_____________________________________________________________________________________________________ *Corresponding author: E-mail: sojihunter@yahoo.com;
www.sciencedomain.org
Serum Levels of Interleukin-6 (IL-6) Cytokine in
Patients with Classic Fever of Unknown Origin, in
Maiduguri, Nigeria
B. S. Oderinde
1,2*, O. O. Agbede
3, S. S. Baba
4, D. N. Bukbuk
3,5, M. M. Baba
1,2,
S. K. Amoka
1, B. B. Ajayi
6, J. S. Dawurung
2, B. A. Denue
7and N. Bukar
81
Department of Medical Laboratory Science, College of Medical Sciences, University of Maiduguri, Nigeria.
2
WHO National Polio / ITD Laboratory, University of Maiduguri Teaching Hospital, Maiduguri, Nigeria.
3
Department of Medical Microbiology and Parasitology, College of Health Sciences, University of Ilorin, Nigeria.
4
Department of Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Maiduguri, Nigeria.
5
Department of Microbiology, Faculty of Science, University of Maiduguri, Nigeria.
6
Department of Immunology, UMTH, Maiduguri, Nigeria.
7
Department of Medicine, College of Medical Sciences, University of Maiduguri, Nigeria.
8
Biotechnology Center, University of Maiduguri, Nigeria.
Authors’ contributions
This work was a collaborative effort. Authors BSO, OOA and SSB designed the study. Authors MMB, SKA, BBA, JSD and NB facilitated the sample collection and data with the laboratory testing with author BAD the clinician. Author DNB carried out the statistical analysis of the data. Authors BSO and OOA wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Article Information
DOI: 10.9734/BJMMR/2016/25116 Editor(s): (1) C. Cameron Yin, Department of Hematopathology, UT MD Anderson Cancer Center, Houston, TX, USA. (2)Philippe E. Spiess, Department of Genitourinary Oncology, Moffitt Cancer Center, USA and Department of Urology and Department of Oncologic Sciences (Joint Appointment), College of Medicine, University of South Florida, Tampa, FL, USA. Reviewers: (1) Anonymous, University of Rwanda, Rwanda. (2)P. Schoenhagen, Cleveland Clinic, USA. (3)Anonymous, University of Sao Paulo Medical School, Brazil. (4)Anonymous, Ain Shams University, Cairo, Egypt. (5)Chia-Li Yu, National Taiwan University Hospital, Taipei, Taiwan. (6)Kamal Shemisa, University of Texas Southwestern Medical Center, Dallas TX, USA. Complete Peer review History:http://sciencedomain.org/review-history/14694
ABSTRACT
Aim: To assess whether a significant correlation exists with serum Interleukin-6 cytokine levels in
patients with Classic Fever of Unknown Origin (FUO) compared with healthy controls. The levels of IL-6 cytokine were tested as a proinflammatory cytokine which may belong to microorganisms that cause disease.
Study Design: This is a case control, single center study.
Place and Duration of Study: The study was conducted in the University of Maiduguri Teaching
Hospital, Maiduguri, Borno State, Nigeria in patients with classic FUO who were treated between 2009 and 2012 were included in this study.
Methodology: A total of 75 patients with classic FUO within the age range of 15 – 65 years were
tested for IL-6 cytokine in comparison with 75 healthy controls. The sample size was calculated using Fisher’s formula.
Results: In patients with FUO, 39% of patients demonstrated elevated levels of IL-6 while 20% of
healthy controls demonstrated elevated levels (>200 pg/ml) of serum IL-6, while 12% and 15% respectively were in standard range of 0 - 6.4 pg/ml which is considered low. Gender differences were noted and mean values of IL-6 were higher in males (FUO mean±SD 136.7±161.8; Healthy controls mean±SD 132.3±183.5) than in females (FUO mean±141.6±133.9; Healthy controls mean±SD 97.8±134.2). Healthy male control had a value of 520.5 pg/ml with little difference to the male patients with FUO (575 pg/ml), p=0.91) while IL-6 concentrations in the female group who had FUO (407.5 pg/ml vs 367.5 pg/ml, p=0.16) showed greater difference in value in comparison to the healthy which trended towards statistical significance. We found that most patients (39%) with FUO have higher values of ≥ 200 pg/ml than the healthy controls.
