1071-412X/96/$04.0010
Copyrightq1996, American Society for Microbiology
Novel Enzyme-Linked Immunosorbent Assays for the Detection
of Anti-Fc Gamma Receptor Autoantibodies
ARMELLE LAMOUR,
1ROZENN LE CORRE,
1CHRISTOPHE JAMIN,
1CLAUDE SOUBRANE,
2DAVID KHAYAT,
2AND
PIERRE YOUINOU
1*
Laboratory of Immunology, Brest University Medical School Hospital, Brest,
1and Department of Oncology,
La Pitie´-Salpe´trie`re Hospital, Paris,
2France
Received 8 November 1995/Returned for modification 2 January 1996/Accepted 9 February 1996
There is a substantial interest in the role of antineutrophil antibodies since Fc gamma receptors (Fc
g
Rs)
have been identified as the target for the majority of such autoantibodies. Antineutrophil antibodies have long
been detected by an indirect immunofluorescence technique. Following optimization of the flow cytometric
method of detection, we developed three enzyme-linked immunosorbent assays (ELISAs), each specific for
autoantibodies against one of the three classes of human Fc
g
R. Fc
g
RI and Fc
g
RII were purified from cultured
cells, and Fc
g
RIII was produced as a recombinant molecule. These were then used as capture agents in the
respective ELISAs. When applied in parallel to a sizeable group of patients with primary Sjo
¨gren’s syndrome,
both methods established that anti-Fc
g
R autoantibodies were heterogeneous. This finding indicates that
different populations of partly cross-reactive antibodies are detectable by these two methods.
Neutrophil-reactive autoantibodies have long been
de-scribed in patients with a variety of disease states with immune
neutropenia. In those early studies, the detection of
autoanti-bodies was achieved by an indirect immunofluorescence (IIF)
technique (17) with control neutrophils as the substrate (19).
Subsequently, the receptors for the immunoglobulin G (IgG)
Fc domain (Fc gamma receptors [Fc
g
Rs]), and occasionally
their allotypic determinants, were shown to belong to the
tigenic targets of these antibodies (4, 16). More recently,
an-tibodies against different classes of Fc
g
R, expressed or not on
resting neutrophils, were found in sera from patients with
autoimmune diseases, including primary Sjo
¨gren’s syndrome
(pSS) and rheumatoid arthritis, irrespective of neutropenia.
These anti-Fc
g
R autoantibodies appear to be a rather
heter-ogeneous population reactive with one or several classes of
Fc
g
Rs. We have developed direct methods for the detection of
such autoantibodies by enzyme-linked immunosorbent assays
(ELISAs) that use each type of Fc
g
R separately as the
sub-strate. Given the description of sera positive by the Fc
g
RIII
ELISA and negative by IIF, these assays might complement
very usefully the IIF screening test with neutrophils and
iden-tify all types of anti-Fc
g
R antibodies so that distinct
autoanti-bodies would be characterized.
MATERIALS AND METHODS
Sera.Sera were collected from 66 patients with pSS, with all patients fulfilling the European preliminary criteria for the disease (20). Normal controls consisted of 34 sex- and age-matched healthy subjects. Three patients with idiopathic neutropenia were also tested and served as positive controls in the IIF screening test.
Immune complex assessment.The ELISA used to measure immune com-plexes (ICs) has been described previously (1). Briefly, ICs were allowed to bind to C1q-coated plates, and IgG-containing ICs were detected by incubating the wells with horseradish peroxidase (HRP)-conjugated F(ab9)2anti-human IgG
(Dakopatts, Copenhagen, Denmark).
Screening by flow cytometry.Sera were screened for neutrophil-reactive au-toantibody by the IIF technique. Normal neutrophils were isolated from blood samples drawn into EDTA-containing tubes prepared in the laboratory. Cell suspensions containing more than 90% neutrophils were obtained by dextran
T500 sedimentation (Pharmacia, Uppsala, Sweden); this was followed by Ficoll-Hypaque (Eurobio, Paris, France) density gradient centrifugation and hypotonic lysis of residual erythrocytes. The cell viability exceeded 98%, as determined by staining with ethidium bromide (Sigma Chemical Co., St. Louis, Mo.).
Since some antineutrophil antibodies have been shown to recognize allospeci-ficities on FcgRIIIb, neutrophil donors had previously been phenotyped for the NA1/NA2 and NB11/NB12systems (5). This preliminary phenotyping was performed by using specific polyclonal antisera of the First International Gran-ulocyte Serology Workshop to identify NA11/NA22/NB11, NA12/NA21/ NB11, and NA11/NA21/NB12individuals (15). We thus identified heterozy-gous NA11NA21NB11or NA11NA21NB12and homozygous NA11NA11
NB11or NA21NA21NB11individuals (J. Cartron, Kremlin-Biceˆtre Hospital, Paris, France) for the ensuing IIF assays.
