INTRODUCTION
Tooth number is highly regulated in mammals. Species such as mice have a highly reduced dentition consisting of only molars and incisors, whereas the development of other mammalian tooth types has been lost during evolution. Evidence from three-dimensional reconstructions of early tooth development in mouse embryos has identified tooth primordia in the diastema, a toothless region between the incisors and molars, which are initiated but are later suppressed by apoptosis (Tureckova et al., 1996; Peterkova et al., 2002). These primordia are believed to be the vestiges of teeth lost during mouse evolution and suggest that a mechanism for suppression of tooth formation in the diastema involves selective apoptosis of tooth primordia (Peterkova et al., 2003). In the early oral cavity, Shhexpression is restricted to the thickenings of oral epithelium (dental placodes), from which the tooth germs arise (Bitgood and McMahon, 1995; Dassule and McMahon, 1998; Hardcastle et al., 1998). A loss of Shh activity in these regions at embryonic day (E)10 results in a lack of epithelial cell proliferation and a failure of tooth bud formation (Cobourne et al., 2001). Once the early tooth bud has formed, continued Shh activity is required within discrete regions of the tooth germ epithelium and mesenchyme for normal growth and morphogenesis of the developing tooth (Dassule et al., 2000; Gritli-Linde et al., 2002; Jeong et al., 2004). Significantly, Shhtranscriptional activity is lacking in the diastema and, thus, during normal development of the
murine dentition, suppression of Shh pathway activity in the diastema may play an important role in controlling primary tooth number (Cobourne et al., 2004).
Primary cilia have recently been shown to play a crucial role in Shh signalling (Rohatgi et al., 2007; Caspary et al., 2007; Singla and Reiter, 2006; Huangfu and Anderson, 2005). The Shh receptor Ptch1, its activator Smo and the transcriptional effector Gli1 are all found to localize in cilia, suggesting that the Shh signalling pathway is active in cilia and that cilia are required for Shh signal transduction (Rohatgi et al., 2007; Haycraft et al., 2005). Intraflagellar transport (IFT) proteins are highly conserved in all ciliated eukaryotic cells, and mutation of the proteins that comprise the IFT process result in defects in cilia formation in all organisms studied to date (Rosenbaum and Witman, 2002; Pan et al., 2005; Scholey and Anderson, 2006). Mice with null mutations in Ift88, which encodes the IFT protein polaris, lack cilia on all cells and die mid-gestation with severe defects in neural tube patterning and closure, polydactyly, and left-right axis determination (Murcia et al., 2000; Zhang et al., 2003). Defects in the neural tube have been associated with loss of Shh signalling regulation through disruption of Gli activation, whereas the limb patterning defects are associated with a gain of Shh activity as a result of a loss of Gli3 repression (Huangfu and Anderson, 2005; Haycraft et al., 2005; Michaud and Yoder, 2006). Similar defects have also been reported for mice with severe mutations in other IFT proteins (Huangfu and Anderson, 2005; Liu et al., 2005; May et al., 2005). Tg737orpk is a hypomorphic allele of polaris; homozygous Tg737orpk mice exhibit a complex pathology, including kidney and pancreatic cysts, preaxial polydactyly, and supernumerary teeth (Zhang et al., 2003).
We examined the development of vestigial diastema tooth primordia in Tg737orpkmice and found ectopic tooth formation that correlates with ectopic Shh signalling activity in the diastema mesenchyme. Using Wnt1-Cre and keratin(K)5-Cre to conditionally inactivate polaris in tooth mesenchyme and epithelium, respectively, we show that this cilia-mediated Shh signalling is required only in
Primary cilia regulate Shh activity in the control of molar
tooth number
Atsushi Ohazama1, Courtney J. Haycraft2, Maisa Seppala1, James Blackburn1, Sarah Ghafoor1, Martyn Cobourne3, David C. Martinelli4, Chen-Ming Fan4, Renata Peterkova5, Herve Lesot6, Bradley K. Yoder2and Paul T. Sharpe1,*
Primary cilia mediate Hh signalling and mutations in their protein components affect Hh activity. We show that in mice mutant for a cilia intraflagellar transport (IFT) protein, IFT88/polaris, Shh activity is increased in the toothless diastema mesenchyme of the embryonic jaw primordia. This results in the formation of ectopic teeth in the diastema, mesial to the first molars. This phenotype is specific to loss of polaris activity in the mesenchyme since loss of Polaris in the epithelium has no detrimental affect on tooth development. To further confirm that upregulation of Shh activity is responsible for the ectopic tooth formation, we analysed mice mutant for Gas1, a Shh protein antagonist in diastema mesenchyme. Gas1mutants also had ectopic diastema teeth and
accompanying increased Shh activity. In this context, therefore, primary cilia exert a specific negative regulatory effect on Shh activity that functions to repress tooth formation and thus determine tooth number. Strikingly, the ectopic teeth adopt a size and shape characteristic of premolars, a tooth type that was lost in mice around 50-100 million years ago.