Conclusions: Serum IL-6 cytokine level is generally higher in males than in females and most
patients (39%) with FUO have a higher value of ≥ 200 pg/ml than the healthy controls. IL-6 levels trended towards statistical significance and may help to discriminate females with FUO as compared their healthy controls.
Keywords: Interleukin-6 (IL-6); cytokines; FUO; blood donors.
1. INTRODUCTION
Several undefined clinical syndromes are increasingly being observed in health care institutions that care for Fever of Unknown Origin (FUO). FUO is a condition defined in adult patient as the presence of a temperature higher than 38.3°C, lasting more than three weeks with an undetermined source despite appropriate investigations [1]. The four categories of FUO are classic, nosocomial, immune deficiency and human immunodeficiency virus. While the four subgroups of the differential diagnosis are infectious, malignancies, autoimmune conditions, and numerous rheumatic diseases (e.g., systemic lupus erythematosus [SLE], juvenile rheumatoid arthritis [JRA], rheumatoid arthritis [RA]) [2]. The spectrum of diseases found in several studies examining FUO shows some variation, but overall, infections continue to be the most important cause of FUO accounting for about 20-40% [1,3-8].
Circulating cytokines which are considered the endogenous mediators of fever in response to administration of a exogenous pyrogen. This concept about the pathogenesis of fever [9] is
mainly based on three experiments; Lipopolysaccharide (LPS) trigger the release of a number of cytokines in the bloodstream which produce the fever response. Tumor necrosis factor alpha (TNF-α) is the first cytokine which appears in the circulation [9,10], followed by traces of interleukin- 1β (1L-1β) [10], and high amounts of IL-6 [11,12], IL-8 [13] as well as other cytokines such as, e.g. macrophage inflammatory protien-1 (MIP-1) [14].
Among all cytokines which are measurable in blood plasma during LPS-induced fever, circulating levels of IL-6 show the best correlation with the febrile changes of the body temperature [11,12]. The measurement of IL-6 in the clinical setting of FUO as a marker for ongoing infection is therefore an attractive area of study. It is our aim to assess whether the level of IL-6 cytokine release into the blood in the course of viral infections correlates well in FUO.
2. MATERIALS AND METHODS
2.1 Study Area
with classic FUO and 75 apparently healthy blood donors who visited University of Maiduguri Teaching Hospital (UMTH), a tertiary health institution which serves as a referral centre for six states in northeastern Nigeria and neighbouring African countries (Chad to the northeast, Niger to the North and Cameroon to the East).
2.2 Study Population
The Ethical Committee of University of Maiduguri Teaching Hospital, Maiduguri gave approval to the study protocol. Only patients with classic FUO and apparently healthy blood donors with no known immune disorder or who had been treated with immune-suppressive drugs or radiotherapy in the previous twelve months or had received systemic corticoids in the last three months or had received immunoglobulin or blood transfusion in the last three months were excluded from the study. After obtaining informed consent from those subjects, willing participants were enrolled consecutively in the study. Demographic data including age and sex of the patients; the date of onset of symptoms were obtained through interview at the time of sample collection.
2.3 Sample Size and Collection
A total number of 75 patients with classic fever of unknown origin consulted between 2009 and 2012 were recruited for this study and their blood collected with 75 apparently healthy blood donors as matching controls in General out-Patient Department (GOPD) University of Maiduguri Teaching Hospital (UMTH), Nigeria. This sample size was estimated using the Fisher’s formula.