Two procedures were used to obviate irrelevant binding of circulating IgG to the cells. Firstly, circulating ICs were removed from the sera by precipitation with 2% polyethylene glycol (PEG; Merck, Darmstadt, Germany). PEG was dissolved in a buffer containing 0.1 M boric acid, 0.025 M disodium tetraborate, and 0.075 M NaCl (pH 8). The tubes were agitated, left on ice for 1 h, and centrifuged. In addition, the neutrophils used in the IIF assay were fixed to prevent binding of IgG-containing ICs or IgG aggregates to FcgR via their Fc domains. The fixation was carried out by a 5-min incubation at room temperature (RT) with 1% paraformaldehyde (PFA) in cold phosphate-buffered saline (PBS). In order to evaluate the effect of PFA treatment, fixed and unfixed neutrophils were incu-bated with heat-aggregated IgG for 1 h at 378C. After three washes in PBS supplemented with 2% bovine serum albumin (BSA) (PBS-BSA), bound IgG was revealed as described for the autoantibodies in the IIF assay (see below). Aggregated IgG was prepared by heating Cohn fraction II (Sigma) at a concen-tration of 20 mg/ml in PBS for 1 h at 638C.
Furthermore, all serum samples were extensively absorbed with a pellet of established human endothelial cells expressing major histocompatibility complex class I and class II antigens but devoid of FcgR, as described in every detail elsewhere (13).
Finally, the IIF assay was performed with suspensions of neutrophils adjusted to 53106/ml in PBS-BSA. One hundred microliters of cell suspension was
incubated with 50ml of serum diluted 1/10 in PBS. After 30 min at 378C, the cells were washed three times and were incubated with a previously absorbed fluo-rescein isothiocyanate (FITC)-conjugated F(ab9)2anti-human immunoglobulin
(Tago, Burlingame, Calif.) for 30 min at RT. This reagent had been incubated with a pellet of neutrophils, cleared by centrifugation, and tested by direct immunofluorescence to ensure that it did not bind to neutrophils. The cells were then washed another three times and were immediately analyzed by flow cytom-etry. An Elite flow cytometer (Coultronics, Hialeah, Fla.) equipped with a 488-nm argon laser was used to analyze 10,000 neutrophils per test. Positive cells were determined by defining a threshold with reference to an irrelevant anti-CD3 FITC-conjugated monoclonal antibody (MAb) and to the fluorescent second layer reagent applied without test serum (see Fig. 1A1 and A2). Control sera were used to set the normal threshold (see below).
Preparation of pure FcgRs.FcgRI and FcgRII were isolated from the mono-cytic cell line U937 (14) as described by Kulczycki (7) and Suzuki (18) with minor modification. The U937 cell line was purchased from the American Type Culture Collection (Rockville, Md.) and was maintained in culture in RPMI 1640
(Eu-* Corresponding author. Mailing address: Laboratory of Immunol-ogy, Brest University Medical School Hospital, BP 824, F 29 609 Brest Cedex, France. Phone: (33) 98-22-33-84. Fax: (33) 98-80-10-76.
315
on August 17, 2020 by guest
http://cvi.asm.org/
robio) supplemented with 10% heat-inactivated fetal calf serum (LabSystem, Sergy-Pontoise, France), 100 U of penicillin per ml, 100mg of streptomycin per ml, and 200 mmol of glutamine per liter (Biomerieux, Marcy l’Etoile, France) at 378C with 5% CO2. To increase the density of FcgRI on their membranes, U937
cells were treated with 250 IU of gamma interferon (Genzyme, Cambridge, Mass.) per ml for 18 h.
To extract the FcgR, U937 cells were solubilized. A pellet of 109cells was
washed three times in cold PBS. The resulting cell suspension was chilled in an ice-water bath and lysed with 0.5% Nonidet P-40 (Sigma) in the presence of protease inhibitors (10 mM iodoacetamide [Merck-Clevenot, Nogent-Sur-Marne, France] and 0.3 IU of aprotinin per ml, 1mM peptstatin, and 2 mM phenylmethylsulfonyl fluoride [the last three compounds were from Sigma]). After 1 h, the lysates were clarified by centrifugation at 30,0003g for 40 min at
48C. The U937 lysates were then sequentially applied to a Sepharose 4B column (Sigma) and a Sepharose-BSA column to remove nonspecific debris. The flowthrough was collected, and FcgRI and FcgRII were affinity purified on an IgG-Sepharose column. FcgRI was then separated from FcgRII by using an anti-FcgRII column (anti-CD32 MAb, clone 2E1, kindly donated by Michel Hirn, Immunotech, Marseille, France).