KEY WORDS: Intraflagellar transport, Cilia, Shh, Supernumerary tooth, Tooth development, Tooth number, Orpk, Tg737, Gas1, Epithelium, Mesenchyme, Mouse
Development 136, 897-903 (2009) doi:10.1242/dev.027979
1Department of Craniofacial Development, Dental Institute, King’s College London,
London SE1 9RT, UK. 2Department of Cell Biology, University of Alabama at
Birmingham, Birmingham, AL 35294-0005, USA. 3Department of Craniofacial
Development and Orthodontics, Dental Institute, King’s College London, UK.
4Department of Embryology, Carnegie Institution of Washington 3520 San Martin
Drive, Baltimore, MD 21218, USA. 5Department of Teratology, Institute of
Experimental Medicine, Academy of Sciences of the CR, Prague, Czech Republic.
6INSERM UMR977, Dental School, Strasbourg University, Strasbourg, France.
*Author for correspondence (e-mail: paul.sharpe@kcl.ac.uk)
Accepted 21 January 2009
D
E
V
E
LO
P
M
E
N
the mesenchyme. We further show that mutants in the Shh regulatory protein Gas1 also develop diastema teeth as a result of ectopic Shh activity in diastema mesenchyme. Therefore, in the context of control of tooth number, cilia are involved in the repression of Shh activity in diastema mesenchyme cells.
MATERIALS AND METHODS
Production and analysis of mice
Tg737orpkand Tg737Δ2-3β-galwere produced as described previously (Moyer et al., 1994). Gas1mutant mice, polarisLoxPmice, K5-Cre and Wnt1-Cre mice were produced as described previously (Chai et al., 2000; Lee et al., 2001; Ramirez et al., 2004; Haycraft et al., 2007).
In situ hybridisation
Radioactive section in situ hybridisation using 35S-UTP radiolabelled riboprobes was carried out as described previously (Ohazama et al., 2008). Whole-mount in situ hybridisation was carried out as described previously (Pownall et al., 1996).
Micro-CT analysis
Heads were scanned with Explore Locus SP (GE Pre-clinical imaging) high resolution Micro-CT with a voxel dimension of 8 μm. Three-dimension reconstruction was performed by the three structure analysis software Microview (GE Pre-clinical imaging).
β-Galactosidase (β-gal) staining
Embryo heads were processed as previously described (Taulman et al., 2001).
Epithelial three-dimension reconstruction
Epithelial three-dimension reconstruction of mandible dental and adjacent oral epithelium were performed as previously described (Lesot et al., 1996).
Transmission electron microscopy (TEM) analysis
Heads were fixed in 2.5% glutaraldehyde (phosphate buffer) overnight at 4°C and postfixed in 2% osmium tetroxide (Millonigs buffer) after washing by buffer. Specimens were dehydrated through a graded series of ethanols and embedded in Epon 812-equivalent (TAAB Lab). Semithin sections (1 μm) were stained with Toluidine Blue for light microscopy analysis. Ultrathin sections (40-90 nm) were cut, stained with uranyl acetate and lead citrate and examined with a Hitachi H7600 transmission electron microscope.
Immunohistochemistry
Sections were incubated with antibody to acetylated α-tubulin or γ-tubulin (Sigma). Alexa488 or Alexa594 was used (Molecular probe) for detecting primary antibody. Immunohistochemistry for Polaris or Shh were performed as previously described (Rosenbaum and Witman 2002; Martinelli and Fan, 2007; Gritli-Linde et al., 2001).
RESULTS AND DISCUSSION Shh activity in the diastema
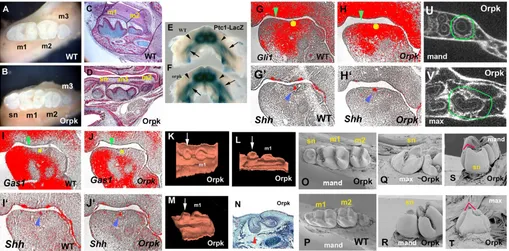
[image:2.612.53.562.360.611.2]Primary cilia have been shown to be important mediators of Shh activity and, as Shh controls tooth initiation, we examined tooth development in Tg737orpkadult mice, which contain a hypomorphic mutation in the IFT protein polaris. We identified teeth mesial to the
Fig. 1. Lower molar tooth phenotypes and Shhsignalling in Tg737orpkhomozygous mutant mice.(A-D) Whole teeth (A,B) and sagittal sections (C,D) show that supernumerary teeth develop mesial to the first molars (sn in B,D) in mutants. (E,F) Increase of Ptch1-lacZstaining in the mandible of Tg737orpkmutants with Ptch1-lacZallele at E13.5. Ptch1-lacZstaining was expanded into the diastema region (arrowhead). Arrows
indicate molar germs. (G-J) Radioactive in situ hybridisation on sagittal sections showing Gli1(G,H), Gas1(I,J) and Shh(G’,H’,I’,J’) expression in mandibles of embryo heads at E12.5. (G,H,I,J) Yellow circles represent positions of Shhexpression in adjacent sections (blue arrowheads in G’,H’,I’,J’). Gli1expression (H) was expanded into the diastema and Gas1(J) was downregulated in the diastema of Tg737orpkmutants (green
arrowheads). (K-N) Sagittal sections (N) and three-dimensional reconstructions (K-M) of the dental epithelium of the mandible molar region at E15.5 (N) and E16.5 (K-M) in Tg737orpkmutants. The supernumerary tooth buds (arrows in K-M) were observed in the position of the mesial swellings
(arrowhead in N). (O-T) SEM analysis shows that the lingual cusp of maxillary supernumerary tooth (red line in T) is more prominent in comparison with that of mandibular supernumerary tooth (red line in S). (U,V) Horizontal micro-CT sections of Tg737orpkshow that maxillary supernumerary
teeth have concave roots (circle in V) and in mandibles show round roots (circle in U). m1, first molar; m2, second molar; m3, third molar; mand,
mandible jaw; max, maxillary jaw.
