERSTEDDE, 1993 Identification of a chicken RAD52 h o m e
logue suggests conservation of the RAD52 recombination path-
way throughout the evolution of higher eukaryotes. Nucleic Acids Res. 21: 5945-5949.
BOUNDY-MILIS, K. L., and D. M. LMNCSTON, 1993 A Saccharomyces cerevisiae RAD52 allele expressing a Gterminal truncation
protein: activities and intragenic complementation of missense mutations. Genetics 133: 39-49.
CAO, L., E. ALAN1 and N. KLECKNER, 1990 A pathway for generation and processing of double-strand breaks during meiotic recom- bination in S . cerevisiae. Cell 61: 1089-1101.
CHLEBOWCZ, E., and W. J . J A C H Y M C Z ~ , 1979 Repair of MMSinduced DNA double-strand breaks in haploid cells of Saccharomyces cer- evisiae, which requires the presence of a duplicate genome. Mol. Gen. Genet. 167: 279-286.
CLARKE, L., and J. CARBON, 1980 Isolation of the centromere-linked