Criteria for selecting Patients with FUO are age > 15 years; Fever for at least 3 weeks but no more than 12 weeks; Fever > 38.3°C at least twice with failure reaching diagnosis in 1 week of inpatient investigation. Exclusion Criteria for Patients with FUO are: Patients with Tuberculosis; Intra abdominal abscesses; Known infection with HIV; Patients on Immunosuppressive treatment within 2 months or granulocyte count < 109/L for any reason; Cancer; Nosocomial fevers; Patients with known history of heart disease.
2.3.1 Blood sample
Five mililiters of blood was obtained by venipuncture with plain container, transported to the laboratory and allowed to clot and later
centrifuged at 1500 rpm for 10 minutes. The sera were aspirated after centrifugation and stored in nunc tubes at -70°C until tested. However, some of the patients samples were collected in EDTA bottle of which plasma was aspirated into nunc tubes and stored until tested.
2.4 Laboratory Testing
2.4.1 Testing for interleukin-6 cytokine
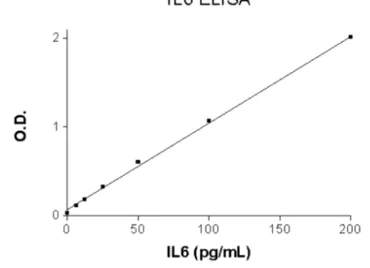
Sample analysis was carried out by Sandwich ELISA method, using commercially produced enzyme-linked immunosorbent assay (ELISA) 6.25-200 pg/ml from ELI-Pair (Diaclone Besancon Cedex, France). All samples and standards were run in duplicates and the average value considered after reading at optical density (OD) of 450 nm.The recommended IL-6 standard range was 6.25-200 pg/ml. Using standard wells values, each standard OD (Y axis) versus the corresponding standard concetration (x-axis). A standard curve was drawn on linear graph paper manually to obtain the best linear/linear curve to give the most accurate results. Concentrated samples (i.e.>200 pg/ml) are diluted with standard diluent buffer and the OD result obtained was multiplied by the correponding dilution factor.
2.5 Refrence Curve for Human IL-6,
Optical Density (OD) against
Concetration (pg/ml)
Tests were based on the test standard and dwarfed standard curve to calculate the IL-6 value in specimens.
Fig. 1. IL-6 Standard Curve ranging from 6.25 to 200 pg/ml
2.6 Statistical Analyses
expressed as mean ± SD or numbers and percentages. Chi squared test or Fisher's exact test were used to compare categorical variables. Student's t-test was used to compare continuous variables between the two variable or groups. A
P-value < 0.05 was considered to be statistically
significant.
3. RESULTS
Table 1: Shows the IL-6 serum levels mean standard deviation of patients with FUO and healthy controls. Statistically significant differences were not observed in the serum level
of IL-6 cytokine in patients with FUO and the healthy control.
Table 2.Shows the IL-6 range in patients with FUO in comparison with healthy controls in relation to sex. The male healthy control has a value of 520.5 pg/ml which compared to male patients with FUO (575 pg/ml), p=0.91 was not statistically significant. There was a greater difference in the female group where the FUO patients has a value of 407.5 pg/ml in comparison to the healthy with 367.5 pg/ml, p=0.16 which may suggest greater discrimination in females than male patients who have FUO.
