Prediction of Bainite Intervened in Ferrite-Pearlite Forging Steel
I. Modeling
Kouji Tanaka
1;2, Yasuhiro Yogo
1, Koukichi Nakanishi
1;2and Carlos Capdevila
31
Toyota Central R&D Labs., Inc., Nagakute, Aichi 480-1192, Japan 2CREST, Japan Science and Technology Agency, Tokyo 100-0004, Japan
3Centro Nacional de Investigaciones Metalu´rgicas (CENIM), CSIC, Avda. Gregorio del Amo, 8. 28040, Madrid, Spain
The successive ferrite () + pearlite (P) transformations from austenite () were modeled to predict the presence of bainite in as-forged medium-carbon manganese steels. The kinetics of diffusional transformations were calculated based on classical nucleation and growth theory coupled with CALPHAD multi-component thermodynamics. The description of the growth rate of proeutectoid-includes a time dependence due to the carbon enrichment in the remaining. The/interface was assumed to be in negligible-partitioned local equilibrium (NPLE). The kinetics calculation ofPnucleating on thesurface was integrated into themodel. Given the transformation temperature range in continuous cooling, the growth rate ofPwas also expressed in the NPLE constraint for/cementite. The concentration of untransformed(U) can be monitored and should be dependent on the extent of the preceding transformations. Thus, the energies available for the nucleation and diffusionless growth of bainitic-were evaluated from the thermodynamics of theUsingle-phase system, which is proposed as a method to predict the inclusion of bainite in the finalþPmicrostructure. [doi:10.2320/matertrans.MRA2008331]
(Received November 25, 2008; Accepted December 4, 2008; Published January 21, 2009)
Keywords: phase transformations, kinetic modeling, medium-carbon manganese steel, continuous-cooling, multicomponent thermodynamics, proeutectoid ferrite, pearlite, bainite
1. Introduction
Medium-carbon manganese steels (MCMS) are used in large quantities as raw materials for various hot-forged auto parts. Their microstructure consisting of proeutectoid ferrite () and pearlite (P) is controlled mostly by the C and Mn content of the steels and the cooling rates. The/Pfraction has a primary effect on the average hardness which should be balanced under the requirement of proof strength and mass productivity. The bainite (B), a mixture of bainitic ferrite (B) and fine cementite, is comparatively hard and undesired in theþPmicrostructure because it degrades machinabil-ity in finishing final products. From the standpoint of global production, it is of concern that the fluctuation range of chemical compositions of supplied steels has become larger. In addition, a faster cooling is expected due to space-saving factory design, and even a slightly higher Mn content, which has not been a problem to date, may cause the problem ofB
in the near future. Thus, a computational prediction of B
transformation would be very effective in reducing trial forgings with modified steel compositions and/or cooling schedules.
Many researchers followed the subunit model proposed by Bhadeshia and coworkers1–6)for the description of isothermal
B kinetics from austenite () grain boundaries. Still, the fundamentals of the transformation mechanism remain controversial. Apart from the complexity of the kinetics, the energies for the nucleation and growth of bainitic-(B) have been interpreted simply, and the criteria for B formation has been formulated from experimental BS temperatures.1,7)In light of the MCMS grades and practical cooling rates, it is reasonable to assume that the rest of the volume occupied by proeutectoidandPwould result inB. The chemistry of untransformed austenite (U) is altered by the partitioning during the preceding transformation. However, the above B criteria were calibrated for an
undercooled single phase, and there is no evidence as to whether they can be applied to a detached volume ofU. In order to develop a simple treatment for the Bfraction, this first paper aims to build a model that can accurately calculate the evolution of theþP fraction and the thermodynamic energies for the transformation of U into B during continuous cooling.
2. Model and Calculation Methods
2.1 Framework of the model
The inputs for the present model are chemical composi-tions,grain size (d0) and cooling rate.d0is strongly related to hot-working conditions and can be estimated using empirical equations.8) Yogo9) proposed a modified expres-sion that accounts for a short holding time in the forging process. Recently, the effect of the distribution ofd0has been incorporated into this kind of kinetic calculations.10) This should have a large effect, which will be discussed in the second paper.