The F(ab9)2anti-CD32 fragments were recovered following a 2-h pepsin
di-gestion by passing the fraction over a protein G column and then a G-100 column (Pharmacia). The purities of the F(ab9)2fragments were checked by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and their bind-ing abilities were ascertained by testbind-ing the reagent with neutrophils in the flow cytometer with FITC-conjugated F(ab9)2anti-mouse IgG (Tago) as a second
layer. The anti-CD32 column was made by standard procedures, and the mixture of FcgRI and FcgRII was run through the column three times. FcgRI was
collected in the effluent, and FcgRII eluted with 0.5 N CH3COOH and 0.05%
Nonidet P-40 in Tris (pH 8.6). Both the FcgRI and the FcgRII preparations were homogeneous, as judged by SDS-PAGE, in which proteins were silver stained and confirmed by Western blotting (immunoblotting) with anti-CD32 (Immuno-tech) and anti-CD64 (Serotec, Oxford, United Kingdom) MAbs to develop the blots. Purified FcgRI and FcgRII were quantitated by the Micro-BCA protein assay (Pierce, Rockford, Ill.).
The production of recombinant human FcgRIIIb has been fully described elsewhere (4a). Briefly, a CD16-encoding full-length complementary DNA-containing plasmid was used as the template, and two oligonucleotides (Xo 123, 59-GAATTCGGATCCTTAGTACCAGGTGGAGAGAATGATGA-39, and Xo124, 59-AAGCTTGAATTCATATGCGGACTGAAGATCTCCCAAAGG-39) were prepared to amplify the segment corresponding to the extracellular part of the molecule. A BamHI fragment was inserted into the vector M13 mp18 at the
EcoRI site. One of the recombinant clones that expressed large amounts of
recombinant FcgRIIIb as inclusion bodies was selected to prepare a 608-bp fragment, and the fragment was inserted into the vector pET3a at the NdeI and
BamHI sites and transfected into Escherichia coli C600. Following growth in
culture, bacterial cells were digested with lysozyme and DNA was lysed with 50 U of benzonase per ml. The homogenate was centrifuged so that the pellet represented CD16-enriched inclusion bodies. These were dissolved, and the solubilized material was filtered through an 8-mm-pore-size Millipore membrane and a 0.2-mm-pore-size filter. SDS-PAGE showed a silver-stained major band with a molecular mass of approximately 27 kDa. Automated NH2-terminal
se-quencing established the presence of the nine expected amino acid residues.
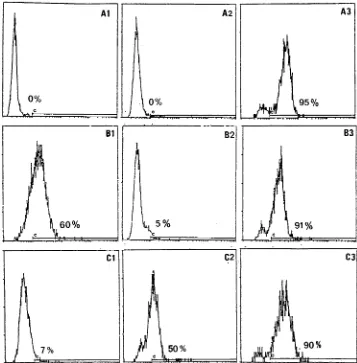
Anti-FcgR autoantibody analysis by ELISAs.Three ELISAs were developed by using purified FcgRI, purified FcgRII, and recombinant FcgRIIIb, respec-FIG. 1. Profiles of control and test sera in the IIF assay with normal neutrophils as the substrate. Fluorescence was analyzed on a logarithmic scale. (A) As negative controls, cells were directly incubated with an FITC-conjugated nonspecific MAb (A1) or an FITC-conjugated anti-human Ig (A2); the serum from a patient with idiopathic neutropenia was used as a positive control (A3). (B) Binding of heat-aggregated IgG on unfixed cells (B1) was prevented by paraformaldehyde treatment of the cells (B2), but the control described above for panel A3 remained positive with fixed cells (B3). (C) Representative profiles of a normal serum sample (C1), a weakly positive serum sample from a patient with pSS (C2), and a strongly positive serum sample from a patient with pSS (C3). Sera were tested on fixed cells, and the percentage of stained cells was calculated according to the threshold defined for panel A1.
on August 17, 2020 by guest
http://cvi.asm.org/
tively, as capture agents. To prevent binding of IgG through its Fc portion in the IgG autoantibody test, FcgRs were denatured by heating them at 958C for 5 min and treating them withb-mercaptoethanol, which was subsequently removed by dialysis. Successful denaturation was evaluated by incubating serial dilutions of monomeric or aggregated human IgG instead of sera in the three ELISAs.
Appropriate conditions for coating the FcgRs were defined in preliminary experiments in which increasing amounts of each FcgR were diluted in either citrate or carbonate-bicarbonate buffer. In the subsequent anti-FcgRIII autoan-tibody test, microtiter plates (Dynatech, Marne-la-Coquette, France) were coated with 100ml of FcgRIII diluted to 1mg/ml in citrate buffer (pH 3.5) for an overnight incubation at 48C. In the anti-FcgRI and anti-FcgRII autoantibody tests, microtiter plates (Nunc, Roskilde, Denmark) were coated with 100ml of either antigen diluted to 5mg/ml in carbonate-bicarbonate buffer (pH 9.6) for 2 h at 378C and overnight at 48C. The results were obtained by subtracting the background optical density (OD) from that of the test serum.
Rheumatoid factor (RF) was depleted from all test sera by using a column of IgG Sepharose 4B. Bound RF was eluted from the column with acetate, and the flowthrough was again passed over the column.
The next steps were conducted in the three ELISAs. Once coated, the plates were washed four times with PBS containing 0.05% Tween (PBS-T) and the free sites were blocked with PBS–3% BSA for 1 h at 378C. Following another four washes, 100ml of serum samples diluted 1/100 in PBS-T was dispensed into the wells, and the sera were left in the wells for 90 min at RT. After washing, bound antibodies were detected by flooding the wells with HRP-conjugated F(ab9)2
anti-human total immunoglobulin or anti-human IgG or IgM (Dakopatts). After a 30-min incubation at RT and development, the A492was read on a Titertek
Multiskan microplate reader (Flow Laboratories, McLean, Va.). MAbs IgM directed toward FcgRIII (Becton Dickinson, Mountain View, Calif.), IgG di-rected toward FcgRIII (3G8; Immunotech), and IgG directed toward FcgRI (Serotec) or FcgRII (Immunotech) were used as positive controls in the corre-sponding assays. They were revealed by HRP-conjugated goat anti-mouse IgM or IgG antibodies (Jackson, West Grove, Pa.). Each assay was performed in tripli-cate, and the results were averaged.
RESULTS
Optimization of the IIF screening test.
The IIF screening
validation process began with the definition of positive sera.
The negative and positive controls were analyzed by using
heterozygous NA1
1
NA2
1
NB1
1
unfixed neutrophils as the
substrate, to define the cutoff proportion of positive cells on
the logarithmic fluorescence scale. According to this threshold,
no stained cells were detected by the FITC-conjugated
anti-CD3 MAb (Fig. 1A1) or the reagent in the second layer (Fig.
1A2), while up to 90% stained cells were obtained with the
three positive controls (Fig. 1A3). Whereas nonspecific
stain-ing of unfixed neutrophils by aggregated IgG was efficiently
prevented by the PFA treatment (Fig. 1B1 and B2), these
positive controls showed comparable profiles when PFA-fixed
neutrophils were used (Fig. 1B3).
Following the definition of these conditions, 34 serum
sam-ples from healthy subjects were tested as described earlier. The
mean proportion of positive cells was 5.5%
6
6.5%. This led us
to set the normal threshold at 20% stained cells, i.e., the mean
plus two standard deviations. Test sera, diluted 1/10, were thus
considered positive when they stained at least 20% of the cells.
Positive sera were then serially diluted, and the autoantibody
titer was defined as the highest dilution giving at least this
percentage of stained cells. Representative profiles of sera
from a healthy control (Fig. 1C1) and two patients (Fig. 2C2
and C3) are also shown.
When comparing the results of the IIF screening before and
after the removal of circulating ICs by PEG precipitation of 66
serum samples from pSS patients, 13 serum samples were
positive before, and 10 of these remained positive thereafter
(Table 1). Three serum samples appeared to be positive in the
IIF test, and indeed, they displayed high levels of
IgG-contain-ing ICs, as established by the specific ELISA (ODs
5
0.710,
0.632, and 0.438, respectively). However, another three
IgG-IC-containing serum samples were negative in the original IIF
assay (ODs
5
0.602, 0.376, and 0.311, respectively).
To address the question of whether the NA1/NA2 and
NB1
1
/NB1
2
phenotype of the neutrophil donor may
influ-ence the assay, 10 serum samples from pSS patients (found to
be positive with heterozygous NA1
1
NA2
1
NB1
1
and
follow-ing PEG precipitation) were tested again with heterozygous
NA1
1
NA2
1
NB1
2
and homozygous NA1
1
NA1
1
and NA2
1
NA2
1
neutrophils. As shown in Tables 1 and 2, the results of
the IIF assay remained unchanged with NA2
2
or NB1
2
cells,
whereas five serum samples did not react with NA1
2
cells. Our
conclusion is that it is unnecessary to consider the NB1
1
/
NB1
2
phenotype of the neutrophil donor, while the use of NA
homozygous neutrophils as the substrate in the IIF screening
would miss some autoantibody-positive serum samples.
Fi-nally, 10 of 66 serum samples from pSS patients and no serum
samples from healthy subjects were considered to be positive.
Consequently, the IIF screening for neutrophil-reactive
anti-body had to be performed by using fixed cells from NA
het-erozygous donors with sera from which ICs had been removed.
Validation of the anti-Fc
g
R ELISAs.
The conditions of the
three ELISAs were optimized by testing three concentrations
of the coated antigen in carbonate-bicarbonate and citrate
buffers. Standard curves were drawn with increasing amounts
of MAb anti-Fc
g
RI, anti-Fc
g
RII, and anti-Fc
g
RIII to define
the optimum conditions. Figure 2 shows the curves obtained in
the anti-Fc
g
RIII assay.
It was essential to avoid binding of circulating IgG to the
coated Fc
g
R via the Fc portion. A treatment that combined
heating and
b
-mercaptoethanol incubation was therefore
ap-plied to the Fc
g
Rs. Serial dilutions of human IgG prepared
from Cohn fraction II were tested for their ability to recognize
treated and untreated Fc
g
Rs. Human IgG had previously been
heat aggregated in the Fc
g
RII- and Fc
g
RIII-specific assays.
We observed that this pretreatment of the Fc
g
Rs efficiently
TABLE 1. Remaining percentages of stained cells following the precipitation of ICs from the serum of 13 patients with pSS
Serum sample no.
% stained cells in the IIF assay
Interpretation Before IC
precipitation
After IC precipitation
1 92.9 92.3 1
2 49.7 30.4 1
3 44.6 32.5 1
4 92.6 77.0 1
5 47.5 55.7 1
6 63.0 31.0 1
7 43.7 38.3 1
8 88.0 76.5 1
9 60.0 55.3 1
10 49.0 47.2 1
11 32.5 6.8 2
12 29.0 1.3 2
13 41.2 1.9 2
TABLE 2. Allospecificities recognized by 10 antineutrophil-reactive serum samples defined by the IIF test
Target neutrophil
Result for the following serum sample no.a:
1 2 3 4 5 6 7 8 9 10
NA11NA21NB11 1 1 1 1 1 1 1 1 1 1
NA11NA21NB12 1 1 1 1 1 1 1 1 1 1
NA11NA11NB11 1 1 1 1 1 1 1 1 1 1
NA21NA21NB11 2 1 2 2 2 1 2 1 1 1
a
Five serum samples did not react with NA1-negative cells (serum samples 1, 3 to 5, and 7).
on August 17, 2020 by guest
http://cvi.asm.org/
prevented the nonspecific binding of IgG (Fig. 3) but did not
affect the reactivity of the MAb specific for each Fc
g
R except
for the IgG anti-Fc
g
RIII MAb (3G8), which showed a
dramat-ically decreased OD when it was tested with denaturated
Fc
g
RIII (data not shown). This prompted us to select the IgM
anti-Fc
g
RIII as a positive control in the corresponding ELISA.
The assays were then applied to 20 serum samples from
healthy subjects. They were tested at a previously determined
optimal dilution of 1/100. Their mean anti-Fc
g
RI and
anti-Fc
g
RII activities were 0.050
6
0.030 (mean
6
1 standard
deviation) and 0.092
6
0.047, respectively. Their IgM and IgG
anti-Fc
g
RIII activities were 0.096
6
0.008 and 0.088
6
0.008,
respectively. Test sera were considered positive when they gave
an OD higher than the mean for sera from healthy subjects
plus 2 standard deviations. As defined, the anti-Fc
g
RI and
anti-Fc
g
RII cutoff values were 0.110 and 0.186, respectively.
For the anti-Fc
g
RIII assay, the IgM and IgG cutoff values were
0.190 and 0.174, respectively. The reproducibility of the ELISA
was established by calculating the interassay coefficients of
variation, which were 7 and 6% for the IgM and IgG
anti-Fc
g
RIII tests, respectively, and 4 and 7% for the anti-Fc
g
RI
and the anti-Fc
g
RII antibody assays, respectively.
Application to the patients’ sera.
Sixty-six serum samples
from pSS patients were screened for neutrophil-reactive
anti-body by the IIF technique and for anti-Fc
g
RIII antibody in the
ELISA. Ten serum samples were positive by the IIF technique,
as shown above (with titers ranging from 1/80 to 1/1,280); 30
serum samples were found to contain at least one isotype of
anti-Fc
g
RIII antibody in the ELISA (24 IgM and 17 IgG). Five
of the 10 IIF assay-positive serum samples were thus positive in
the anti-Fc
g
IIIb ELISA. Interestingly, IgM and IgG
autoanti-bodies were detected in four and three of these serum samples,
respectively. Three distinct profiles of autoantibody-containing
sera were observed: those positive in both assays and those
positive in only one of them. Twenty serum samples were
positive in the anti-Fc
g
RIII-specific ELISA and negative in the
IIF assay. Ten of these 20 serum samples were further tested in
the Fc
g
RI- and Fc
g
RII-specific ELISAs. Autoantibodies from
one serum sample recognized the three receptors (serum
sam-ple 1 in Table 3), those from six serum samsam-ples reacted with
Fc
g
RII in addition to Fc
g
RIII (serum samples 2 through 7 in
Table 3), and those from the remaining three serum samples
(serum samples 8 through 10 in Table 3) proved to be specific
for Fc
g
RIII.
DISCUSSION
Our observations suggest that the ‘‘gold standard’’ IIF
method for the detection of total anti-Fc
g
R antibody activity
must be associated with ELISAs with Fc
g
Rs as soluble
anti-gens. Indeed, we found that anti-Fc
g
R autoantibodies could be
identified with a given form of Fc
g
R but not with other forms.
The variety of epitopes targeted by anti-Fc
g
R autoantibodies is
likely to account for this apparent discrepancy between the IIF
assay and the ELISA. One may assume that those patients
testing negative by the former assay but positive by the latter
one have autoantibodies to the Fc
g
RIIIb intracellular domain.
Alternatively, changes to the receptor may occur after
shed-ding upon activation in patients with connective tissue diseases,
such as pSS (11) or rheumatoid arthritis (12). Prior to testing,
all of the serum samples were extensively absorbed with human
endothelial cells from clones established in our laboratory (13)
and were shown to express major histocompatibility complex
class I and class II antigens but were shown to be devoid of
Fc
g
R (as determined by using CD16, CD32, and
anti-CD64 MAbs). Given that platelets and B lymphocytes express
Fc
g
RIIa and Fc
g
RIIb, respectively (6), these cells would
de-plete sera of anti-Fc
g
RIIIb antibodies cross-reactive with
Fc
g
RII, as established in pilot experiments.
FIG. 2. Standard curves obtained with an FcgRIII-specific IgM MAb in microtiter plates coated with 1mg (squares), 2mg (circles), or 5mg (triangles) of recombinant FcgRIIIb per ml. Antigen was diluted in citrate (pH 3.5) (closed symbols) or carbonate-bicarbonate (pH 9.6) (open symbols). The MAb was revealed by an HRP-conjugated anti-mouse IgM diluted 1/2,500.
FIG. 3. Standard curves obtained with serial dilutions of heat-aggregated IgG incubated in microtiter plates coated with FcgRI (circles), FcgRII (trian-gles), or FcgRIII (squares). FcgR had been denatured (open symbols) or not denatured (closed symbols) prior to coating.
on August 17, 2020 by guest
http://cvi.asm.org/
The distinction of IgG bound to Fc
g
R via the Fc fragment
from autoantibodies attached to the cells through their Fab
regions is a prerequisite for the detection of
neutrophil-reac-tive autoantibodies. To inhibit binding of IgG to the cells, PFA
treatment of neutrophils has already been used by several
investigators, leading to divergent results (15, 19). As an
addi-tional precaution, ICs were therefore precipitated with PEG,
which reduced the number of IIF assay-positive serum samples
from 13 to 10. Three serum samples became negative.
How-ever, the IC ELISA data for these three serum samples
indi-cated that the amount of aggregated IgG was far less than the
amount of aggregated IgG which was used to test the
neutro-phil PFA fixation. It would thus have been interesting to know
whether the ICs of these three patients had anti-Fc
g
R
reactiv-ities. Assuming that Fc
g
Rs might be incorporated into the
target of antineutrophil autoantibodies, it was necessary to
validate this method in order to ensure whether it inhibits Fc
binding of IgG without affecting the specific recognition of
Fc
g
Rs by the corresponding autoantibodies. We confirmed
this point in the present study, since the procedure reduced
background fluorescence but had no effect on the antigenic
epitopes of the Fc
g
RIIIb. Inasmuch as the NA1 allotypic
de-terminant seems to be a target of some anti-Fc
g
RIII
antibod-ies, we proposed that neutrophil donors heterozygous in the
NA1/NA2 allotypic system be selected for the IIF screening of
anti-Fc
g
R antibodies. Five patients (patients 1, 3, 4, 5, and 7 in
Table 2) showed apparent NA1 specificity. These are
autoan-tibodies, not alloanautoan-tibodies, because all of them were NA1
homozygotes.
We have developed two additional ELISAs in which Fc
g
RI
and Fc
g
RII, purified from the surfaces of U937 cells, were
used to coat the plates. Our purification method provides
func-tional receptors or, at least, receptors that are still able to bind
to aggregated human IgG. Such receptors may be potentially
useful in affinity or function studies by using inhibition
exper-iments. In return, we had to denature them before using them
to coat the microtiter plates. We described in this report a very
simple treatment sufficient to achieve this. It is advisable to
apply it even in the IgM autoantibody assay, in which RF may
interfere with the anti-Fc
g
R activity. Indeed, anti-Fc
g
R
au-toantibodies have been described in various systemic
autoim-mune diseases in which RF is commonly present. We must,
however, acknowledge that by denaturing the Fc
g
R antigens to
prevent ligand binding, we limit ourselves to the detection of
antibodies reactive with linear amino acid epitopes.
Physiolog-ically relevant three-dimensional complex epitopes may thus
be missed. This conclusion is supported by our finding that the
reactivity of MAb 3G8 was reduced after the denaturation of
Fc
g
RIII. In addition, the expression of rhuFc
g
RIIIb in E. coli
prevents glycosylation and thus detection of antisera that
in-volve glycosylated residues, a major feature of native Fc
g
RIIIb.
These antibodies could possibly react with neutrophils in the
IIF assay, because some serum samples bound to the
neutro-phil-extracted NA1
1
receptor but did not bind to its
deglycos-ylated form in the ELISA (8). However, neutrophil antibodies
from other serum samples reacted with the nonglycosylated
rhuFc
g
RIIIb, as shown previously (10) by the ELISA and
Western blotting (immunoblotting) methods.
Our third ELISA was based on the use of a whole human
recombinant Fc
g
RIII. Recently, Boros et al. used truncated
murine (2) or human (3) recombinant Fc
g
RII and Fc
g
RIII
and described anti-Fc
g
R activity in autoimmune patients. All
of these methods for the detection of specific anti-Fc
g
R
anti-bodies could be used in parallel in order to further elucidate
the implicated epitopes. Interestingly, the presence of such a
reactivity is related to the clinical presentation of the disease
(9), although neutropenia is not always due to autoantibodies.
Overall, three antibody profiles could be defined (8): 5
se-rum samples were positive in both assays, 5 sese-rum samples
were positive in the IIF test and negative in the ELISA, and 20
serum samples were positive in the ELISA and negative in the
IIF test. Previous absorption experiments (8, 10) established
that the IIF assay and ELISA were directed to the same
anti-gen. Alternatively, steric hindrance may be incriminated.
Given that 20 serum samples were positive in ELISA and
negative in the IIF assay, another potential explanation relates
to the fact that the sensitivity of the ELISA was too high,
although five serum samples were negative by the IIF assay and
positive by the ELISA.
ACKNOWLEDGMENTS
We gratefully acknowledge M. Colonna and M. Hirn for kindly providing reagents. Thanks are also due to S. Forest and A. Paul for secretarial assistance.
REFERENCES
1. Bendaoud, B., Y. L. Pennec, A. Lelong, J. F. Le Noach, G. Magadur, J.
Jouquan, and P. Youinou.1991. IgA-containing immune complexes in the circulation of patients with primary Sjo¨gren’s syndrome. J. Autoimmun.
4:177–184.
2. Boros, P., T. Muryoi, H. Spiera, C. Bona, and J. C. Unkeless. 1993. Autoan-tibodies directed against different classes of FcgR are found in sera of autoimmune patients. J. Immunol. 150:2018–2024.
3. Boros, P., J. A. Odin, J. Chen, and J. C. Unkeless. 1994. Specificity and class distribution of FcgR-specific autoantibodies in patients with autoimmune disease. J. Immunol. 152:302–305.
4. Bux, J., G. M. Robertz-Vaupel, A. Glasmacher, H. J. Dengler, and C.
Muel-ler-Eckhardt.1991. Autoimmune neutropenia due to NA1 specific antibod-ies in primary biliary cirrhosis. Br. J. Haematol. 77:121–126.
4a.Colonna, M., C. Soubrane, and D. Khayat. Production of recombinant Fc-gamma receptor IIIb. Submitted for publication.
5. Huizinga, T. W., M. Kleijer, P. A. Tetterro, D. Roos, and A. E. G. K. von dem
Borne.1990. Biallelic neutrophil NA-antigen system is associated with a polymorphism on the phosphoinositol-linked Fc gamma receptor III (CD16). Blood 75:213–217.
6. Hullet, M. D., and M. Hogarth. 1994. Molecular basis of Fc receptor func-tion. Adv. Immunol. 57:1–127.
7. Kulczycki, J. R. 1983. Purification of Fcεreceptors and Fcgreceptors. Meth-ods Enzymol. 93:178–189.
8. Lamour, A., D. Baron, C. Soubrane, J. Cartron, D. Khayat, Y. Adler, P. Le
Goff, and P. Youinou.1995. Anti-Fc gamma receptor III antibodies correlate to the levels of cell-free Fc gamma receptor III in rheumatoid arthritis serum and synovial fluid. J. Autoimmun. 8:249–265.
9. Lamour, A., R. Le Corre, Y. L. Pennec, J. Cartron, and P. Youinou. 1995. Heterogeneity of neutrophil antibodies in patients with primary Sjo¨gren’s syndrome. Blood 86:3553–3559.
10. Lamour, A., R. Le Corre, Y. L. Pennec, and P. Youinou. 1995. The presence of anti-Fc gamma receptor autoantibodies is related to the clinical
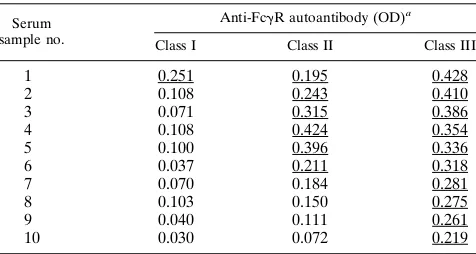
presen-TABLE 3. IgG autoantibodies to FcgRI, FcgRII, and FcgRIII in 10 serum samples from patients with pSS as evaluated by
specific ELISAs
Serum sample no.
Anti-FcgR autoantibody (OD)a
Class I Class II Class III
1 0.251 0.195 0.428
2 0.108 0.243 0.410
3 0.071 0.315 0.386
4 0.108 0.424 0.354
5 0.100 0.396 0.336
6 0.037 0.211 0.318
7 0.070 0.184 0.281
8 0.103 0.150 0.275
9 0.040 0.111 0.261
10 0.030 0.072 0.219
aUnderscores indicate positive results. The cutoff for class I was 0.110, the
cutoff for class II was 0.186, and the cutoff for class III was 0.174.
on August 17, 2020 by guest
http://cvi.asm.org/
tation of primary Sjo¨gren’s syndrome. J. Rheumatol. 22:2241–2245. 11. Lamour, A., R. Le Corre, C. Soubrane, D. Khayat, and P. Youinou. Anti-Fc
gamma receptor autoantibodies from patients with Sjo¨gren’s syndrome do not react with native receptor on human polymorphonuclear leukocytes. J. Autoimmun., in press.
12. Lamour, A., C. Soubrane, M. Ichen, Y. L. Pennec, D. Khayat, and P.
Youi-nou.1993. Fc-gamma receptor III shedding by polymorphonuclear cells in primary Sjo¨gren’s syndrome. Eur. J. Clin. Invest. 23:97–101.
13. Le Tonque`ze, M., C. Jamin, M. Bo¨hme, R. Le Corre, M. Dueymes, and P. Youinou.Establishment and characterization of permanent human endothe-lial cell clones. Lupus, in press.
14. Looney, R. J., G. N. Abraham, and C. L. Anderson. 1986. Human monocytes and U937 cells bear two distinct Fc receptors for IgG. J. Immunol. 136:1641– 1647.
15. Lucas, G. F., and P. A. Carrington. 1990. Results of the First International Granulocyte Serology Workshop. Vox Sang. 59:251–256.
16. Rothko, K., T. S. Kickler, M. E. Clay, R. J. Johnson, and D. F. Stroncek. 1989. Immunoblotting characterization of neutrophil antigenic targets in autoimmune neutropenia. Blood 74:1698–1703.
17. Smidt-Melbye, A., A. Kolstad, and K. Hannestad. 1985. Antibodies to gran-ulocytes detected by an indirect immunofluorescence method not requiring clinical modification of cells. Transfusion 25:165–169.
18. Suzuki, T. 1983. Isolation and characterization of biologically active Fcg
receptors of human B lymphocytes. Methods Enzymol. 93:219–230. 19. Verheugt, F. W. A., A. E. G. K. von dem Borne, J. C. van Noord-Bokhorst,
and C. P. Engelfriet.1978. Autoimmune granulocytopenia: the detection of autoantibodies with the immunofluorescence test. Br. J. Haematol. 39:339– 350.
20. Vitali, C., S. Bombardieri, H. M. Moutsopoulos, G. Balestrieri, W.
Benciv-elli, R. Bernstein, J. Coll, S. de Vita, P. Y. Hatron, F. C. Hay, D. A. Isenberg, A. Janin, J. R. Kalden, Y. T. Konttinen, P. Maddison, R. N. Maini, R. Manthorpe, O. Meyer, P. Ostuni, Y. L. Pennec, J. U. Prause, B. Sauvezie, M. Schiodt, M. Sciutto, Y. Shoenfeld, F. N. Skopouli, J. S. Smolen, M. Snaith, A. Tincani, M. Tishler, S. Todesco, G. Valesini, P. J. W. Venables, M. J. Wattiaux, and P. Youinou.1993. Diagnostic criteria for Sjo¨gren’s syndrome: results of a European multicentre prospective study. Arthritis Rheum. 36: 340–347.