D
E
V
E
LO
P
M
E
N
first molar in all four jaw quadrants with 100% penetrance that were not present in wild-type animals (Fig. 1A-D) (Zhang et al., 2003). Because the development of ectopic digits in Tg737orpkmutants is due to alternations in Shh signalling in the limb bud, we examined the expression of Ptch1and Gli1, which are upregulated in response to Shh signal transduction, in the region of ectopic tooth development in the Tg737orpkhomozygous mutants at E12.5 and E13.5. To determine the expression pattern of Ptch1, we generated Tg737orpkhomozygous mutants that were also heterozygous for a Ptch1-lacZallele and examined the activity of β-gal. At E13.5, Ptch1-lacZstaining was increased in the incisor and molar areas of Tg737orpkhomozygous mutants and expanded into the diastema, which is normally devoid of Shh signalling activity (Fig. 1E,F). Gli1 expression was also expanded into the diastema region (Fig. 1G-H⬘) and expression of Gas1, a Shh antagonist in tooth development that is downregulated by Shh activity, was reduced (Fig. 1I-J⬘) (Cobourne et al., 2004; Martinelli and Fan, 2007). The expansion of Ptch1and Gli1 expression into the diastema correlates with the placement of premolar-like tooth buds that develop in the Tg737orpk mutants. These observations suggest that an absence of the IFT protein polaris results in a Shh gain-of-function phenotype. In limb buds, hypomorphic mutation of polaris leads to a series of changes in Shh signalling that include disruption of Gli3 processing, resulting in severe polydactyly similar to that found in Gli3mutants (Haycraft et al., 2005; Litingtung et al., 2002). The expression of Shh is downregulated in the midline of polaris null mutants, which show severe midline defects (Murcia et al., 2000). It has been suggested that the loss of Gli activators, which play a major role in neural tube
patterning, is consistent with the loss of ventral neural tube cells in the IFT protein mutant mice, whereas loss of Gli3 repressor function, which is more important in digit patterning, is consistent with the formation of extra digits in the IFT protein mutants (Huangfu and Anderson, 2005; Michaud and Yoder, 2006). The absence of an IFT protein can therefore affect both positive and negative aspects of Shh signalling, depending upon the particular Gli activity. In the context of the control of tooth number, increased Shh activity is thus consistent with loss of Gli3 repressor which is prominently expressed in the wild-type diastema (see Fig. S1 in the supplementary material) (Huangfu and Anderson, 2005; Haycraft et al., 2005; Michaud and Yoder, 2006).
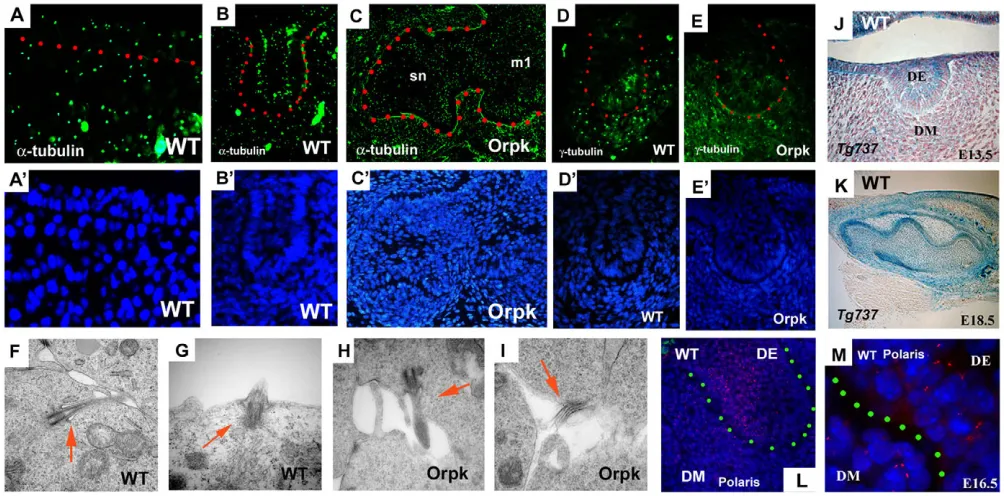
[image:3.612.57.559.383.631.2]Cilia and cilia proteins in tooth development To investigate the cells that require fully functional cilia during tooth formation, we first determined the location of cilia in tooth germs using a cilia marker, acetylated α-tubulin and a marker of basal bodies of cilia, γ-tubulin. Cilia were found in tooth epithelium and mesenchyme cells at early stages of development (Fig. 2A,B,D). Cilia were observed on both epithelial and mesenchymal cells of supernumerary and endogenous tooth germs in Tg737orpkmice (Fig. 2C,E). Transmission electron microscopy (TEM) was performed to investigate the structure of the cilia on tooth cells. Cilia were found on dental epithelial and mesenchymal cells and no obvious morphological differences were visible between Tg737orpkand wild-type cells (Fig. 2F-I). We next examined Tg737expression and polaris protein localisation in tooth development to confirm that this protein is a component of cilia on dental cells. Tg737was expressed