Much effort has been devoted to understanding the kinetics of various phase transformations in steels. There are some integrated models11–14) that can reportedly predict the evolution of , P, widmansta¨tten-, and B. These models were based on classical nucleation and growth theories (CNGT), developed for low-carbon steel strips, and thus, focused on the prediction of the grain size which affects significantly the mechanical strength and elongation. For the sake of simplicity of analytical treatment, the present model basically follows the equations in CNGT. In particular, to predict the =P fractions accurately, the effects of Mn content of MCMS have been studied extensively with respect to the partitioning between the and product phases.
assumption of the additivity rule. More specifically, the increases in the transformedandPvolume were summed for small isothermal time-step along cooling schedules. And the thermodynamic condition was simultaneously evaluated forUrenewed in each time-step.
2.2 Multicomponent thermodynamic calculation It is important to calculate the thermodynamic effect of Mn on the stability of , a decisive factor for the phase transformation kinetics of steels. The CALPHADmethod is now the standard for calculations in multicomponent ther-modynamics. One of the commercial packages,ThermoCalc, was employed in the present modeling work with the aid of the programming interfaceTQ-i.
Coupled with the database TCFe3 and MOB2, the maximum driving force (Gmax) for precipitation, the equilibrium C composition sets (XC=, XC=, XC=), and the C diffusivities in (DC) are calculated for a required composition in the Fe-C-Si-Mn-Cr system. The para-equi-librium (PE) calculation subroutine inTQ-iwas used to get
XC=;PE and XC=;PE. Using other subroutines, we can set and delete diverse equilibrium conditions such as the status of partitioning in a precipitating phase. It has been found that the following procedures successfully yield the interface compositions under negligible-partitioned local equilibrium (NPLE), in this case, with reference to Mn.
(1) Calculate the initial þ equilibrium for the alloy compositions.
(2) Fix thestatus to zeromole. Delete the condition of initial C composition (XC0).
(3) Calculate equilibrium and the new bulk compositions will be on the=ðþÞboundary.
(4) Set the concentration of non-partitioning elements in (XMn) to the same value as the initial composition (XMn0). Delete the condition ofXMn0 for bulk.
(5) Calculate equilibrium and the new composition set for and will give the NPLE relation (XC=;NPLE
-XC=;NPLE).
(6) Get the activity of the system. Set the same for the activity of. Delete the condition set in (2) and change thestatus suspended (single phase).
(7) Calculate equilibrium and the bulk composition moves along the isoactivity line and gives the highest possible C concentration in for the-to-transformation to proceed (XC=;NPlimit).
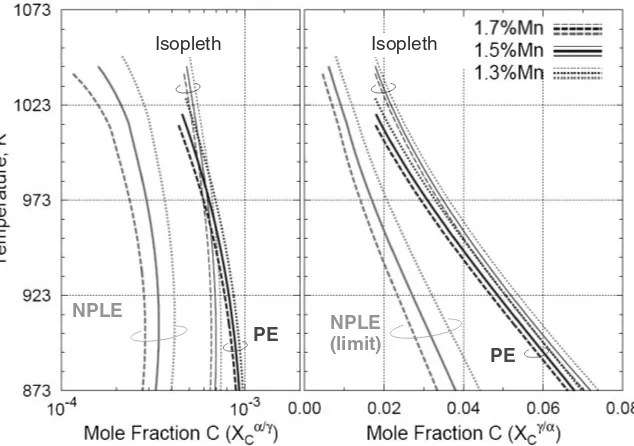
Figure 1 is an example of the calculated special / equilibrium interpolated on the Fe-Mn-C isopleth, vertical section of the constant Mn content. The alloy compositions are Fe-0.39%C-1.3%, 1.5%, 1.7%Mn, respectively, and clearly, this small difference in Mn content promotes a larger shift inXC=;NPlimit thanXC=;PE.