Table 1. Interleukin-6 levels mean standard deviation in serum samples of patients with FUO and healthy control
IL- 6 Level FUO patients Healthy control F statistics P-value
Number Mean ± SD Number Mean ± SD
IL -6≥ 200 pg/ml (Elevated)
29 (39) 15 (20)
IL-6(0-6.4 pg/ml) (Low)
9 (12) 11 (15)
IL-6 (6.5-199.5) (Normal)
37 (49) 49 (65)
Total 75 139.1±147.3 75 115±160.90 0.91 0.34
Percentage values in parentheses
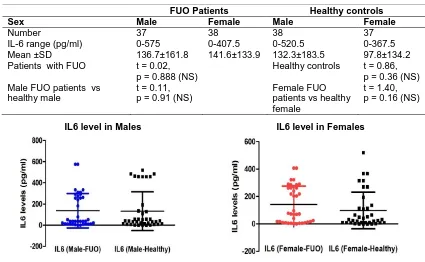
Table 2. Interleukin-6 cytokine maximum levels in FUO patients and healthy controls according to sex
FUO Patients Healthy controls
Sex Male Female Male Female
Number 37 38 38 37
IL-6 range (pg/ml) 0-575 0-407.5 0-520.5 0-367.5 Mean ±SD 136.7±161.8 141.6±133.9 132.3±183.5 97.8±134.2 Patients with FUO t = 0.02,
p = 0.888 (NS)
Healthy controls t = 0.86, p = 0.36 (NS) Male FUO patients vs
healthy male
t = 0.11, p = 0.91 (NS)
Female FUO patients vs healthy female
t = 1.40, p = 0.16 (NS)
IL6 level in Males IL6 level in Females
Fig. 2. Scatter plot of IL6 level (Mean with SD) by sex
4. DISCUSSION
This study demonstrates the utility of IL-6 cytokine in the evaluation of Classic Fever of Unknown Origin (FUO) as compared with apparently healthy controls. IL-6 cytokine in this study indicated that its level cannot be used solely as a marker in diagnosis of classical FUO as only 39% of the patients show IL-6 serum level above standard (>200 pg/ml) and 20% of the healthy blood donors as well. However, it is expected that the serum IL-6 will be higher above standard in the majority of the FUO cases due the body temperature and fever exhibited as symptoms as it also plays a vital role in the production of acute response proteins (ARP) and fever response [15]. In conjecture, the stage or time after infection is a very critical factor in the Serum IL-6 level evaluation.
Table 1 show that there is no statistical significance in the IL-6 in patients with FUO in comparison to the healthy control with P-value of 0.34. In relation to sex IL-6 Table 2 reflects that male healthy control has a high value of 520.5 pg/ml with little difference to the male patients with FUO (575 pg/ml), p=0.91 but there is higher difference in IL-6 Level in the female group where the FUO patients has a value of 407.5 pg/ml in comparison to the healthy with 367.5 pg/ml, p=0.16 which trended towards statistical significance and a discriminator in females with FUO in comparison to their healthy controls. Regarding the higher proinflammation showing in males, studies have shown that sexes differ in the intensity, prevalence, and pathogenesis of infections caused by viruses, bacteria, parasites, and fungi. Males and females of species ranging from humans to horses and rodents differ in their responses to and the outcome of diverse pathogenic infections [16,17]. For each of these infectious diseases, there are numerous and diverse ways in which sex and gender can impact differential susceptibility between males and females. For example, human studies reveal that women have over 40% less human immunodeficiency virus (HIV) RNA in circulation than men inferring that female can be less susceptible to inflammation which is evident in the IL-6 values obtained in both sex where females are lower. Sex differences in the pathogenesis of infectious diseases may reflect differences in the immune responses during infection. Males and females differ in their innate immune responses, suggesting that some sex differences are germ line-encoded. Innate detection of nucleic acids by pattern recognition
receptors (PRRs) differs between the sexes [18]. Females tend to generate stronger innate and adaptive immune response compared with males [19,20], which may raise the discriminatory between patients with FUO and healthy controls where that of the female is extremely higher in comparison to male.
Fig. 2 Shows the Scatter plot of IL-6 level (Mean with SD) by sex, in males the mean virtually at same level while it is higher in Female FUO than their healthy counterpart as reflected in the values. This reveal that most healthy controls have higher values of IL-6 than in FUO patients that are more in number in this group than the healthy controls which might be as a result of other diseases or factors not considered in this study.