Fig. 2. Cilia, and Tg737 expression and localisation in tooth germs.(A-C) Acetylated α-tubulin-positive cells (immunohistochemistry) are found in both tooth epithelium and mesenchyme of wild type (A,B). There are no significant differences between wild-type and Tg737orpkmice, or
between first molar (m1) and supernumerary tooth (sn) in Tg737orpkmice (C). (D,E)γ-Tubulin-positive cells are observed in both tooth epithelium
and mesenchyme of wild-type (D) and Tg737orpkmice (E). (F-I) TEM analysis of molar teeth show that cilia were found in both epithelium (G) and
mesenchyme (F) of molar tooth germs in wild-type (F,G) and in molar tooth epithelium (H) and mesenchyme (I) in Tg737orpk(arrows). (J,K)β-Gal
expression in molar of Tg737Δ2-3β-galshow that Tg737 was expressed in both epithelium and mesenchyme. (L,M) Immunohistochemistry sections
also show that polaris protein is localised in both dental epithelium (DE) and dental mesenchyme (DM). Frontal sections (A,B,D-J,L,M) and sagittal sections (C,K) of lower molars at E10.5 (A), E13.5 (B,D-J,L), E14.5 (C), E16.5 (M) and E18.5 (K) of wild-type littermates (A,B,D,F,G,J-M) and Tg737orpkmutants (C,E,H,I). Dots represent boundaries between epithelium and mesenchyme (A,M). Tooth epithelium is outlined in red (B-E) or
green (L). Nuclei are shown in blue by DAPI (A’-E’,L,M).
D
E
V
E
LO
P
M
E
N
throughout the dental epithelium and in the underlying mesenchyme, as determined by expression of β-gal in embryos heterozygous for the Tg737Δ2-3β-galallele (Fig. 2J,K). In agreement with the β-gal expression pattern, immunolocalisation of polaris showed cilia were present on both tooth epithelial and underlying dental mesenchyme cells (Fig. 2L,K).
Polaris in epithelial and mesenchymal cells of tooth germs
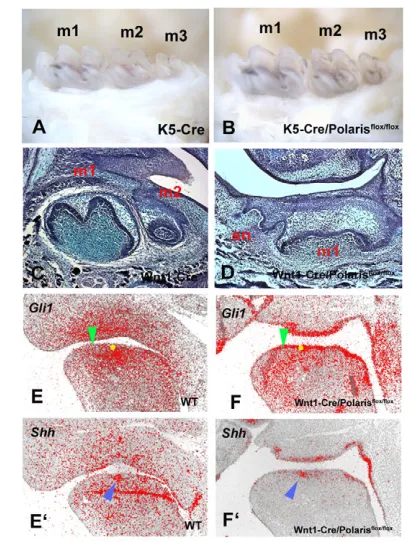
As cilia are present on both epithelial and mesenchymal cells of early tooth germs, the effect of reduction in polaris in Tg737orpk mice could be mediated in either or both cell types. Conditional loss-of-function of polaris in epithelial cells was investigated by crossing polaris floxed mice with Cre mice. The K5-Cre/polarisflox/floxmice survived but there was no evidence of supernumerary teeth, although they did show polydactyly (Fig. 3A,B; data not shown). To test whether polaris is required in dental mesenchyme, we crossed polaris floxed mice with Wnt1-Cre mice. Wnt1-Wnt1-Cre/polarisflox/floxmice died at birth and showed severe craniofacial abnormalities (data not shown). Supernumerary tooth germs were observed mesial to first molars in the Wnt1-Cre/polarisflox/floxmice (Fig. 3C,D). The expansion
of Gli1 expression into the diastema was seen in the Wnt1-Cre/polarisflox/floxmice as also observed in Tg737orpkmice (Fig. 3E-F⬘). This confirmed that the formation of supernumerary teeth results from loss of polaris in dental mesenchyme. Although Shh is only expressed in the epithelium during tooth development, Ptch1and other Shh pathway molecules are expressed in both epithelial and mesenchymal cells in early tooth primordia. Moreover, a role for Shh in dental epithelial cells is confirmed by abnormal cell differentiation in K14-Cre/Shh and K14-Cre/Smo conditional mutant mice; however, the teeth that develop in K5-Cre/polarisflox/floxmice undergo apparently normal morphogenesis and cytodifferentiation (Dassule et al., 2000; Gritli-Linde, 2002). This suggests that cilia-mediated Shh activity mediated by polaris is dispensable in dental epithelial cells, whereas Shh signalling in early dental mesenchyme requires polaris-mediated cilia function. Despite the expectation that cilia would be absent in tooth mesenchymal cells in Wnt1-Cre/polarisflox/floxmice, α-tubulin immunohistochemistry did show cilia present but in reduced numbers (see Fig. S2 in the supplementary material). This may represent incomplete expression of Cre or a contribution of non-neural crest cells (Chai et al., 2000).
Tooth phenotype in Gas1 mutant mice
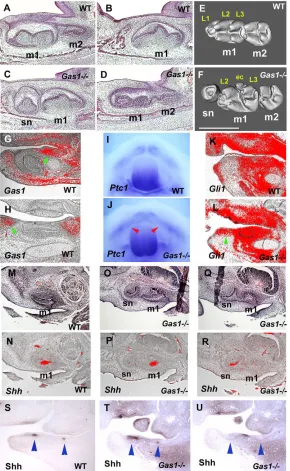
In order to confirm that ectopic Shh signalling in the diastema was the direct cause of supernumerary tooth formation, we examined the dentition of mice with a targeted disruption in the Gas1gene, which we has previously identified as an inhibitor of Shh in
diastema mesenchyme. Gas1 is expressed in diastema
mesenchyme and non-dental mesenchyme, but not in tooth germs (Fig. 4G,H) (Cobourne et al., 2004). Supernumerary teeth in the diastema region of both the maxilla and mandible were observed with 100% penetrance in Gas1mutants (Fig. 4A-F). These teeth, positioned mesial to the first molars, were identical to those observed in Tg737orpk mice. The presence of these teeth was associated with ectopic Shh signalling activity within the diastema region, demonstrated by analysis of Ptch1, Gli1 and Shh expression, and Shh protein localization, as observed in the Tg737orpkmutants (Fig. 4I-U). Significantly, we also found that Gas1expression was downregulated in diastema mesenchyme of Tg737orpkmutant embryos (Fig. 1I-J⬘). Ectopic Shh activity in the
diastema mesenchyme was thus observed in two different mutants, both resulting in ectopic tooth formation mesial to the first molars. In Gas1mutants, the effect on Shh signalling can be attributed to a direct consequence of loss of an antagonist, whereas Tg737orpkmay be indirect following reduction in polaris that results in loss of Gas1.
[image:4.612.337.542.57.328.2]In the developing tooth, Shh signalling is required for initiation and subsequent downgrowth of the dental epithelium into the underlying mesenchyme to control development of the teeth (Sarkar et al., 2000; Cobourne et al., 2001); in the diastema, Shh signalling is normally specifically inhibited by Gas1 (Cobourne et al., 2004). When Gas1 is lost either directly, or indirectly in the mesenchyme of Tg737orpkmice, Shh signalling is increased. As a result, the vestigial tooth rudiments mesial to the first molar do not undergo apoptosis, but survive and develop into a functional tooth. This suggests that modulation of Shh signalling has played an important part in the evolution of dental patterns by regulating tooth number. Interestingly, in the talpid2chick mutant, ectopic activation of Shh signalling in the developing oral cavity correlates with the formation of reptile-like teeth [reminiscent of avian ancestors (Harris et al., 2006)]. The requirement for Shh signalling in the mesenchyme to regulate events in the epithelium also identifies a mesenchyme-to-epithelium interaction in this process.
Fig. 3. Molar teeth of K5-Cre/polarisflox/floxmice and
Wnt1-Cre/polarisflox/floxmice.(A,B) There are no supernumerary teeth in
K5Cre (A) and K5-Cre/polarisflox/flox(B) mice. (C) Sagittal sections showing first molar (m1) and second molar (m2) in mandible. (D) Supernumerary teeth (sn) were observed in Wnt1-Cre/polarisflox/flox mice. (E-F⬘) Radioactive in situ hybridisation on sagittal sections showing Gli1(E,F) and Shh(E’,F’) expression in mandibles of embryo heads. (E,F) Yellow circles represent positions of Shhexpression in adjacent sections (blue arrowheads in E’,F’). Gli1expression (E,F) was expanded into the diastema of Wnt1-Cre/Polarisflox/floxmice (green arrowheads). Images show lower mandibles of E11.5 (E-F’), newborn (C,D) and adult (A,B).
D
E
V
E
LO
P
M
E
N
Ectopic tooth formation mesial to the first molars has been observed in mice with mutation of the BMP/Wnt antagonist ectodin (Wise) and following misexpression of Eda and Edar (Mustonen et al., 2003; Kassai et al., 2005; Tucker et al., 2004). In both these cases, development of the molars is impaired. The formation of diastema teeth in the context of normal molar development is observed in Sprouty2 and Sprouty4 mutant mice, and has been interpreted as a role for suppression of FGF signalling in suppression of diastema tooth formation. In Sprouty4mutants, ectopic teeth are restricted to one quadrant with low penetrance; in Sprouty2mutants, 92% have bilateral, 5% have unilateral and 3% have no ectopic teeth in the mandible, whereas ectopic teeth are observed in less than 5% of the maxilla (Klein et al., 2006) (J.-M. Courtney and P.T.S, unpublished). We show here that ectopic Shh signalling in the diastema produces ectopic teeth with 100% frequency in all four jaw quadrants without affecting molar development and thus we propose
that Shh is the direct determinant of tooth initiation and that the roles of FGF, BMP and other signalling pathways in tooth suppression are mediated through Shh.
Premolar formation in the diastema
[image:5.612.51.341.60.531.2]Mouse first molar tooth buds form from a fusion of four epithelial swellings, the most mesial of which is excluded from the first molar tooth bud and undergoes apoptosis (Peterkova et al., 2002). We used histology and 3D reconstruction of E15.5 embryos to show that the supernumerary tooth primordia were observed in the position of the former mesial swellings, indicating that rather than being suppressed, these swellings continued to develop into independent teeth (Fig. 1K-N). The cyclin-dependent kinase inhibitor p21 provides a marker for diastema bud apoptosis and in Tg737orpkhomozygous embryos we found that p21expression was downregulated in diastema
Fig. 4. Molar tooth phenotypes of Gas1mutant mice. (A-F) Sagittal sections (A-D) and micro-CT analysis (E,F) show that supernumerary teeth develop mesial to the first molars (sn in C,F). Micro-CT analysis showed that supernumerary teeth were smaller than first molars and had fewer cusps (F). At the section level of the second molar, the supernumerary teeth were not visible (D). (G,H) Radioactive in situ hybridisation on sagittal sections, showing Gas1expression in mandibles of embryo heads at E13.5. Gas1was expressed in the diastema region (arrowheads), whereas it was absent in tooth regions (incisor in G; molar in H). (I,J) Whole-mount in situ hybridisation showing Ptch1expression in the mandibular process of embryo heads at E12.5. Ptch1expression was expanded into the diastema region in Gas1mutants (arrowheads in J). (K,L) Radioactive in situ hybridisation on sagittal sections showing Gli1expression in mandibles of embryo heads at E14.5. Gli1expression was expanded into the diastema region of Gas1mice (arrowhead in L). (N,P,R) Radioactive in situ hybridisation on sagittal sections showing Shhexpression in maxillae of embryo heads at E14.5. Shhexpression was found in both supernumerary (sn in R) and the first molars in Gas1 mutants (m1 in P). (M,O,Q) Haematoxylin and Eosin stained sections adjacent to N, P and R, respectively. Shh expression could not be seen in the first molar at the section level of the supernumerary tooth (R).
(S-U) Immunohistochemistry on sagittal sections showing Shh protein localisation in mandibles of embryo heads at E12.5. Shh localisation was found in the diastema region of Gas1mice (between arrowheads in U), as well as endogenous tooth germs (arrowheads in T); it was not observed in the diastema of wild type (between arrowheads in S). Shh protein deposition could not be seen in the incisors and molars at the section level of the diastema showing Shh expression (U). m1, first molar; m2, second molar; sn, supernumerary tooth. Lingual cusps (L1-L3) and extra cusp (ec). Scale bar: 500μm.
D
E
V
E
LO
P
M
E
N
epithelial buds (see Fig. S3 in the supplementary material). The premolar-like teeth in Tg737orpkthus form from the survival of vestigial swellings.
To verify whether the supernumerary teeth developed with a molar-like or incisor-like programme, we performed in situ hybridisation at E15.5 for Barx1, which is specifically expressed in developing molars, in addition to dHAND(Hand2 – Mouse Genome Informatics) and Islet1 (Isl1– Mouse Genome Informatics), which are expressed in incisors (Thomas et al., 1998; Tucker et al., 1998; Mitsiadis et al., 2003). The supernumerary teeth expressed Barx1in a similar manner to the developing first and second molars, but did not express Hand2 or Isl1 (see Fig. S4 in the supplementary material). These data support the identification of these ectopic teeth as molar-like, and confirm they develop from oral epithelium in the molar region.
In several other mouse mutants that have been reported to develop extra teeth, the form and position of the molars are highly abnormal, which makes analysis of the morphology of diastema teeth difficult (Mustonen et al., 2003; Kassai et al., 2005). Interestingly, the molar and incisor teeth of homozygous Tg737orpk mutants showed no major abnormalities in shape, size or position. The supernumerary teeth were smaller than the first molars, had fewer cusps and a cusp pattern that was consistent with a premolar-like identity. The lingual cusps of maxillary supernumerary teeth were more prominent in comparison with the lingual cusps of mandibular supernumerary teeth (Fig. 1O-T). Furthermore, the supernumerary teeth in the maxilla showed a concave-shape root, whereas those in the mandible had a round root shape (Fig. 1U,V). These differences in root and cusp shapes are a consistent feature of human premolars (Ash and Nelson, 2003; Berkovitz et al., 2002).
The fact that the extra teeth that develop as a result of ectopic Shh form a shape that is appropriate for their position indicates that despite having lost the ability to form a premolar tooth shape over 50-100 million years ago, the mouse embryo has retained all the genetic information necessary to make this tooth type (Meng et al., 1994; Ji et al., 2002). This supports the concept that loss of tooth types during evolution results from suppression of tooth initiation. This is also consistent with the specification of tooth shape being provided by expression of specific homeobox genes such as Dlxand Barx1in the mesenchyme prior to initiation (Tucker and Sharpe, 2004). These gene expression domains in the mesenchyme of the facial primordia provide cells with positional information. This information is used to direct cells to follow particular pathways of hard tissue morphogenesis. In animals where tooth development is suppressed, such as birds or the mouse diastema, this information is still required for bone and cartilage morphogenesis. Thus, what has been ‘lost’ in the evolution of mice is the ability to initiate and maintain epithelial tooth buds, mesial to the first molars. When ectopic tooth development is stimulated as a result of ectopic Shh signalling, the tooth primordia develop from mesenchyme cells with the information appropriate for that position.
We thank Karen Liu and Isabelle Miletich for critical reading of the manuscript, Deepak Srivastava for dHANDplasmid, Andrew McMahon for Shhplasmid, Ken Brady for TEM analysis, and Chris Healy for micro-CT analysis. Maisa Seppala is a European Union Marie Curie Early Stage Fellow (grant number MEST-CT-2004-504025). Funding for this work was provided by the Medical Research Council, The Wellcome Trust, GACR-304/07/0223 and MSM 0021620843. Atsushi Ohazama is an Research Councils UK Fellow. Deposited in PMC for release after 6 months.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/6/897/DC1
References
Ash, M. M. and Nelson, S. J.(2003). Wheeler’s Dental Anatomy, Physiology, and Occlusion. Philadelphia, PA: W. B. Saunders.
Berkovitz, B. K. B., Holland, G. R. and Moxham, B. J.(2002). Oral Anatomy, Histology and Embryology. St Louis, MO: Mosby.
Bitgood, M. J. and McMahon, A. P.(1995). Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 172, 126-138.
Caspary, T., Larkins, C. E. and Anderson, K. V.(2007). The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell12, 767-778.
Chai, Y., Jiang, X., Ito, Y., Bringas, P., Jr, Han, J., Rowitch, D. H., Soriano, P., McMahon, A. P. and Sucov, H. M.(2000). Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development127, 1671-1679.
Cobourne, M. T., Hardcastle, Z. and Sharpe, P. T.(2001). Sonic hedgehog regulates epithelial proliferation and cell survival in the developing tooth germ. J. Dent. Res. 80, 1974-1979.
Cobourne, M. T., Miletich, I. and Sharpe, P. T.(2004). Restriction of sonic hedgehog signalling during early tooth development. Development131, 2875-2885.
Dassule, H. R. and McMahon, A. P.(1998). Analysis of epithelial-mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev. Biol.
202, 215-227.
Dassule, H. R., Lewis, P., Bei, M., Maas, R. and McMahon, A. P.(2000). Sonic hedgehog regulates growth and morphogenesis of the tooth. Development
127, 4775-4785.
Gritli-Linde, A., Lewis, P., McMahon, A. P. and Linde, A.(2001). The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev. Biol. 236, 364-386.
Gritli-Linde, A., Bei, M., Maas, R., Zhang, X. M., Linde, A. and McMahon, A. P.(2002). Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development129, 5323-5337.
Hardcastle, Z., Mo, R., Hui, C. C. and Sharpe, P. T.(1998). The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development
125, 2803-2811.
Harris, M. P., Hasso, S. M., Ferguson, M. W. and Fallon, J. F.(2006). The development of archosaurian first-generation teeth in a chicken mutant. Curr. Biol. 16, 371-377.
Haycraft, C. J., Banizs, B., Aydin-Son, Y., Zhang, Q., Michaud, E. J. and Yoder, B. K.(2005). Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 1, e53.
Haycraft, C. J., Zhang, Q., Song, B., Jackson, W. S., Detloff, P. J., Serra, R. and Yoder, B. K.(2007). Intraflagellar transport is essential for endochondral bone formation. Development134, 307-316.
Huangfu, D. and Anderson, K. V.(2005). Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA 102, 11325-11330.
Jeong, J., Mao, J., Tenzen, T., Kottmann, A. H. and McMahon, A. P.(2004). Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 18, 937-951.
Ji, Q., Luo, Z. X., Yuan, C. X., Wible, J. R., Zhang, J. P. and Georgi, J. A.(2002). The earliest known eutherian mammal. Nature416, 816-822.
Kassai, Y., Munne, P., Hotta, Y., Penttila, E., Kavanagh, K., Ohbayashi, N., Takada, S., Thesleff, I., Jernvall, J. and Itoh, N.(2005). Regulation of mammalian tooth cusp patterning by ectodin. Science309, 2067-2070.
Klein, O. D., Minowada, G., Peterkova, R., Kangas, A., Yu, B. D., Lesot, H., Peterka, M., Jernvall, J. and Martin, G. R.(2006). Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev. Cell11, 181-190.
Lee, C. S., May, N. R. and Fan, C. M.(2001). Transdifferentiation of the ventral retinal pigmented epithelium to neural retina in the growth arrest specific gene 1 mutant. Dev. Biol. 236, 17-29.
Lesot, H., Vonesch, J. L., Peterka, M., Tureckova, J., Peterkova, R. and Ruch, J. V.(1996). Mouse molar morphogenesis revisited by three-dimensional reconstruction. II. Spatial distribution of mitoses and apoptosis in cap to bell staged first and second upper molar teeth. Int. J. Dev. Biol. 40, 1017-1031.
Litingtung, Y., Dahn, R. D., Li, Y., Fallon, J. F. and Chiang, C.(2002). Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature418, 979-983.
Liu, A., Wang, B. and Niswander, L. A.(2005). Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132, 3103-3111.
Martinelli, D. C. and Fan, C. M.(2007). Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 21, 1231-1243.
May, S. R., Ashique, A. M., Karlen, M., Wang, B., Shen, Y., Zarbalis, K., Reiter, J., Ericson, J. and Peterson, A. S.(2005). Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol. 287, 378-389.
Meng, J., Wyss, A. R., Dawson, M. R. and Zhai, R.(1994). Primitive fossil rodent from Inner Mongolia and its implications for mammalian phylogeny.
Nature370, 134-136.
D
Michaud, E. J. and Yoder, B. K.(2006). The primary cilium in cell signaling and cancer. Cancer Res. 66, 6463-6467.
Mitsiadis, T. A., Angeli, I., James, C., Lendahl, U. and Sharpe, P. T.(2003). Role of Islet1 in the patterning of murine dentition. Development130, 4451-4460.
Moyer, J. H., Lee-Tischler, M. J., Kwon, H. Y., Schrick, J. J., Avner, E. D., Sweeney, W. E., Godfrey, V. L., Cacheiro, N. L., Wilkinson, J. E. and Woychik, R. P.(1994). Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science264, 1329-1333.
Murcia, N. S., Richards, W. G., Yoder, B. K., Mucenski, M. L., Dunlap, J. R. and Woychik, R. P.(2000). The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development127, 2347-2355.
Mustonen, T., Pispa, J., Mikkola, M. L., Pummila, M., Kangas, A. T., Pakkasjarvi, L., Jaatinen, R. and Thesleff, I.(2003). Stimulation of ectodermal organ development by Ectodysplasin-A1. Dev. Biol. 259, 123-136.
Ohazama, A., Johnson, E. B., Ota, M. S., Choi, H. J., Porntaveetus, T., Oommen, S., Itoh, N., Eto, K., Gritli-Linde, A., Herz, J. et al.(2008). Lrp4 modulates extracellular integration of cell signaling pathways in development. PLos One3, e4092.
Pan, J., Wang, Q. and Snell, W. J.(2005). Cilium-generated signaling and cilia-related disorders. Lab. Invest. 85, 452-463.
Peterkova, R., Peterka, M., Viriot, L. and Lesot, H.(2002). Development of the vestigial tooth primordia as part of mouse odontogenesis. Connect Tissue Res.
43, 120-128.
Peterkova, R., Peterka, M. and Lesot, H.(2003). The developing mouse dentition: a new tool for apoptosis study. Ann. NY Acad. Sci. 1010, 453-466.
Pownall, M. E., Tucker, A. S., Slack, J. M. and Isaacs, H. V.(1996). eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development122, 3881-3892.
Ramirez, A., Page, A., Gandarillas, A., Zanet, J., Pibre, S., Vidal, M., Tusell, L., Genesca, A., Whitaker, D. A., Melton, D. W. et al.(2004). A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis 39, 52-57.
Rohatgi, R., Milenkovic, L. and Scott, M. P.(2007). Patched1 regulates hedgehog signaling at the primary cilium. Science317, 372-376.
Rosenbaum, J. L. and Witman, G. B.(2002). Intraflagellar transport. Nat. Rev. Mol. Cell. Biol. 3, 813-825.
Sarkar, L., Cobourne, M. T., Naylor, S., Smalley, M., Dale, T. and Sharpe, P. T.
(2000). Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc. Natl. Acad. Sci. USA 97, 4520-4524.
Scholey, J. M. and Anderson, K. V.(2006). Intraflagellar transport and cilium-based signaling. Cell 125, 439-442.
Singla, V. and Reiter, J. F.(2006). The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313, 629-633.
Taulman, P. D., Haycraft, C. J., Balkovetz, D. F. and Yoder, B. K.(2001). Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol. Biol. Cell 12, 589-599.
Thomas, T., Kurihara, H., Yamagishi, H., Kurihara, Y., Yazaki, Y., Olson, E. N. and Srivastava, D.(1998). A signaling cascade involving endothelin-1, dHAND and msx1 regulates development of neural-crest-derived branchial arch mesenchyme.Development125, 3005-3014.
Tucker, A. and Sharpe, P.(2004). The cutting-edge of mammalian development: how the embryo makes teeth. Nat. Rev. Genet. 5, 499-508.
Tucker, A. S., Matthews, K. L. and Sharpe, P. T.(1998). Transformation of tooth type induced by inhibition of BMP signaling. Science282, 1136-1138.
Tucker, A. S., Headon, D. J., Courtney, J. M., Overbeek, P. and Sharpe, P. T.
(2004). The activation level of the TNF family receptor, Edar, determines cusp number and tooth number during tooth development. Dev. Biol. 268, 185-194.
Tureckova, J., Lesot, H., Vonesch, J. L., Peterka, M., Peterkova, R. and Ruch, J. V.(1996). Apoptosis is involved in the disappearance of the diastemal dental primordia in mouse embryo. Int. J. Dev. Biol. 40, 483-489.
Zhang, Q., Murcia, N. S., Chittenden, L. R., Richards, W. G., Michaud, E. J., Woychik, R. P. and Yoder, B. K.(2003). Loss of the Tg737 protein results in skeletal patterning defects. Dev. Dyn. 227, 78-90.