2.3 Kinetics of proeutectoid ferrite
The incubation time () of the isothermaltransformation was calculated in eq. (1) according to the pillbox model.15) The /interfacial energy (=) and the volume occupied
by an iron atom () inwere assumed to be 0.705 J/m2and 8:7851030m3, respectively, as in Ref. 16). The average lattice parameter (a) was estimated from the experimental polynomials17)including the effect of alloying elements. The driving force per unit volume of (GV) is equal to the Gmaxdivided by themolar volume (Vm).XC0is the initial C content and the other symbols have their usual meanings.
¼ 12kTa
4=
DCX0
C2GV2
ð1Þ
The nucleation rate of(I) on thegrain boundary surface was calculated in eq. 2 following the expression in Ref. 11). The parameter K1; 1:7391026K0:5/m4, is related to the density of nucleation sites, and K2; 6:6108J3/mol3, includes the geometry factor of the nuclei as well as the ratio of the interfacial energies for / and/.18)As we discuss later when we examine the C enrichment in , the XC in eq. 4 was recursively input after calculating the volume increment of in a previous time-step. The nucleation, however, saturates soon and the enrichment has NPLE
NPLE (limit)
PE
PE
Isopleth Isopleth
[image:2.595.140.457.72.295.2]no significant effect on I compared with the effect on the growth rate.
I¼K1ð1X
CÞD
C ffiffiffiffi
T
p exp K2
RTG2 V
ð2Þ
Assuming carbon-diffusion-controlled growth, the parabolic growth rate constant (1) was expressed in eq. 3, which can be solved numerically.19)Interfacial C compositions (X
C=,
XC=) are conventionally estimated under PE for the transformations of low-carbon steels. Recently, the issue has reemerged and non-constant interface compositions have been proposed20–23) even those operating in isothermal transformations. The authors20) have reported that the calculated kinetics of under the PE condition was far beyond the experimental fraction in 0.37C-1.45Mn steel isothermally transformed at 973 K. A transition model from the PE to NPLE can be fitted to the experimental result, but no criteria for the transition to occur has been found for Fe-C-Ni22)nor Fe-C-Mn alloys.
[image:3.595.132.462.71.296.2]We also investigated the isothermal transformation behaviors in a series of 0.39C-Mn, and revealed the large effect of a small fluctuation in Mn content on the evolution of fraction. To explain the experimental results we relied on Fig. 1, which clearly shows that the Mn content in the range of interest alters the XC=;NPlimit interfacial composition much more than XC=;PE. Thus, the NPLE condition was adopted in the model.
2 ffiffiffiffiffiffiffi
DC
s
XC=XC
X=C X=C ¼1exp
12
4DC
erf 1
2pffiffiffiffiffiffiDC
( )
ð3Þ
The soft impingement effect (SIE) is rather pronounced in medium-carbon steels and has to be properly modeled for growth. The regressive, mean-field approximation has been implemented to calculate C enrichment by the growth of film-likefrom thegrain boundary. In eq. 3, the C content away from the interface (XC) is conveniently represented by
the average C content in (XCbar). Accordingly, 1 is constantly updated with a new volume fraction of (Vf) using the relation in eq. 4. This makes 1 time-dependent, and in this sense, other parameters such asIandDCare also recalculated by inputting the newXCbar.
XC XCbar ¼X 0 CVfX
=
C 1Vf
ð4Þ
The volume fraction of proeutectoid-(Vf) was calculated in the following manner.11)A continuous cooling schedule was divided into small isothermal time-steps, t, during which the temperature isTi(i¼1;. . .k;. . .n). The thickness (R) of nucleating atTkand grown untilTn is given by:
R2ðTn;TkÞ ¼
Xn
i¼k
f12ðTiÞ tg ð5Þ
Usually the grains are assumed to be ellipsoidal hemi-spheres having a three times longer axis along the grain boundary. Consider a parallel plane at a distance ofyfrom the grain boundary. The extended area of the cross-section (Yex) ofhemispheres and the plane is expressed in eq. 6. Thus, theVfatTncan be calculated by the integral ofyfrom 0 to the maximumRvalue multiplied by thegrain boundary area per unit volume (SV).
Yex¼0 fory>RðTn;TkÞ
¼X
k
i¼1
ItX n
i¼k
f9R2ðTn;TkÞ y2g
fory<RðTn;TkÞ 9 > > > = > > > ;
ð6Þ
VfðTnÞ=V eq f ðTnÞ
¼1exp 2SV
ZRðtn;t1Þ
0
f1expðYexÞgdy
ð7Þ
SV is related to d0, and is estimated to be 2.37/d0 for truncated octahedron grains. Finally,Vf must be normalized with respect to the equilibrium fraction Vfeq defined by the lever rule.
Gγγ−>αβαβ
Gγ−>γ'+ α α
GN
GN Gγ − > αβαβ
Gγ−>γ'+α
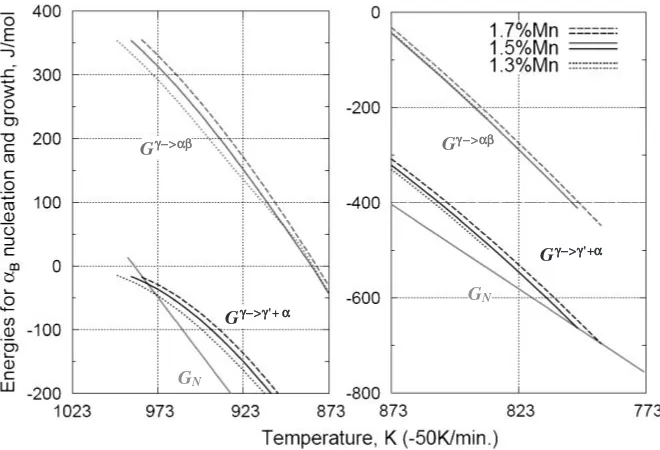
Fig. 2 Calculated free energy change for the nucleation and diffusionless growth ofB(see text for symbols) during continuous cooling
[image:3.595.326.550.638.737.2]2.4 Kinetics of pearlite
Before the start ofPtransformation, thegrain boundaries in MCMS are in most cases covered with the preceding . Thus,Pkinetics have been modeled following the compre-hensive work by Capdevilaet al.,24)in whichPnodules are predominantly formed from attached to the grains. In general, as a thermodynamic condition, thePtransformation is possible from with XCbar higher than the =ðþÞ boundary;
XC=XCbar ð8Þ
The present model determines the start of P when the additional kinetic condition is fulfilled. ForPnodule to form on the surface of growing grain, the velocity of the migrating/interface (=) should be low enough to allow the nucleation of cementite () particle. The critical velocity has been analyzed by Aaronsonet al.25)
In eqs. 9, a is the lattice parameter in , G
V is the volume free energy change associated with nucleation given conveniently by eq. 10. K is a constant that equals 1:0104and=is the/interfacial energy for which a
value of 0.68 J/m2 was adopted.
= ða
Þ3D
CX
=
C ðG
VÞ 3
16ð1==2=ÞpffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffikTð=Þ5K
= ¼1=2 ffiffi
t
p
9 > = >
; ð9Þ
GV¼6:914108 ðAe1TÞ=Ae1; J/m3 ð10Þ
The nucleation rate of pearlite (IP) was formulated in a manner similar to that proposed by Reed and Bhadehia26)for thenucleation from grain surfaces, edges, and corners.
A series of papers have discussed the diffusion-controlled growth of P in the Fe-Mn-C system,24,27,28) and it was proposed that either the growth rate with full partitioning of Mn or the growth rate in NPLE operated depending on the temperature. Considering a narrow temperature range and a small possibility of Mn partitioning at a practical cooling rate, we have taken the NPLE expression forPgrowth rate (G). The/interface C concentrations in eq. 11 should be given in the NPLE calculation as described for the / interface previously. The expressions for lamellar spacingS,
Sc, were taken from Ref. 29).
G¼ D
C 0:72
S
SS
XC=XC=
XC=XC=
1Sc
S0
ð11Þ
VPðTnÞ=VPeq¼1exp S
=
V
Xn
i¼k
GðTiÞtfðG;IP;TnÞ
( )
fðG;IP;TnÞ ¼ Z1
0
1exp
3IP
Xn
i¼k
G2ðTiÞt3ð13 22 3Þ
( )
" #
d
9 > > > > > = > > > > > ;
ð12Þ
In eq. 12, the volume fraction ofP(VP) is analyzed assuming a semi-spherical geometry. The / interface area (S=)
depends onVf and can be estimated by a simple expression:
SVð1VfÞ2=3. The is an integral constant that equalsy/G, whereyis the normal distance from the surface of thegrain.
VPeqis the equilibrium fraction ofPdefined in the relation;19)
VPeq¼ XC
0X
C=ðTnÞ
XC=ðTnÞ XC=ðTnÞ
ð13Þ
This expression has been revised in this work and it equals the rest of Vfeq at the start and becomes unity at the temperatureT whereX
C0¼XC=.
2.5 Start condition of bainite
The energetic criteria for the start ofBfrom undercooled
are:
(1) the driving force (Gmax) for nucleation becomes larger than the function GN irrespective of the steel composition, and
(2) the free energy change associated with diffusionless growth (G!B) exceeds the stored energy of bainite. In the course of and P kinetics calculation, the average composition in the remainingis constantly monitored from the change inXCbar. Ifgrowth so far results in a highVf, the carbon swept byenriches in the remaining, and the Gm for nucleation should be small. This prevents the formation ofBfromUand helps avoidBformation. Thus, these criteria have been applied to theUsingle-phase system
having the enriched C composition ofXCbar.
The GN recently reviewed by Mateo7) for high-carbon steels was considered appropriate for the above purpose of predicting B from U. In his work, these energies were evaluated using MTDATA, and the chemical free energy change (G!0þ
) was used conveniently to avoid the complexity of the numerical analysis of Gmax. We have interpreted G!0þ
as the difference between the free energy of the U single phase with all the other phases suspended and of theU0þsystem in PE.G!Bcould be calculated by taking the difference in free energies ofU and the forced B single-phase system. These changes of phase status were controlled byTQ-i.
3. Calculation Test
A test calculation was designed to see if the present combination of kinetic models reveals the difference in the energies concerning theBfromU. The compositions of the model alloys are Fe-0.39%C-1.3%, 1.5%, 1.7% Mn, which lets us focus on the effect of small Mn fluctuations. The histories of the energies are compared in Fig. 2 for continuous cooling at a rate of 50 K/min. The calculation starts at each Fs temperature of the alloy and terminates when the total transformed fraction becomes unity.
is predicted at this critical temperature. On the other hand, G!B is positive at the start of transformation and diffusionless transformation becomes possible below around 873 K. Initially, 1.7%Mn steel has the highest positive energy and, as transformation proceeds, the order of G!B reverses owing to the difference in C enrichment. As a result, the entire volume transforms into þP in 1.3%Mn steel before the criteria of400J/mol is reached. In contrast, the 1.5%Mn and 1.7%Mn steels fulfill the second criteria at around 798 K. On the basis of this result alone, it cannot be expected that these energy calculations onUwould provide an accurateBfraction; however, at least it demonstrates the possibility ofBformation depending on the composition and cooling rate.
4. Conclusions
The model forBformation fromUhas been proposed, in particular for continuous cooling of MCMS. New treatments including the time-dependent growth rate and NPLE at the/ interface are intended a Mn-content-sensitive description of the preceding transformation kinetics. This has been enabled by the recursive evaluation of C enrichment inand a direct link with CALPHAD thermodynamic calculations. The energy criteria for nucleation and diffusionless growth of B were applied to U during þP transformations. This method showed the ability to predict the different possibility ofBcaused by minor fluctuations in Mn content.
Acknowledgements
The authors acknowledge the financial support of Japan Science and Technology Agency (JST). K. Tanaka would like to thank the members of the Phase Transformation Group in CENIM for fruitful discussions.
REFERENCES
1) H. K. D. H. Bhadeshia: Acta Metall.29(1981) 1117–1130. 2) G. I. Rees and H. K. D. H. Bhadeshia: Mater. Sci. Technol.8(1992)
985–993.
C5 41–46.
4) M. Azuma, N. Fujita, M. Takahashi, T. Senuma, D. Quidort and T. Lung: ISIJ Int.45(2005) 221–228.
5) M. J. Santofimia, F. G. Caballero, C. Capdevila, C. Garcı´a-Mateo and C. Garcı´a de Andre´s: Mater. Trans.47(2006) 2473–2479.
6) M. J. Santofimia, F. G. Caballero, C. Capdevila, C. Garcı´a-Mateo and C. Garcı´a de Andre´s: Mater. Trans.47(2006) 2465–2472.
7) C. Garcia-Mateo and H. K. D. H. Bhadeshia: Mater. Sci. Eng. AA378 (2004) 289–292.
8) T. Senuma, H. Yada, Y. Matsumura and T. Nimura: Tetsu-to-Hagane 70(1984) 2112–2119.
9) Y. Yogo, K. Ishikawa and H. Yoshida: CAMP-ISIJ14(2001) 424. 10) A. Samoilov, Y. Titovets, N. Zolotorevsky, G. Hribernig and A.
Pichler: Mater. Sci. Forum539–543(2007) 4584–4589.
11) M. Umemoto: Proc. Int. Symp. on Mathematical Modeling of Hot Rolling of Steel, (Montre´al, 1990) 404–423.
12) M. Suehiro, T. Senuma, H. Yada, Y. Matsumura and T. Ariyoshi: Tetsu-to-Hagane73(1987) 1026–1033.
13) S. J. Jones and H. K. D. H. Bhadeshia: Acta Mater.45(1997) 2911– 2920.
14) S. V. Parker: PhD thesis, University of Cambridge, (1997).
15) W. F. Lange, M. Enomoto and H. I. Aaronson: Metall. Trans.19A (1988) 427–440.
16) C. Capdevila, C. G. Andre´s and F. G. Caballero: Scr. Mater.44(2001) 129–134.
17) H. K. D. H. Bhadeshia, S. A. David, J. M. Vitek and W. Reed: Mater. Sci. Technol.7(1991) 686–689.
18) X. Liu, P. Kayjalainen and J. S. Perttula: Proc. 2nd Int. Conf. on Modeling of Metal Rolling Processes, eds. J. H. Beynon, P. Ingham, T. Teichert and K. Waterson, (London, UK, 1996) 489–500. 19) C. Capdevila: private communication.
20) K. Tanaka, M. Hara, Y. Yogo, K. Nakanishi and C. Capdevila: CAMP-ISIJ. Proc. 155th ISIJ Meeting21(2008) 469–470.
21) J. Sietsma and S. van der Zwaag: Acta Mater.52(2004) 4143–4152. 22) C. R. Hutchinson, A. Fuchsmann and Y. Bre´chet: Metall. Mater. Trans.
A37(2006) 1711–1720.
23) C. R. Hutchinson, H. S. Zurob and Y. Bre´chet: Metall. Mater. Trans. A 35(2004) 1211–1221.
24) C. Capdevila, F. G. Caballero and C. G. Andre´s: Acta Mater.50(2002) 4629–4641.
25) H. I. Aaronson, M. R. Plichta, G. W. Franti and K. C. Russell: Metall. Trans. A9A(1978) 363–378.
26) R. C. Reed and H. K. D. H. Bhadeshia: Mater. Sci. Technol.8(1992) 421–435.
27) M. Hillert and L. Ho¨glund: Scr. Mater.50(2004) 171–173. 28) C. Capdevila, F. G. Caballero and C. G. Andre´s:50(2004) 175–177. 29) M. Takahashi: Reaustenitisation from bainite in steels, Ph. D Thesis,