5. CONCLUSION
In conclusion, this study has provided evidence of elevated serum IL-6 cytokine in more patients with FUO than healthy blood donors, Further studies are required to determine the role of IL-6 in cardiovascular infarction, and other related diseases that might cause elevated IL-6 in healthy humans, and. However, IL-6 cannot be used as sole diagnostic marker for FUO but high throughput metagenomic technologies can be applied in unique diagnostics to detect microorganisms responsible for inflammation and further correlation with IL-6 levels.
ACKNOWLEDGEMENT
We acknowledge the World Health Organization, WHO Technical Support.
COMPETING INTERESTS
Authors have declared that no competing interests exist.
REFRENCES
1. Peterdorf RG, Beeson PB. Fever of unexplained origin: Report on 100 cases. Medicine (Baltimore).1961;40:1-40. 2. Allan R, Roth DO, Gina M, Bassello DO.
Approach to the adult patient with fever of unknown origin. Am. Fam. Physician. 2003;68:2223-2228.
4. Larson EB, Featherstone HJ, Petersdorf RG. Fever of undetermined origin: Diagnosis and follow up of 105 cases, 1970-1980. Medicine. 1982;61:269-292. 5. Knockaert DC, Vanneste LJ, Vanneste SB,
Bobbaers HJ. Fever of unknown origin in 1980’s. An update of the diagnostic spectrum. Arch Intern Med.1992;152:51-55.
6. Sharma BK, Kumari S, Varma SC, Sagar S, Singh S. Prolonged undiagnosed fever in North India. Trop Geogr Med. 1992;44: 32-36.
7. De Kleijn EM, Vanderbroucke JP, Vander Meer JM. Fever of unknown origin (FUO): A prospective multicenter study of 167 patients with FUO, using fixed epidemiology entry criteria. Medicine. 1997;76:392-400.
8. Handa R, Singh S, Singh N, Wali JP. Fever of unknown origin: A prospective study. Trop Doct. 1996;26:168-170. 9. Kluger MJ. Fever: Role of pyrogens and
cryogens. Physiological Reviews.1991;71: 93-127.
10. Jansky L, Vybiral S, Pospisilowa D, Roth J, Dornand J, Zeisberger E, Kaminkowa J. Production of systemic and hypothalamic cytokines during the early phase of the endotoxin fever. Neuroendocrinology. 1995;62:55-61.
11. LeMay LG, Vander AJ, Kluger MJ. Role of interleukin-6 in fever in the rat. American Journal of Physiology. 1990;258:R798-R803.
12. Roth J, Conn CA, Kluger MJ, Zeisberger E. Kinetics of systemic and intrahypothalamic IL-6 and tumor necrosis
factor during endotoxin fever in the guinea pig, American Journal of Physiology. 1993;258: R653-658.
13. Van Zee KJ, De Forge LE, Fisher E, Marano MA, Kenney JS, Remick DG, Lowry SF, Moldawer LL. IL-8 in septic shock, endotoxema, and after IL-1 administration. Journal of Immunology. 1991;146:3478-3482.
14. Ziegler SF, Tough TW, Franklin TL, Armitage RJ, Alderson M. Induction of macrophage inflammatory protein-1 gene expression in human monocytes by lipopolysaccharide and IL-7. Journal of Immunology. 1991;147:2234-2239.
15. Banks WA, Kastin AJ, Guitierrez EG. Penetration of interleukin 6 across the murine blood-brain barrier. Neurosci Lett. 1994;179:53-56.
16. Klein SL, Roberts CW, editors. Sex and gender differences in infection and treatments for infectious diseases Switzerland: Springer International Publishing; 2015.
17. Klein SL, Roberts CW, editors. Sex hormones and immunity to infection. Berlin: Springer-Verlag; 2010.
18. Berghofer B. Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006;177(4):2088-96.
19. Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases.J. Autoimmun. 2007;28(1):1-6.
20. Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. Genetic and hormonal factors in female-biased autoimmunuty. J. Autoimmun Rev. 2010;9(7):494-498. _________________________________________________________________________________ © 2016 Oderinde et al.; This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Peer-review history: