ABSTRACT
BITTNER, RICHARD JOHN. Investigating the Mechanism of Resistance to Bacterial Wilt, Caused by Ralstonia solanacearum, in Tobacco Cultivars. (Under the direction of Dr. Asimina Mila).
Bacterial wilt, also known as Granville wilt (GW), is a destructive disease of many
crops, including tobacco. Use of resistant cultivars is one of the most effective means to
reduce losses from Ralstonia solanacearum (Rs), but little is known about the mechanism of
resistance in tobacco. It has been shown that the severity of bacterial wilt in resistant tomato
cultivars increases when temperature is above 28ºC. We examined the effect of temperature
on resistance to Rs using six tobacco cultivars. Level of resistance of cultivars K346,
Speight 168, NC 71, K326, RJR15, and RJR75 were compared at 10, 15, 20, 25, 30, and
35ºC under controlled conditions. Four strains of Rs, isolated from tobacco plants in North
Carolina in 2008, were used. The highest GW incidence was observed in all cultivars at
30 and 35ºC 18 days after inoculation. No disease symptoms were observed when plants
were incubated at 10 and 15ºC. Plants from different temperatures were placed in 30ºC
for an additional 18 days. Disease symptoms were observed on all cultivars at the end of
this period regardless of the temperature in which they were incubated during the initial days
0 to 18. Temperature (P<0.0001), cultivar (P<0.0001), and strain (P<0.0001) were
significant factors explaining disease incidence, measured as area under the disease
progress curve (AUDPC). The same six cultivars were evaluated in on-farm trials and
their resistance to Rs under these natural conditions was quantified with the use of
an effect on resistance to Rs under natural conditions, similar to the results obtained under
controlled conditions, cumulative Degree-Days (DD) were calculated with three
thresholds (DD25 ºC, DD28 ºC, and DD30ºC) and regressed against DI. Cumulative
DD30ºC had no significant effect (P=0.0657) on the DI of any of the cultivars studied.
However, cumulative DD28ºC had a significant effect on the DI for cultivars: K346, NC71,
RJR15, and RJR75. Lastly, cumulative DD25ºC had a significant effect on the DI of all
cultivars. The growth of the four Rs strains, measured as colony forming units per ml, was
examined at 10, 20, and 30ºC. Temperature (P<0.0001) had a significant effect on Rs
growth. Rs strains incubated at 10 and 20ºC grew at these suboptimal temperatures.
Based on our results we hypothesize that temperature affects not only the growth of the
pathogen but also its interaction with tobacco.
The mechanism of resistance in the same six cultivars was further studied by
histological studies, with the use of a Ralstonia solanacearum (Rs) - strain AW1-gfp38 –
that expressed green fluorescent protein (GFP). Bacteria were observed using fluorescent
microscopy. Bacterial infection was studied at 10, 20, and 30°C. When incubated at
30°C, low resistance cultivars K326 and RJR15 had the highest number of observed stem
infections. At 20°C most infections were latent. Between 0.5 and nine days after
inoculation the low resistance cultivars K326 and RJR15 had the most plants with vascular
tissue infections. After nine days all six cultivars had similar levels of vascular infection,
however. When incubated at 10°C, bacteria rarely progressed into the secondary root xylem
vessels. The effect of temperature (10, 20, and 30°C) on Rs strain AW1-gfp38 growth was
AW1-gfp38 incubated at 20 and 30°C had similar growth rates. When incubated at 10°C, the
bacterial population significantly decreased each day of observation. Based on our studies
we suggest that the mechanism of resistance to Rs in flue-cured tobacco cultivars is
associated with plant tolerance of latent root infections. Discovery of these latent root
Investigating the Mechanism of Resistance to Bacterial Wilt, Caused by Ralstonia solanacearum, in Tobacco Cultivars
by
Richard John Bittner
A thesis submitted to the Graduate Faculty of North Carolina State University
in partial fulfillment of the requirements for the degree of
Master of Science
Plant Pathology
Raleigh, North Carolina
2011
APPROVED BY:
_____________________________ ______________________________ Dr. Frank Louws Dr. Ramsey Lewis
________________________________ Dr. Asimina Mila
BIOGRAPHY
Richard John Bittner grew up on a farm in Kempton, PA. His interests in science
were enhanced by his high school biology classes. After completing high school he
attended Susquehanna University, pursing an undergraduate degree in biology. His
interests in plant pathology were due to his experiences on his family’s farm, as well as
his research at Susquehanna University, under the direction of Dr. Alissa A. Packer.
After graduation, with a degree in biology, he decided to pursue a master’s degree in plant
pathology. Once accepted to North Carolina State University, under the direction of Dr.
ACKNOWLEDGEMENTS
The author would like to thank his committee chair, Dr. Asimina Mila, for giving
him a terrific research project to work. He would also like to thank her for her help and
support throughout his master’s degree. The author would also like to thank his
committee members Dr. Frank J. Louws and Dr. Ramsey S. Lewis for their suggestions
and help regarding the research project. The author would also like to thank John
Radcliff for his help and knowledge regarding field work. He would also like to thank
Melanie Katawczik for her instruction of lab techniques important to the completion of this
thesis.
He would also like to mention Dr. Alissa Packer from Susquehanna University,
who gave him the opportunity to work on a research project and for her help and
support throughout his undergraduate career. The author would also like to thank his
undergraduate advisor, Dr. David Richard. The author would especially like to thank Dr.
Tom and Dr. Peggy Peeler for suggesting that he apply to North Carolina State University.
If it was not for their recommendations he would not have applied to NCSU.
Lastly, the author would like to thank his family for their constant help and support.
He would especially like to thank his parents who have worked very hard to give him
TABLE OF CONTENTS
LIST OF TABLES... ...v
LIST OF FIGURES... ...vi
CHAPTER 1. The effect of temperature on resistance to Bacterial wilt, caused by Ralstonia solanacearum, in tobacco cultivars... ....1
Abstract...1
Introduction...3
Materials and Methods...6
Results...12
Discussion...15
Literature Cited...19
CHAPTER 2. Describing the mechanism of resistance to Bacterial wilt, caused by Ralstonia solanacearum, in tobacco cultivars with histological studies... ...33
Abstract...33
Introduction...35
Materials and Methods...39
Results...43
Discussion...48
LIST OF TABLES
Chapter 1
Table 1. Tobacco cultivars used in on-farm trials between the 2005 and 2009
growing seasons in North Carolina... ...21
Table 2. Effect of temperature, flue-cured tobacco cultivar, strain, and their
interaction on Area Under the Disease Progress Curve (AUDPC), 18 days
post-inoculation... ...21
Table 3. Effect of temperature, flue-cured tobacco cultivar, strain, and their
interaction on Area Under the Disease Progress Curve (AUDPC), 18 days
after incubation in 30ºC... ...22
Table 4. Effect of temperature, strain, and day on growth rate, measured as
Log CFU ml-1 of four Ralstonia solanacearum strains... ...22
Table 5. Effect of cumulative degree-days (DD) and flue-cured tobacco cultivar on
bacterial wilt disease index (DI) based on a mixed model analysis.. ...23
Table 6. Effect of cumulative degree-days (DD) on bacterial wilt disease index (DI)
for each flue-cured tobacco cultivar... ...24
Chapter 2
Table 1. Effect of temperature and day on the growth rate of Ralstonia
LIST OF FIGURES
Chapter 1
Figure 1. The arrangement of the six tobacco cultivars in a tissue culture plate. 1, K326; 2, K346; 3, NC71; 4, SP168; 5, RJR 15; and 6, RJR 75... ...25 Figure 2. Area Under the Disease Progress Curve (AUDPC) for each cultivar. The
left side of the graphs presents the AUDPC from 0 to 18 days post
inoculation, when cultivars were at 35ºC(A), 30ºC(B), 25ºC(C), 20ºC(D),
15ºC(E), and 10ºC(F). The right side of the graphs presents the AUDPC
from day 19 to 36, when cultivars from all temperatures were incubated at
30ºC. Bars with the same letters do not significantly differ according to
Fisher’s least significant difference test (P≤0.05)... ...26
Figure 3. Growth rate of strains 4, 7, 25, and 46 of Ralstonia solanacearum at 30ºC
(A), 20ºC (B), and 10ºC (C). The vertical bars represent the standard error
of the mean of the two experiments... ...30
Chapter 2
Figure 1. Infection of tobacco root epidermis and cortex by Ralstonia solanacearum
strain AW1-gfp38. (A) Bacterial colonies formation on the outside of the
epidermal cells and colonization of epidermal cell grooves. (B) Two
epidermal cells completely colonized in a secondary root. (C) Epidermal
and cortical cells colonized by bacteria in a damaged secondary root.
cells and root hairs of a secondary root. (E) Bacteria colonizing a root hair
(arrow) and the connecting epidermal cell. (F) Epidermal and cortical
cells colonized by the pathogen in an undamaged root tip... ...55
Figure 2. Penetration into xylem vessels and colonization of the stem by Ralstonia
solanacearum strain AW1-gfp38.(A) Multiple xylem vessels of a
secondary root colonized with bacteria. (B) Single xylem vessel colonized
through the entire secondary root. (C) Three secondary roots colonized
with the pathogen. Infection spreads into hypocotyl (arrow) via the
secondary roots. (D) Colonization by bacteria of one side of the plant
stem. (E) Complete colonization of the hypocotyl and stem tissue by the
pathogen... ...56
Figure 3. The frequency of secondary root xylem tissue infected by Ralstonia
solanacearum strain AW1-gfp38 at 30, 20, and 10°C. Each point
represents the mean of six tobacco cultivars examined on each observation
day. The vertical bars represent the standard error of the mean of the six
cultivars... ...57
Figure 4. The average number of tobacco plants with bacterial stem infection when
incubated at 30°C. The studies were repeated once. The vertical bars
represent the standard error of the mean of the two studies... ...58
Figure 5. The frequency of epidermal cell colonization by Ralstonia solanacearum
strain AW1-gfp38 in wounded and non-wounded tobacco plants when
examined on each observation day. The vertical bars represent the
standard error of the mean of the six cultivars... ...59
Figure 6. The average number of tobacco plants with xylem vessel infection when
incubated at 20°C. The studies were repeated once. The vertical bars
represent the standard error of the mean of the two studies... ...60
Figure 7. Growth rate of Ralstonia solanacearum strain AW1-gfp38 at 30, 20, and
10°C. The studies were repeated once. The vertical bars represent the
CHAPTER 1. The effect of temperature on resistance to Bacterial Wilt, caused by
Ralstonia solanacearum, in tobacco cultivars
ABSTRACT
Bacterial wilt, also known as Granville wilt (GW), caused by Ralstonia solanacearum
(Rs), is an important disease of tobacco (Nicotiana tabacum L.) in the southeastern United
States. Currently, the use of resistant cultivars is one of the most cost effective means to
manage the disease. It has been shown that exposure to temperatures above 28°C increases
GW severity in resistant tomato cultivars. We examined the effect of six temperatures (10,
15, 20, 25, 30, and 35ºC) on resistance to Rs on tobacco. Six flue-cured tobacco cultivars
were studied: highly resistant cultivars K346 and Speight 168, moderately resistant cultivar
NC 71, and low resistance cultivars K326, RJR15, and RJR75. The studies were conducted
with four Rs strains, isolated from tobacco plants in North Carolina in 2008. Temperature
(P<0.0001), cultivar (P<0.0001), and strain (P<0.0001) all had a significant effect on disease
incidence, measured as area under the disease progress curve (AUDPC).The highest GW
incidence was observed in all tobacco cultivars at 30 and 35ºC, 18 days after inoculation,
regardless of the Rs strain used. In contrast, no disease symptoms were observed at 10
and 15ºC. After 18 days of incubation in different temperatures, all tobacco plants from
all temperatures were placed in 30ºC for an additional 18 days. At the end of this period
GW symptoms were observed in plants that were previously incubated at 10 and 15ºC
or higher temperatures. The growth rate of the four Rs strains, measured as colony
forming units per ml, was studied at 10, 20, and 30ºC. Temperature (P<0.0001) had a
significant effect on the pathogen’s growth rate. Rs strains when incubated at 10 and
20ºC grew at an equal rate as when the strains were incubated at 30ºC. Cumulative
Degree-Days (DD), using three thresholds (25, 28, 30°C), were calculated to investigate
the effect of temperature on the GW disease index (DI) of the six cultivars in North
Carolina on-farm trials. Cumulative DD30ºC had no significant effect on the DI of all
cultivars studied. In contrast, cumulative DD28ºC did have a significant effect on the DI
for cultivars: K346, NC71, RJR15, and RJR75. Lastly, cumulative DD25ºC had a
significant effect on the DI of all six cultivars. Our results suggest that temperature
affects the growth rate of the pathogen, the expression of resistance of a cultivar to Rs,
INTRODUCTION
Ralstonia solanacearum (Rs), the causal agent of bacterial wilt, also known as
Granville wilt (GW),is a soil-borne plant pathogen that infects several hundred species of
plants, including tobacco (Nicotiana tabacum L.) and is distributed throughout tropical and
subtropical regions worldwide (Grimault et al, 1994; Hayward, 1991). Rs strains have been
divided into five races based on host range and five biovars based on capability to use and/or
oxidize multiple hexose alcohols and disaccharides (Hayward, 1991). Rs race 1 biovar 1 is
prevalent throughout the southeastern United States and is a pathogen to many
ornamentals, vegetables, and tobacco. Rs penetrates the root surface through wounds and
natural openings resulting from nematode damage, agricultural equipment, and the
emergence of secondary roots (McGarvey et al, 1999). The pathogen infects tobacco
roots by colonizing the xylem tissue, resulting in root necrosis, vascular tissue necrosis,
one-sided leaf wilting, yellowing and necrosis between veins and leaf margins, stunting,
and inevitable plant death (Lucas, 1975). In North Carolina, annual yield losses due to
GW range from 1 to 3%, resulting in millions of dollars of lost revenue for growers (Mila
and Radcliff, 2009).
Currently, GW is managed using an integrated approach which includes
fumigation, crop rotation, destruction of tobacco roots and stalks, and the use of resistant
cultivars (Mila and Radcliff, 2009). The use of resistant cultivars is one of the most
successful and cost-efficient strategies to manage GW on tobacco. However, none of
these resistant cultivars are completely immune to disease. GW will be observed in resistant
varies between seasons (Mila and Radcliff, 2009). Currently, there are numerous flue-cured
tobacco cultivars with quantitative resistance to Rs in the United States. The resistance
originated from the Nicotiana tabacum line T.I.448A, an accession collected from Columbia
and maintained by the U.S. Nicotiana germplasm collection (Clayton and Smith 1942). The
resistance has been speculated as being polygenic in inheritance (Burk and Heggestad, 1966).
Clayton and Smith (1942) found that by crossing two moderately resistant cultivars, a highly
resistant genotype could be developed. The first bacterial wilt-resistant flue-cured cultivar,
Oxford 26, was developed from a cross involving T.I. 448A and 400 in 1935 (Valleau, 1952).
Recently, new cultivars originating from Zimbabwe have been introduced to the United
States, but little is known about their resistance to Rs.
Environmental and climatic conditions are known to influence resistance to
bacterial, viral, fungal, and nematode infections in plants (Wang et al, 2009). The most
important environmental factor that affects host-pathogen interactions is temperature
(Hayward, 1991). It has been shown that resistant host plants may become more
susceptible to the pathogen at high temperatures (Dropkin, 1969). This has been
observed in several pathosystems such as root-knot nematodes in tomato cultivars. In the
case of root-knot nematodes, resistance is mediated by the Mi-1 gene, which at
temperatures above 28ºC becomes inactive (Jablonska et al, 2007). Similar temperatures
above 28ºC have been shown to inactivate the N gene which induces resistance to Tobacco
mosaic virus (TMV) on tobacco (Samuel, 1931). Resistance to tobacco ringspot virus
(TRSV) in burley tobacco breeding line L8 has been shown to be temperature dependent,
1972).
Host resistance to Rs is difficult to maintain in environments with high humidity
and temperature (Hayward, 1991). An increase of temperature to a range of 30 to 35ºC is
associated with an increase in severity of the disease caused by Rs in several hosts
(Hayward, 1991). That is, plants resistant to Rs at moderate temperatures become more
susceptible at high ambient temperatures (Hayward, 1991). For instance in tomato, the
pathogen rapidly moves through the plant at temperatures above 28ºC and thus cultivars that
seem resistant in lower temperatures become susceptible when exposed to temperatures
higher than 28ºC (Prior et al, 1996). Another study has shown disease severity to be
significantly greater at 32.2ºC than at 26.6ºC in Rs resistant tomato cultivars (Krausz and
Thurston, 1975). Understanding host-pathogen interactions and the effect of temperature on
disease development may offer information to advance breeding and disease management
strategies.
The objectives of this study were to investigate the effect of temperature on: (i)
resistance to Rs in six flue-cured tobacco cultivars under controlled conditions, (ii)
growth of Rs strains under controlled conditions and (iii) resistance to Rs in tobacco
cultivars under field conditions. Experiments on the effect of temperature on tobacco
cultivars and Rs growth rate were conducted in growth chambers that control the ambient
temperature. The field experiments were conducted in fields naturally infested with Rs
MATERIALS AND METHODS
Tobacco cultivars used and inoculum preparation. Six flue-cured tobacco cultivars with varying levels of Rs resistance were used: K346 (high resistance),
Speight 168 (high resistance), NC71 (moderate resistance), K326 (low resistance), RJR15
(low resistance), and RJR75 (low resistance). K346, K326, Speight 168, and NC71 have
resistance originating from line T.I. 448A and RJR15 and RJR75 have a low level of
resistance derived from an unknown pedigree. Resistance of these cultivars to Rs has been
determined in seven replicated field evaluations conducted since 2005 in nurseries
naturally infested with Rs in North Carolina (Mila and Radcliff, 2009). Rs strains 4, 7, 25,
and 46 (previously characterized as race 1 biovar 1) from our collection were used. Strains
were collected in North Carolina counties in 2007 from stems of diseased tobacco plants,
isolated on tetrazolium chloride (TZC) medium (Kelman, 1954) and stored in 20% glycerol
at -80ºC. Strains 4 and 7 were isolated from tobacco cultivar K326 in a single field of
Edgecombe County. These Rs strains have different molecular profiles based on rep-PCR.
Strains 25 and 46 were isolated from different tobacco cultivars, K346 and K394
respectively, in Johnston County. In contrast, these strains have the same molecular profile.
For the laboratory experiments, inoculum was prepared by growing the strains
on TZC medium for 48 h at 28ºC. Bacterial cells were suspended in sterile distilled water
and adjusted to 108 CFU/ml using a spectrophotometer. Roots of tobacco seedlings were
injured before inoculation to create entry points for the pathogen by stabbing a sterilized
scalpel around each seedling, separating pieces of the root from the seedling.
added to each cell of the plate.
Effect of temperature on resistance to R. solanacearum in laboratory experiments. The laboratory experiments were conducted using the method described by Katawczik and Mila (Katawczik and Mila, 2011). The six tobacco cultivars were grown in
12-cell tissue culture (TC) plates (Corning Incorporated, Corning, NY). In each cell, 3.5
cm3 of perlite (<2 mm in size) and 1.5 ml of deionized water was added. Four to six
seeds were added to each cell (one cultivar per two cells; Fig. 1). Deionized water was
added to the space between the cells for additional moisture. Seeded plates were
incubated at room temperature (20 to 25ºC) under 12 h of light and 12 h of darkness. A
0.5 ml solution of 200-ppm N fertilizer (Bulldog water-soluble fertilizer 20-10-20;
Chilean Nitrate Corporation, Northfolk, VA) per cell was added to each plate 10 days
after seeding and once a week thereafter. TC plates were checked once a week for excess
water. Seed germination was evaluated 14 days after seeding to determine the number of
plants per cell. Twenty-five day old seedlings were inoculated per cell with 0.5 ml of
bacterial suspension, one strain per TC plate. Each strain was used to inoculate 12 TC
plates (48 TC plates total). Following inoculation, TC plates were placed into 52-by-27-
by-16-cm plastic containers (Sterlite Co., Townsend, MA) and covered with a 55-by-46-
cm polyvinyl-chloride laboratory wrap (Fisher Scientific Company, Pittsburgh, PA) to
create a moisture chamber. The plastic containers were then incubated in a growth chamber
at 28ºC with 13 h of light and 11 h of dark for 48 h. Then, the plastic containers were
that two TC plates per strain were placed in each individual growth chamber. One TC plate
with non-inoculated seedlings was also placed in each growth chamber (i.e. negative
control). After 18 days of incubation all plates were moved into a 30ºC growth chamber. TC
plates were then incubated for an additional 18 days. The experiment was repeated twice.
Disease incidence was evaluated 4 days after inoculation and every 3-4 days
thereafter, totaling 12 evaluations. Each individual cell from the TC plates was assessed
for diseased seedlings. The total number of diseased seedlings was recorded for each
cell, during each count. Tobacco seedlings with general necrosis, wilting, dark roots, and
death were considered to be infected by Rs. The area under the disease progress curve
(AUDPC) was calculated for each cultivar.
The effect of temperature on R. solanacearum growth rate. Studies were conducted to determine the effect of temperatures on the growth rate of the same four Rs
strains (4, 7, 25, and 46). Three temperatures were investigated: 10, 20, and 30ºC.
Bacteria were incubated in a 50 ml centrifuge tube (Becton, Dickinson and Company,
Franklin Lakes, NJ). A 1 μl loopful of bacteria grown on TTC medium was added to 30
ml per centrifuge tube of casamino acid peptone glucose (CPG) broth (Ji et al, 2007). Each
strain was grown in a separate tube for each temperature investigated. The number of
bacterial cells as colony forming units (CFU) in each tube was determined by dilution plating
onto nutrient agar (8 g nutrient broth and 20 g granulated agar in 1 L of distilled water) and
plate incubation at 28 ºC for 48 h. Dilution plating was conducted on day 0, 2, 4, 6, 8, 10,
12, and 20 after initiation of incubation. The experiment was repeated once.
From 2004 to 2009 tobacco-growing seasons, 15 field studies were conducted in
seven counties of North Carolina (Duplin, Edgecombe, Harnett, Johnston, Robeson,
Wake, and Wayne Counties) (Table 1). The six tobacco cultivars used in the laboratory
experiments were evaluated in field studies: K326, K346, NC71, Speight168, RJR15, and
RJR75. Tobacco cultivars were set in a randomized complete block design consisting of
a one-row plot (1.17 m wide and 15.24 m long) with four replications for each cultivar.
Flue-cured tobacco plants were transplanted between April 16 and May 14 of each
season. The stand count of the plots was evaluated three weeks after transplanting.
Throughout the growing season standard cultural practices for the area were followed
with regards to cultivation, use of insecticides and fungicides, sucker control, and
topping. Disease evaluations were conducted once every two weeks, beginning four to
six weeks after transplanting. Bacterial wilt incidence was evaluated at each field by
counting tobacco plants with the characteristic aboveground symptom of wilting on one
side of the plant leaf or one side of the plant. The final evaluation was completed 14 to
18 weeks after transplanting for a total of five evaluations. The disease index (DI), a
modification of the AUDPC, was calculated for each cultivar in each field experiment.
For the five evaluations the formula for DI is: DI = [Єi = i – 5 Xi (100-[i-1])(100/5)]/N,
where X is the number of diseased plants since the previous count, i is the ordinal
evaluation number, and N is the total number of tobacco plants (stand count) (Csinos et al,
1986).
temperature on bacterial wilt DI observed in on-farm trials. Three thresholds were used
to calculate DD: 30, 28, and 25°C. DD30 and 25°C were selected because the results
collected from the laboratory experiments indicated that these temperatures were important
to Rs resistance in tobacco cultivars. DD28°C was examined because literature suggests that
temperatures above 28°C can break Rs resistance in tomato cultivars (Prior et al, 1996).
Temperature data was obtained from the National Climactic Data Center (NCDC). Data used
included the daily maximum and minimum temperatures from the nearest regional weather
station for the months of April, May, June, July, and August. Daily DD were calculated by
averaging the maximum and minimum temperatures for each day, and then subtracting the
base temperature of 30, 28 and 25°C from the daily, calculated average. Negative values
were set to zero. The daily DD were calculated between the transplant date and the final
disease evaluation for each location in a particular year. Daily DD values were then
summed, resulting in 15 location-year DD values.
Statistical analysis. Data from the laboratory experiments were analyzed using the general linear model procedure (PROC GLM) of the Statistical Analysis System
(version 9.1; SAS Institute Inc., Cary, NC). F tests were used to determine the significant
effects in the mean AUDPC values. Effects examined were temperature, flue-cured
tobacco cultivar, strain, and their interactions. Bartlett’s test for homogeneity of
variances was conducted to determine if there was a significant difference in the AUDPC
data between the three laboratory experiments. Data from experiments on Rs growth
rates were analyzed with analyses of variance using the PROC GLM of the Statistical
effects on the growth rate of the strains. Effects examined were temperature, strain, and
day. Bartlett’s test for homogeneity of variances was conducted to determine if there
were significant differences between the data collected from the two experiments.
For the on-farm trials, a mixed linear model analysis of variance (PROC MIXED)
of the Statistical Analysis System (SAS, version 9.1) was used to determine the effects of
DD (DD30°C, DD28°C, and DD25°C) and cultivar on DI. DI data was logarithmically
transformed before analysis. Fixed effects were cultivar, DD30°C, DD28°C, and
DD25°C. The location was set as a random effect. F tests were used to determine the
significant effects on the DI. The effect of DD was also examined separate for each
cultivar using PROC MIXED of the Statistical Analysis System (SAS, version 9.1).
Fixed effects were DD30°C, DD28°C, and DD25°C. The random effect was location.
Flue-cured tobacco cultivar was considered a group variable. Bacterial wilt DI data was
logarithmically transformed. F tests were used to determine if the effects significantly
RESULTS
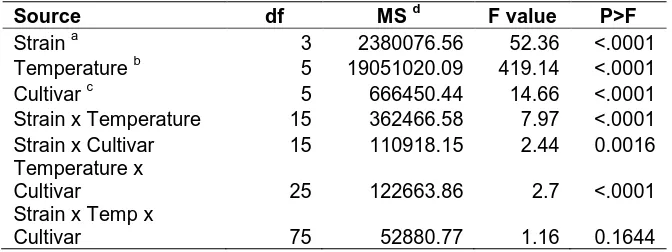
Effect of temperature on resistance to R. solanacearum in laboratory experiments. Temperature (P<0.0001), cultivar (P<0.0001), strain (P<0.0001), and their interactions all had a significant effect (P<0.05) on the AUDPC 18 days post inoculation
(Table 2). The highest AUDPC values were observed in all cultivars at 35 and 30ºC, 18
days post-inoculation (Figure 2A and 2B). In contrast, disease symptoms were not
observed in any tobacco cultivars at 10ºC (Figure 2F) whereas no or low GW incidence
was observed in flue-cured tobacco cultivars incubated at 15 ºC (Figure 2E). The highest
AUDPC values were observed at 35ºC regardless of the tobacco cultivar. The AUDPC
values decreased in all cultivars as temperature decreased. Highly resistant cultivar
Speight168 showed the least incidence of all cultivars tested, with AUDPC values higher
at 35 than 30, 25, 20, 15, or 10ºC (Figure 2). At 35ºC, the AUDPC value for Speight168
was not statistically different from the AUDPC value calculated for any other tobacco
cultivar, except for cultivar K326 (Figure 2A). Highly resistant cultivar K346 had the
highest AUDPC value at 30 and 25ºC (Figure 2B and 2C) when compared to all other
cultivars, despite being highly resistant toRs in field trials. Cultivar K346 also had high
AUDPC values when incubated at 35 and 20ºC. Cultivars RJR15 and RJR75 had statistically
similar AUDPC values to K326 and K346 at all temperatures tested.
Temperature (P<0.0001), cultivar (P<0.0001), and strain (P<0.0001) all had a
significant effect on the AUDPC for the additional 18 days when plants were moved to
30ºC (Table 3). However, the interactions between these three factors did not have any
when they were moved from 35, 30, 25, 20, 15 and 10ºC to 30ºC (Figure 2). Plants that
were moved from 10 and 15 ºC to 30ºC showed an increase in GW incidence and thus an
increase in the AUDPC values (Figure 2E and 2F). Highly resistant cultivar Speight168
had a significantly smaller AUDPC value than low resistant cultivar K326 when plants
moved from 10 to 30ºC (Figure 2F).
Effect of temperature on R. solanacearum growth rate. Figure 3 represents the growth curves of Rs strains 4, 7, 25, and 46 at 10, 20, and 30ºC.
Temperature (P<0.0001), day (P<0.0001), and strain (P<0.0001) had a significant effect
on the growth rate ofRs (Table 4). Overall, the growth rates were higher at 30 and 20
than at 10ºC for all strains (Figure 3). At 10°C all strains had a slower growth rate during
the first 6 days (Figure 3C), than when grown at 30 and 20ºC. After the first six days of
incubation at 10ºC, growth rates for all Rs strains raised to levels similar to strains
incubated at higher temperatures (Figure 3C). Interestingly, the growth rate of the four
strains was significantly different from each other (Table 4). Strain 4 had the lowest,
while strain 46 had the highest growth rate at all three temperatures.
Effect of temperature on resistance to R. solanacearum in on-farm trials.
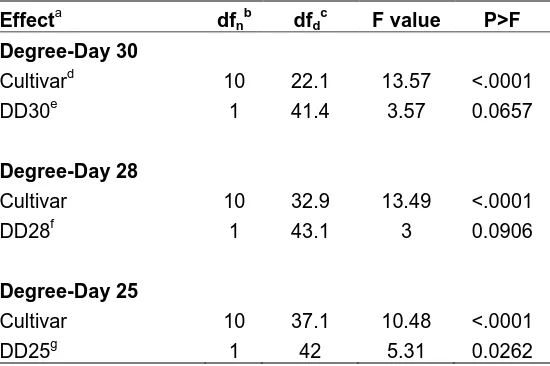
Cumulative DD30ºC (P=0.0657) and DD28ºC (P=0.0906) did not have a significant
effect on the DI (Table 5). Only DD25ºC had a significant effect on DI. The flue-cured
tobacco cultivar (P<0.0001) also had a significant effect on the DI regardless the
threshold of cumulative Degree-Days (Table 5).
cured tobacco cultivars. Cumulative DD30ºC had no significant effect on the DI value of
any of the cultivars (Table 6). In contrast, cumulative DD28ºC had a significant effect on
the DI for the flue-cured tobacco cultivars: K346, NC71, RJR15, and RJR75 (Table 6).
Finally, cumulative DD25ºC had a significant effect on the DI for all six flue-cured
DISCUSSION
Summarizing our results from the growth rate experiments in combination with our
in-field and laboratory experiments on the effect of temperature on tobacco cultivar
resistance, we conclude that temperature likely affects the interaction between the host and
pathogen, rather than the pathogen’s growth and multiplication per se.
Temperature is an important environmental factor that affects multiple
plant pathosystems and their interactions with their hosts (Hayward, 1991). High
ambient temperatures have been shown to induce GW incidence at a faster rate than
moderate temperatures (Ciampi and Sequeira, 1980). The current study discovered the
effect of temperature on tobacco cultivars with different levels of resistance to GW and
on the growth rate of different strains of the pathogen. This study found that a minimum
temperature of 15ºC is required for GW symptom development in all tobacco cultivars in
a controlled environment, demonstrating that temperature does affect disease development in
tobacco cultivars. Our results also indicated that GW symptoms in all tobacco cultivars
incubated at 10ºC were suppressed. This suggests that populations of Rs inside the root
xylem vessels do not advance further into the vascular system when temperatures are
below 15ºC. All tobacco cultivars incubated at 30 and 35ºC had the highest AUDPC
values, when compared to AUDPC values obtained at lower temperatures. These results
confirm that temperatures between 30 and 35ºC significantly increase GW severity in
tobacco as it has been reported in other hosts (Hayward, 1991).
The six tobacco cultivars with varying levels of resistance had significant
values than high resistant cultivars incubated at 20 and 25ºC. On the contrary, incubation
at 35ºC produced no statistically different AUDPC values in most cultivars, which
suggests that the resistance in tobacco cultivars with high resistance, such as Speight168,
will be compromised at high temperatures. Studies by Krausz and Thurston also
demonstrate a relationship between tomato cultivar resistance to Rs and temperature.
They found that disease severity was significantly greater at 32.2ºC than at 26.6ºC, in
resistant tomato cultivars (Krausz and Thurston, 1975). Our results also showed that
highly resistant cultivar K346 had the highest AUDPC value of all cultivars except for
susceptible cultivar K326 when incubated at 30ºC, optimum growth temperature for the
pathogen. These results also suggest that higher temperatures affect the ability of the
plant to retain resistance to GW. In our growth chamber experiments, highly resistant
cultivars K346 and Speight168 had higher AUDPC values at 35 and 30 than 25, 20, 15 or
10ºC. These results demonstrate that as the temperature around the plant increases,
resistance to Rs decreases, increasing the number of plants with visual symptoms.
The disease incidence in all cultivars increased when plants were transferred from
their respective temperatures to 30ºC for an additional 18 days. Plants that were moved
from 10 and 15ºC showed an increase in disease symptoms, resulting in increased
AUDPC values in all tobacco cultivars. We believe that incubation at these low
temperatures suppressed the progression of the pathogen in the infected plants (Chapter
2). When these plants where then exposed to the pathogen’s optimum temperature for
of the tobacco plant and cause disease (Chapter 2).
DI data from on-farm trials was used to examine the effect of temperature on
resistant cultivars and validate our results from the experiments conducted with a few
weeks old tobacco plants in a controlled environment. Cumulative DD were used to
quantify the effect of temperature on the Rs resistant of the six resistant tobacco cultivars.
Our results indicated that cumulative DD30ºC had no impact on the DI for any flue-cured
tobacco cultivar. It is possible that DD30ºC had no effect on DI because there were few
days where the temperature was above 30ºC, leading to a low number of DD30ºC values.
In contrast, our results showed that cumulative DD28ºC and DD25ºC had significant
impacts on the DI of many of the tobacco cultivars examined. Cumulative DD28ºC had a
significant effect on highly resistant cultivar K346, moderately resistant cultivar NC71,
and low resistant cultivars RJR15 and RJR75. Finally, cumulative DD25ºC had a
significant effect on all cultivars tested. Our findings demonstrate that, under field
conditions, the threshold of DD needed to have an effect on disease incidence in resistant
tobacco cultivars is between 25-28 ºC.
Our studies on the effects of temperature on Rs growth showed that temperature
had a significant effect on the bacterial growth. Temperatures below 30ºC did not
prevent bacterial growth and multiplication. Rs strains incubated at 20ºC followed a
similar growth rate to those incubated at 30ºC. On the contrary, strains incubated at 10ºC
showed a hindrance in growth for the first six days of incubation, but then reached
equivalent growth levels (CFUs) to the ones observed at 20 and 30ºC. These results
temperature and get growth levels similar to Rs strains incubated at optimum
temperatures. The effect of low temperatures on growth rate of Rs in culture has been
previously reported by Ciampi and Sequeira (Ciampi and Sequeira, 1980). Their results
indicated that Rs strain K60 (race 1) would sustain growth in liquid culture at low
temperature (16ºC). Similarly in our experiments, we demonstrated that at the low
temperature of 10º C, the four Rs strains (4, 7, 25, and 46), continued to grow and
multiply. Our findings suggest that Rs growth in vitro is not related to an inability of Rs
strains to induce symptoms at low temperatures. Ciampi and Sequeira also reported that
their Rs strain did not cause any disease symptoms in potato cultivars when incubated at
16ºC. They also concluded that Rs growth in vitro is not related to the symptom
development at low temperatures (Ciampi and Sequeira, 1980). We conclude that the results
from the growth rate experiments, in combination with our in-field and laboratory
experiments on the effect of temperature on tobacco cultivar resistance, demonstrate that
temperature likely affects the interaction between the host and pathogen, rather than the
LITERATURE CITED
Burk, L. G., and Heggestad, H. E. 1966. The Genus Nicotiana: A Source of Resistance to Diseases of Cultivated Tobacco. Econ. Bot. 20:76-88.
Ciampi, L., and Sequeira, L. 1980. Influence of temperature on virulence of race 3 strains of
Pseudomonas solanacearum. Am. Potato J. 57:307-317.
Clayton, E. E., and Smith, T. E. 1942. Resistance of tobacco to bacterial wilt (Bacterium solanacearum). J. Agric. Res. 65:547-554.
Csinos AS, Fortnum BA, Gayed SK, Reilly JJ, Shew HD. 1986. Evaluating chemicals for control of soilborne pathogens on tobacco. Pages 231–236, in: Methods for evaluating pesticides for control of plant pathogens. K.D. Hickey, ed. APS Press, St. Paul, MN.
Dropkin, V. H. 1969. The necrotic reaction of tomatoes and other hosts resistant to
Meloidogyne: reversal by temperature. Phytopathology 59:1632-1637.
Grimault, V., Anais, G., and Prior, P. 1994. Distribution of Pseudomonassolanacearum in the stem tissues of tomato plants with different levels of resistance to bacterial wilt. Plant Pathol. 43:663-668.
Hayward, A. C. 1991. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 29:65-87.
Hendrix, J. W. 1972. Temperature-dependent resistance to Tobacco Ringspot Virus in L8, a necrosis-prone tobacco cultivar. Phytopathology 62:1376-1381.
Jablonska, B., Ammiraju, J. S., Bhattarai, K. K., Mantelin, S., Martinez de Iarduya, O., Roberts, P. A., and Kaloshian, I. 2007. The Mi-9 Gene from Solanum arcanum conferring heat-stable resistance to Root-Knot Nematodes is a homolog of Mi-1. Plant Physiology 143:1044-1054.
Ji, P., Allen, C., Sanchez-Perez, A., Yao, J., Elphinstone, J. G., Jones, J. B., and Momol, M. T. 2007. New diversity of Ralstonia solanacearum strains associated with vegetable and ornamental crops in Florida. Plant Dis. 91:195-203.
Katawczik, M. and Mila, A. L. 2011. A laboratory technique to determine tobacco resistance to Ralstonia solanacearum causal agent of Granville wilt. Tob. Sci. (in press).
Krausz, J. P., and Thurston, H. D. 1975. Breakdown of resistance to Pseudomonas solanacearum in tomato. Phytopathology 65:1272-1274.
Lucas, G. B. 1975. Diseases of Tobacco. 3rd ed. Biological Consulting Associates, Raleigh, NC.
McGarvey, J. A., Denny, T. P., and Schell, M. A. 1999. Spatial-temporal and quantitative analysis of growth and EPS I production by Ralstonia solanacearum in resistant and susceptible tomato cultivars. Phytopathology 89:1233-1239.
Mila, A. L., and Radcliff, J. 2009. Managing Diseases. Pages 140-174 in: Flue-Cured Tobacco Guide. N. C. Coop. Ext. Serv. Bull., North Carolina State University, Raleigh.
Prior, P., Bart, S., Leclercq, S., Darrasse, A., and Anais, G. 1996. Resistance to bacterial wilt in tomato as discerned by spread of Pseudomonas (Burholderia) solanacearum in the stem tissues. Plant Pathol. 45:720-726.
Samuel, G. 1931. Some experiments on inoculating methods with plant viruses, and on local lesions. Ann. Appl. Biol. 18:494-507.
Valleau, W. D. 1952. Breeding tobacco for disease resistance. Econ. Bot. 6:69-102.
Table 1. Tobacco cultivars used in on-farm trials between the 2005 and 2009 growing seasons in North Carolina.
Year County Cultivar
Transplant Date
Last Disease Evaluation 2004 Edgecombe K326, K346, NC71, SP168 May 10 July 21 2004 Harnett K326, K346, NC71, SP168 April 27 June 24 2004 Robeson K326, K346, NC71, SP168 April 21 July 22 2004 Wake K326, K346, NC71, SP168 April 16 July 15 2005 Edgecombe K326, K346, NC71, SP168 May 2 July 20 2005 Harnett K326, K346, NC71, SP168 April 27 August 19 2005 Robeson K326, K346, NC71, SP168 April 26 July 21 2005 Wayne K326, K346, NC71, SP168 April 25 August 2
2006 Edgecombe K326, K346,SP168 May 1 July 31
2007 Johnston Site A K326, K346, NC71, SP168, RJR15 May 1 August 8 2007 Johnston Site B K326, K346, NC71, SP168, RJR15 April 30 August 8
2008 Duplin K346, RJR15 , RJR75 May 1 July 31
2008 Edgecombe K346, RJR15 , RJR75 May 6 August 19
2008 Johnston K346, RJR15 , RJR75 May 14 August 11
2009 Edgecombe K346, SP168 April 22 August 4
Table 2. Effect of temperature, flue-cured tobacco cultivar, strain, and their interaction on Area Under the Disease Progress Curve (AUDPC), 18 days post-inoculation.
Source df MS d F value P>F
Strain a 3 2380076.56 52.36 <.0001
Temperature b 5 19051020.09 419.14 <.0001
Cultivar c 5 666450.44 14.66 <.0001
Strain x Temperature 15 362466.58 7.97 <.0001 Strain x Cultivar 15 110918.15 2.44 0.0016 Temperature x
Cultivar 25 122663.86 2.7 <.0001
Strain x Temp x
Cultivar 75 52880.77 1.16 0.1644
a
Rs strains used were isolated from diseased tobacco plants in 2007 in North Carolina.
b
Temperatures examined: 10, 15, 20, 25, 30, and 35ºC.
c
Cultivars used were K346: high resistance, Speight168: high resistance, NC71: moderate resistance, K326: low resistance, RJR15: low resistance, and RJR75: low resistance.
d
Table 3. Effect of temperature, flue-cured tobacco cultivar, strain, and their interaction on Area Under the Disease Progress Curve (AUDPC), 18 days after incubation in 30ºC.
Source df MS d F value P>F
Strain a 3 14094076.7 63.96 <.0001
Temperature b 5 35618027 161.63 <.0001
Cultivar c 5 4376728.4 19.86 <.0001
Strain x Temperature 15 334701.1 1.52 0.0904 Strain x Cultivar 15 291391.7 1.32 0.1801 Temperature x
Cultivar 25 266140.5 1.21 0.2196
Strain x Temp x
Cultivar 75 207861.8 0.94 0.6162
a
Rs strains used were isolated from diseased tobacco plants in 2007 in North Carolina.
b
Temperatures examined: 10, 15, 20, 25, 30, and 35ºC.
c
Cultivars used were K346: high resistance, Speight168: high resistance, NC71: moderate resistance, K326: low resistance, RJR15: low resistance, and RJR75: low resistance.
d
Mean Square (MS) derived from the type III sum of squares.
Table 4. Effect of temperature, strain, and day on growth rate, measured as Log CFU ml-1 of four Ralstonia solanacearum strains.
Source df MS d F value P>F
Temperature a 2 8.29828937 281.41 <.0001 Strain b 3 1.90551212 64.62 <.0001 Day c 6 4.09743284 138.95 <.0001
a
Temperatures examined: 10, 20, and 30ºC.
b
Rs strains used were isolated from diseased tobacco plants in 2007 in North Carolina.
c
Growth rate was measured on day 0, 2, 4, 6, 8, 10, 12, and 20 since the initiation of incubation.
d
Table 5. Effect of cumulative degree-days (DD) and flue-cured tobacco cultivar on bacterial wilt disease index (DI) based on a mixed model analysis.
Effecta dfnb dfdc F value P>F
Degree-Day 30
Cultivard 10 22.1 13.57 <.0001
DD30e 1 41.4 3.57 0.0657
Degree-Day 28
Cultivar 10 32.9 13.49 <.0001
DD28f 1 43.1 3 0.0906
Degree-Day 25
Cultivar 10 37.1 10.48 <.0001
DD25g 1 42 5.31 0.0262
a
Type III tests of fixed effects.
b
Numerator degrees of freedom.
c
Denominator degrees of freedom.
d
Cultivars used were K346: high resistance, Speight168: high resistance, NC71: moderate resistance, K326: low resistance, RJR15: low resistance, and RJR75: low resistance.
e
Cumulative degree days with a base temperature of 30°C.
f
Cumulative degree days with a base temperature of 20°C.
g
Table 6. Effect of cumulative degree-days (DD) on bacterial wilt disease index (DI) for each flue-cured tobacco cultivar.
Effecta dfn b
dfd c
F value P>F
K326
DD30e 1 4.05 1.26 0.3232
DD28f 1 4.00 0.53 0.5056
DD25g 1 10.00 41.13 <.0001 K346
DD30 1 7.21 2.01 0.1979
DD28 1 9.30 21.85 0.0011
DD25 1 4.54 41.95 0.0019
NC71
DD30 1 3.15 0.01 0.9108
DD28 1 4.97 7.73 0.0391
DD25 1 9.00 34.88 0.0002
SP168
DD30 1 6.59 0 0.9935
DD28 1 9.13 1.51 0.2505
DD25 1 5.71 19.39 0.0051
RJR15
DD30 1 1.12 5.86 0.2282
DD28 1 3.99 23.69 0.0083
DD25 1 2.28 72.33 0.0089
RJR75
DD30 1 2.00 4.68 0.1631
DD28 1 2.00 24.05 0.0392
DD25 1 2.00 62.38 0.0157
a
Type III tests of fixed effects. Three cumulative degree-days tested by flue-cured tobacco cultivar group.
b
Numerator degrees of freedom.
c
Denominator degrees of freedom.
e
Cumulative degree days with a base temperature of 30°C.
f
Cumulative degree days with a base temperature of 20°C.
g
Figure 1. The arrangement of the six tobacco cultivars in a tissue culture plate. 1, K326;
Figure 2. Area Under the Disease Progress Curve (AUDPC) for each cultivar. The left side of the graphs presents the AUDPC from 0 to 18 days post inoculation, when
cultivars were at 35ºC (A), 30ºC (B), 25ºC (C), 20ºC (D), 15ºC (E), and 10ºC (F). The
right side of the graphs presents the AUDPC from day 19 to 36, when cultivars from all
temperatures were incubated at 30ºC. Bars with the same letters do not significantly
A 0 200 400 600 800 1000 1200 1400 1600 K32 6 K34 6 NC 71 SP16 8 RJR 15 RJR 75 K32 6 K34 6 NC 71 SP16 8 RJR 15 RJR 75 Cultivar A U D PC a ab
ab ab ab
b a a a a a a 0 200 400 600 800 1000 1200 1400 1600 K32 6 K34 6 NC 71 SP16 8 RJR 15 RJR 75 K32 6 K34 6 NC 71 SP16 8 RJR 15 RJR 75 Cultivar A U D PC a ab
ab ab ab
Figure 3. Growth rate of strains 4, 7, 25, and 46 of Ralstonia solanacearum at 30ºC (A), 20ºC (B), and 10ºC (C). The vertical bars represent the standard error of the mean of the
A
7 7.5 8 8.5 9
2 4 6 8 10 12 20
Day
L
o
g
C
F
U
/m
l
Strain 4 Strain 7 Strain 25 Strain 46
B
7 7.5 8 8.5 9
2 4 6 8 10 12 20
Day
L
o
g
C
F
U
/m
C
7 7.5 8 8.5 9
2 4 6 8 10 12 20
Day
L
o
g
C
F
U
/m
CHAPTER 2. Describing the mechanism of resistance to Bacterial Wilt, caused by
Ralstonia solanacearum, in tobacco cultivars with histological studies
ABSTRACT
The use of resistant cultivars is one of the most successful means to manage
bacterial wilt in tobacco, but little is understood of the mechanism of the resistance. The
mechanism of resistance of six flue-cured tobacco cultivars to bacterial wilt was studied
in histological studies, with the use of a Ralstonia solanacearum (Rs) - strain AW1-
gfp38 - that expressed green fluorescent protein (GFP). Tissues of highly resistant
cultivars K346 and Speight 168, moderately resistant cultivar NC 71, and low resistant
cultivars K326, RJR15, and RJR75 were examined. Plants were incubated at 30, 20, and
10C. Bacterial infection in plant tissue was observed using fluorescent microscopy. All
tobacco cultivars were colonized by Rs at all temperatures investigated. We observed
faster infection progression in all cultivars incubated at 30°C, than 20 or 10°C, an
indication that high temperature affects resistance. Differences between cultivars
incubated at 30°C were only observed in the ability of the pathogen to colonize the stem
of a cultivar. Low resistance cultivars K326 and RJR15 had the highest number of
observed stem infections. Visual disease symptoms were only observed in cultivars
incubated at 30°C. Infections in tobacco cultivars incubated at 20°C were mostly latent
infections, not associated with visual bacterial wilt symptom development. Observations
K326 and RJR15 had the most plants with xylem vessel infections. However,
observations after nine days indicated that all six cultivars had a similar level of vascular
infection. In tobacco cultivars incubated at 10°C, bacteria were confined mainly in the
epidermal cells and root hairs. No differences were observed in disease progression
between cultivars at this temperature. Furthermore, the effect of temperature (30, 20, and
10°C) on Rs strain AW1-gfp38 growth was studied in culture. Temperature (P<0.0001)
had a significant effect on the bacterial growth. Bacteria incubated at 30 and 20°C had
similar growth rates. Overall the bacterial population grew faster when incubated at
20°C, than at 30 or 10°C. The bacterial population significantly decreased each day of
observation, when incubated at 10°C. However, the bacterial population remained alive
after 20 days of incubation. Based on our findings, we hypothesize that the mechanism
of resistance to Rs in tobacco is related to plant tolerance of latent infections.
Identification of these latent infections in tobacco lines could be used to advance tobacco
INTRODUCTION
Bacterial wilt, caused by the soil-borne bacterium Ralstonia solanacearum (Rs),
is a vascular plant pathogen that has a wide range of hosts with over 200 species
belonging to 50 families (Kawasaki et al, 2007). Hosts of Rs include tobacco (Nicotiana
tabacum L.) and other economically important crops such as potato, tomato, pepper,
eggplant, and banana (Saile et al, 1997). Rs is responsible for high economic losses in
these crops in the tropical and subtropical regions of the world (Hayward, 1991). The
pathogen normally gains entrance to a tobacco plant through the roots and then will
colonize the root xylem tissue, resulting in root necrosis (Saile et al, 1997). Once inside
the root xylem, the bacteria progress into the stem and cause vascular necrosis, stunting,
one-sided leaf wilting, yellowing and necrosis between veins and leaf margins, and
finally plant death (Lucas, 1975).
Rs has been found to penetrate the root surface through wounds and natural
openings resulting from nematode damage, agricultural equipment, and the emergence of
secondary roots (McGarvey et al, 1999). Vasse (Vasse et al, 1995) observed that in tomato
cultivars, the bacteria are attracted to these sites and attach to the surface of the epidermal
cells. Bacteria then colonize the longitudinal grooves between the epidermal cells. Rs then
invades the intercellular spaces of the inner cortex and form intercellular micro-colonies
(Vasse et al, 1995). In tomato, the pathogen then moves through the endodermis and
infects the vascular parenchyma that surrounds subsequently xylem vessels. Once the
xylem vessels are colonized, Rs produces high-molecular-mass acidic extracellular
symptoms (McGarvey et al, 1999). Colonization of only a few xylem vessels in each
vascular bundle of the hypocotyl is needed to induce disease symptoms in tomato
cultivars (Vasse et al, 2000).
Management of bacterial wilt is based on an integrated approach which includes
crop rotation, fumigation, destruction of host material, and implementation of resistant
cultivars (Mila and Radcliff, 2009). The use of resistant cultivars is the most cost
effective tool used by growers for managing bacterial wilt (McGarvey et al, 1999).
Resistance in tobacco originated from Nicotiana tabacum line T.I.448A, an accession
collected from Columbia and maintained by the U.S. Nicotiana germplasm collection
(Clayton and Smith 1942). This resistance has been described as being polygenic (Burk and
Heggestad, 1966) and currently, has been incorporated in several of the flue-cured
tobacco cultivars that are commercially available to tobacco growers.
Resistant cultivars are not immune to Rs infection. For instance, Rs resistant
tomato cultivars can develop latent infections, i.e. plants become partially colonized by
the bacteria, without developing any visual bacterial wilt symptoms (Prior et al, 1996).
Latent infections have been observed in Rs resistant potato (Ciampi et al, 1980), tomato,
and peanut (Grimault and Prior, 1993) cultivars. The mechanism of resistance has been
studied extensively in tomato cultivars, where it was determined that resistance was not
associated with bacterial penetration and infection of the tomato roots (Grimault and
Prior, 1993). It was found to be associated with the ability of plants to limit Rs
mechanism of resistance is similar to mechanism observed in tomato cultivars (Grimault
et al, 1994).
Host resistance to plant pathogens can fluctuate because of differences in
environmental and climactic conditions (Grimault et al, 1994). Temperature is the most
important environmental factor influencing host resistance (Hayward, 1991). High
temperatures have been reported to increase susceptibility of resistant plants in many
pathosystems (Dropkin, 1969). For instance, the tobacco N gene, conferring resistance to
Tobacco mosaic virus (TMV) becomes inactive at temperatures above 28°C (Samuel,
1931). Bacterial wilt severity has been shown to greatly increase when plants are
exposed to temperatures between 30-35°C (Hayward, 1991). Previous work has shown
that the higher the ambient temperature, the higher the frequency of vascular tissue
invasion (Prior et al, 1996). In tomato field studies, ambient temperatures above 28°C
have also been shown to increase Rs infection rate due to an increase in root knot
nematode activity, which increases physiological stress on the host (Prior et al, 1996). At
cooler temperatures, Rs invasion becomes limited to mostly latent infections of tomato
secondary roots, and few visual bacterial wilt symptoms are observed (Prior et al, 1996).
Studies of Rs resistance in tomato cultivars show that high temperatures greatly increase
disease severity when compared to moderate temperatures (Krausz and Thurston, 1975).
However, the genetics of the mechanism of resistance to Rs is still unknown.
The objectives of this study were to (i) describe the mechanism of resistance to
bacterial wilt in flue-cured tobacco cultivars based on histological studies, (ii) determine
determine the effect of temperature on growth rate of Rs strain AW1-gfp38 in culture.
Histological studies were conducted using fluorescent microscopy with strain AW1-
gfp38 which expresses green fluorescent protein. Ambient temperature was controlled in
MATERIALS AND METHODS
Tobacco cultivars. Six commercial flue-cured tobacco cultivars with varying levels of R. solanacearum resistance were used: K346 (high resistance), Speight 168
(high resistance), NC71 (moderate resistance), K326 (low resistance), RJR15 (low
resistance), and RJR75 (low resistance). K346, K326, Speight 168, and NC 71 have
resistance originating from line T.I. 448A and RJR15 and RJR75 have a low level of
resistance derived from an unknown pedigree. Resistance of these cultivars to Rs has been
determined in seven replicated field evaluations conducted since 2005 in nurseries naturally
infested with Rs in North Carolina.
Tobacco plants were grown using the method described by Katawczik and Mila
(Katawczik and Mila, 2011). Seeds of the six flue-cured tobacco cultivars were grown in
12-cell tissue culture (TC) plates (Corning Incorporated, Corning, NY). In each cell, 3.5
cm3 of perlite (<2 mm in size) and 1.5 ml of sterile deionized water was added. Four to
six seeds were added to each cell (one cultivar per two cells; Fig. 1). Deionized water
was inserted into the space between the cells for additional moisture. Each TC plate was
covered to create a moist chamber. TC plates were then incubated at room temperature
(20 to 25ºC) with 12 h of light and 12 h of darkness. A 0.5 ml solution of 200-ppm N
fertilizer (Bulldog water-soluble fertilizer 20-10-20; Chilean Nitrate Corporation,
Northfolk, VA) was added to every cell of each TC plate 10 days after seeding and once a
week thereafter. TC plates were watered once a week, or as needed.
al, 2001). Inoculum was prepared by growing the R. solanacearum AW1-gfp38 on
tetrazolium chloride (TZC) solid medium (Kelman, 1954) for 48 h at 28ºC. Bacterial
cells were harvested from the TZC medium and suspended in sterile distilled water. The
bacterial suspension was diluted with sterile distilled water to 2 x 108 cells/ml (108 colony
forming units (CFU)/ml) using a spectrophotometer. Twenty-five day old seedlings were
inoculated with 0.5 ml of the bacterial suspension per cell.
Seedling roots were wounded before inoculation to create entry points for the
pathogen. Tobacco roots were wounded by stabbing a sterilized scalpel around each
seedling, separating pieces of the root from the seedling and creating openings into the
root. Immediately after the tobacco seedling roots were injured, 0.5 ml of the bacterial
suspension was added to each cell of the plate. 15 TC plates were inoculated without
wounding the roots. These plates were used to compare the effects of wounding and non-
wounding on infection progression and it was conducted only at 30ºC.
Plant growth conditions. Following inoculation, TC plates were placed into 52- by-27-by-16-cm plastic containers (Sterlite Co., Townsend, MA). The containers were
lined with wet paper towels and covered with a 55-by-46-cm polyvinyl-chloride
laboratory wrap (Fisher Scientific Company, Pittsburgh, PA) to create a moister chamber.
The plastic containers were separated and incubated in three different growth chambers
set at 10, 20, and 30ºC. One TC plate with non-inoculated tobacco seedlings was placed
into each growth chamber as a negative control. Tobacco plants were watered once a
Sampling and processing for microscopy. The progression of Rs strain AW1- gfp38 in the plant root and stem tissues was observed under an Olympus BX 60
microscope (Olympus Corp., Tokyo) with a GFP filter. For each sampling time, whole roots
and stems of the flue-cured tobacco seedlings were observed. Flue-cured tobacco seedlings
were examined 12 and 24 hours after inoculation and every 24 h for 14 days thereafter,
totaling 15 observations. Three tobacco seedlings from each of the six cultivars were
arbitrarily selected and examined on each observation time. Seedlings were washed in sterile
distilled water to remove excess dirt, perlite, and seed coats from the root system. The
leaves from each seedling were removed, leaving the epicotyl, hypocotyl, and the whole
root system intact. Each plant was placed on a glass slide and observed by fluorescent
microscopy. The histological studies were repeated once.
The effect of temperature on Rs strain AW1-gfp38 growth. Experiments were
conducted to determine the effect of three temperatures (30, 20, 10C) on Rs strain AW1-
gfp38 growth. Rs strain AW1-gfp38 was grown on solid TZC medium. A 1l loopfull
of bacteria was added to 30 ml of casamino acid peptone glucose (CPG) broth in 50 ml
centrifuge tubes (Becton, Dickinson and Company, Franklin Lakes, NJ). Bacterial
growth was measured as colony forming units (CFU) by dilution plating onto nutrient
agar (8 g nutrient broth and 20 g granulated agar in 1 L of distilled water). Plates were
then incubated at 28C for 48 h before counting colonies. Dilution plating was conducted
on days 0, 2, 4, 6, 8, 10, 12, and 20 of incubation at each temperature. The experiment
on Rs strain AW1-gfp38 growth were analyzed using the general linear model procedure
(PROC GLM) of the Statistical Analysis System (version 9.1; SAS Institute Inc., Cary,
NC). F tests were used to determine the significance of temperature and day of
incubation on the growth rate of the bacterial strain. Bartlett’s test for homogeneity of
variances was performed to examine if there were significant differences between the two
RESULTS
Rs infection and progression at 30C. Approximately 12 hours after
inoculation, the bacteria were observed colonizing the surface of the roots, especially the
longitudinal grooves between the epidermal cells (Figure 1A). We also observed that
multiple epidermal cells, especially root hairs of secondary roots, were completely
colonized (Figure 1B). Epidermal cells were often infected around a damaged root site
(Figure 1C). However, frequently epidermal cells appeared to be infected randomly
(Figure 1D). Bacteria that had entered root hairs often colonized the end of the root hair,
where no wounding had occurred (Figure 1D). These observations were similar in all six
tobacco cultivars where seedlings were wounded before inoculation. The bacteria were
also observed colonizing the elongation zone, the axils of emerging secondary roots, and
cells around the root tip (Figure 1F).
One to two days after inoculation the pathogen began to enter the cortical and
xylem tissue of the secondary roots. Cortical infection was primarily seen at the
wounded root sites (Figure 1C) and the axils of the secondary roots. Secondary root
xylem vessel infection primarily began at cut root sites. Wounded root sites were the
sites that the pathogen had its greatest frequency at gaining entry into the vascular tissue.
All tobacco cultivars had xylem infection in a secondary root 2 days after inoculation.
During this time period, we observed that the pathogen had progressed from the outer
tissue infection sites to the inner tissues, specially the xylem vessels (Figure 2A).