metal-organic papers
Acta Cryst.(2006). E62, m133–m135 doi:10.1107/S1600536805041760 Djedouaniet al. [Cu(C
8H7O4)2(C2H6OS)2]
m133
Acta Crystallographica Section E Structure Reports Online
ISSN 1600-5368
Bis[3-acetyl-6-methyl-2
H
-pyran-2,4(3
H
)-dionato]bis(dimethyl sulfoxide)copper(II)
Amel Djedouani,aAbderrahmen Bendaaˆs,aSofiane Bouacida,b,c* Adel Beghidjacand Tahar Douadia
aLaboratoire d0Electrochimie des Mate´riaux Mole´culaires et Complexes, De´partement de Ge´nie des Proce´de´s, Faculte´ des Science de l0Inge´nieur, Universite´ Farhat Abbes de Setif, DZ-19000 Se´tif, Algeria,bDe´partement de
Chimie, Faculte´ des Sciences et Sciences de l0Inge´nieur, Universite´ A. Mira de Be´jaia, Route Targua Ouzmour 06000 Be´jaia, Algeria, and
cLaboratoire de Chimie Mole´culaire, du
Controˆle de l0Environnement et de Mesures Physico-Chimiques, De´partement de Chimie, Faculte´ des Sciences, Universite´ Mentouri 25000, Constantine, Algeria
Correspondence e-mail: bouacida_sofiane@yahoo.fr
Key indicators
Single-crystal X-ray study
T= 294 K
Mean(C–C) = 0.003 A˚
Rfactor = 0.035
wRfactor = 0.093
Data-to-parameter ratio = 22.6
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 1 December 2005 Accepted 13 December 2005
#2006 International Union of Crystallography All rights reserved
In the structure of the mononuclear title complex, [Cu(C8H7O4)2(C2H6OS)2], the Cu
II
atom lies on an inversion centre and has an octahedral coordination geometry of type MO6. The bidentate dehydroacetic acid (DHA) ligands occupy the equatorial plane of the complex in a trans
configuration, and the dimethylsulfoxide (DMSO) ligands are weakly coordinated through their O atoms.
Comment
Mixed d-transition metal–-diketone compounds were used extensively as starting materials in the early days of metallo-cene chemistry (Smith & Andersen, 1996). Dehydroacetic acid [DHA, 3-acetyl-6-methyl-2H-pyran-2,4(3H)-dione], a commercially available compound usually obtained through the auto-condensation of ethyl acetoacetate (Arndt et al., 1936), has been shown to possess modest antifungal properties (Rao et al., 1978). The importance of similar pyrones as potential fungicides is reinforced by the existence of several natural fungicides possessing structures analogous to 5,6-dihydrodehydroacetic acid, such as alternaric acid (Bartels-Keith, 1960), the podoblastins (Miyakado et al., 1982) and lachnelluloic acid (Ayeret al., 1988). Also, it has been shown that the complexes of DHA with zinc and with several other transition metal cations are fungistatic (Raoet al., 1978). This has motivated our study of the structural characterization of complexes of dehydroacetic acid. The complex of DHA with Cu was previously reported by Casabo`et al.(1987), but their characterization of the compound was based only on thermal and elemental analysis, and on IR and NMR spectroscopy. We present here the crystal structure determination of the title complex, [Cu(DHA)2(DMSO)2], (I) (DMSO is dimethyl-sulfoxide).
structures have been already reported with thiosemicarbazone 2-pyridineformamide (Castin˜eiras et al., 2000), and the thio-semicarbazone of acenaphthenequinone (Rodriguez-Argu¨elleset al., 1997).
(I) is isostructural with the two complexes [Zn(DHA)2 -(DMSO)2] and [Cd(DHA)2(DMSO)2] (Zucolotto Chalac¸a et
al., 2002). The O5 Cu bond length for the DMSO in the copper complex, (I) (2.463 A˚ ) is significantly longer than the Zn O and Cd O distances of 2.185 and 2.316 A˚ , respec-tively, found in the above complexes. This difference is due to the Jahn–Teller distortion for CuII.
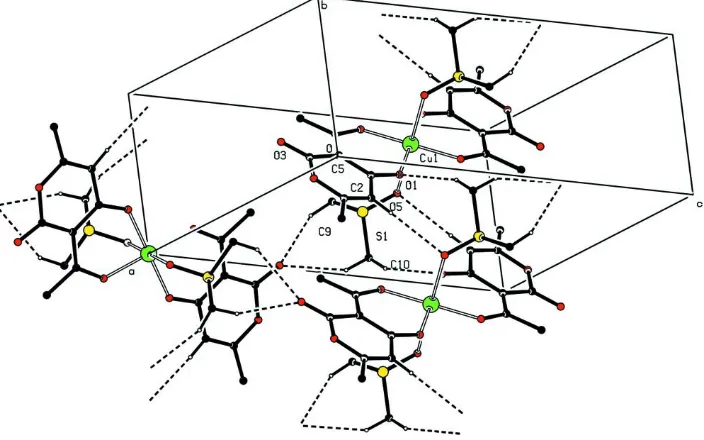
The packing of (I) is stabilized by weak intermolecular C— H O hydrogen bonds (Table 1) which form a three-dimen-sional network (Fig. 2).
Experimental
A solution of copper acetate monohydrate was added, with stirring, to a solution of dehydroacetic acid in absolute ethanol in a 1:2 stoi-chiometric ratio. The title complex precipitated after 1 h. The resulting precipitate of (I) was filtered off and recrystallized by slow evaporation of a dimethylsulfoxide solution.
Crystal data
[Cu(C8H7O4)2(C2H6OS)2]
Mr= 554.07
Monoclinic, P21=n a= 11.580 (5) A˚
b= 6.320 (5) A˚
c= 16.424 (5) A˚ = 92.27 (5)
V= 1201.1 (11) A˚3
Z= 2
Dx= 1.532 Mg m
3 MoKradiation
Cell parameters from 11050 reflections
= 2.1–30.1
= 1.14 mm1
T= 294 K Prism, blue 0.10.10.1 mm
Data collection
Nonius KappaCCD area-detector diffractometer
’scans, and!scans withoffsets Absorption correction: none 11050 measured reflections 3506 independent reflections
2651 reflections withI> 2(I)
Rint= 0.040
max= 30.1
h=13!16
k=6!8
l=22!23
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.035
wR(F2) = 0.093
S= 1.05 3506 reflections 155 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0438P)2 + 0.1877P]
whereP= (Fo2+ 2Fc2)/3 (/)max< 0.001
max= 0.37 e A˚ 3
[image:2.610.317.564.70.281.2]min=0.39 e A˚ 3
Table 1
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
C2—H2 O5i
0.93 2.55 3.475 (3) 170
C9—H9B O3ii
0.96 2.55 3.394 (3) 146
C10—H10B O3ii 0.96 2.53 3.379 (3) 147 C10—H10C O1i
0.96 2.58 3.486 (3) 158
Symmetry codes: (i)xþ1;y;zþ1; (ii)xþ3 2;y
1 2;zþ
1 2.
All H atoms were located in difference Fourier maps but intro-duced in calculated positions and treated as riding on their parent C atoms, with C—H distances of 0.96 A˚ (CH3) and 0.93 A˚ (Caromatic) and withUiso(H) = 1.2Ueq(Caromatic) or 1.5Ueq(CH3).
Data collection: COLLECT (Nonius, 1998); cell refinement: DENZO and SCALEPACK (Otwinowski & Minor, 1997); data
reduction:DENZO and SCALEPACK; program(s) used to solve
structure: SIR2002 (Burla et al., 2003); program(s) used to refine
structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
ORTEPIII (Burnett & Johnson, 1996), ORTEP-3 for Windows (Farrugia, 1997) and PLATON (Spek, 2003); software used to prepare material for publication:WinGX(Farrugia, 1999).
References
Arndt, F., Eistert, B., Scholz, H. & Aron, E. (1936).Berichte Teil B,69, 2373-2380.
Ayer, W. A., Figueroa-Villar, J. D. & Migaj, B. (1988).Can. J. Chem.66, 506– 512.
metal-organic papers
m134
Djedouaniet al. [Cu(C8H7O4)2(C2H6OS)2] Acta Cryst.(2006). E62, m133–m135
Figure 1
[image:2.610.314.567.334.493.2]A view of the title compound, with the atomic labelling scheme. Displacement ellipsoids are drawn at the 50% probability level. H atoms have been omitted for clarity. [Symmetry code: (i)x,y,z.]
Figure 2
[image:2.610.44.298.674.729.2]Bartels-Keith, J. R. (1960).J. Chem. Soc.pp. 1662–1665.
Burla, M. C., Camalli, M., Carrozzini, B., Cascarano, G. L., Giacovazzo, C., Polidori, G. & Spagna, R. (2003).J. Appl. Cryst.36, 1103.
Burnett, M. N. & Johnson, C. K. (1996).ORTEPIII. Report ORNL-6895. Oak Ridge National Laboratory, Tennessee, USA.
Casabo`, J., Marquet, J., Moreno-man˜as, M., Prior, M. & Teixidor, F. (1987).
Polyhedron,6, 1235–1238.
Castin˜eiras, A., Garcia, I., Bermejo, E. & West, D. X. (2000).Polyhedron,19, 1873–1880.
Farrugia, L. J. (1997).J. Appl. Cryst.30, 565. Farrugia, L. J. (1999).J. Appl. Cryst.32, 837–838.
Miyakado, M., Inoue, S., Tanabe, Y., Watanabe, K., Ohno, N., Yoshioka, H. & Mabay, T. (1982).J. Chem. Lett.pp. 1539–1542.
Nonius (1998).COLLECT. Nonius BV, Delft, The Netherlands.
Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276,
Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
Rao, D. S., Ganorkar, M. C., Rao, B. L. S. & John, V. T. (1978).Natl Acad. Sci. Lett.1, 402–404.
Rodriguez-Argu¨elles, M. C., Ferrari, M. B., Fava, G. G., Pellizi, C., Pelosi, G., Albertini, R., Bonita, A., Dall’Aglio, P. P., Lunghi, P. & Pinelli, S. (1997).J. Inorg. Biochem.66, 7–17.
Sheldrick, G. M. (1997).SHELXL97. University of Go¨ttingen, Germany. Smith, M. E. & Andersen, R. A. (1996).J. Am. Chem. Soc. 118, 11119–
11128.
Spek, A. L. (2003).J. Appl. Cryst.36, 7–13.
Zucolotto Chalac¸a, M., Figueroa-Villar, J. D., Ellena, J. A. & Castellano, E. E. (2002).Inorg. Chim. Acta,328, 45–52.
metal-organic papers
Acta Cryst.(2006). E62, m133–m135 Djedouaniet al. [Cu(C
supporting information
sup-1
Acta Cryst. (2006). E62, m133–m135supporting information
Acta Cryst. (2006). E62, m133–m135 [doi:10.1107/S1600536805041760]
Bis[3-acetyl-6-methyl-2
H
-pyran-2,4(3
H
)-dionato]bis(dimethyl
sulfoxide)-copper(II)
Amel Djedouani, Abderrahmen Benda
â
s, Sofiane Bouacida, Adel Beghidja and Tahar Douadi
S1. Comment
Mixed d-transition metal–β-diketone compounds were used extensively as starting materials in the early days of metallocene chemistry (Smith & Andersen, 1996). Dehydroacetic acid [DHA, 3-acetyl-6-methyl-2H-pyran-2,4(3H )-dione], a commercially available compound usually obtained through the auto-condensation of ethyl acetoacetate (Arndt
et al., 1936), has been shown to possess modest antifungal properties (Rao et al., 1978). The importance of similar
pyrones as potential fungicides is reinforced by the existence of several natural fungicides possessing structures
analogous to 5,6-dihydrodehydroacetic acid, such as alternaric acid (Bartels-Keith, 1960), the podoblastins (Miyakado et al., 1982) and lachnelluloic acid (Ayer et al., 1988). Also, it has been shown that the complexes of DHA with zinc and with several other transition metal cations are fungistatic (Rao et al., 1978). This has motivated our study of the structural characterization of complexes of dehydroacetic acid. The complex of DHA with Cu was previously reported by Casabò et al. (1987), but their characterization of the compound was based only on thermal and elemental analysis, and on IR and NMR spectroscopy. We present here the crystal structure determination of the title complex, [Cu(DHA)2(DMSO)2], (I)
(DMSO is dimethylsulfoxide).
The CuII atom of (I), located on an inversion centre, is surrounded by two DHA ligands occupying the equatorial plane.
The two apical positions are occupied by O atoms of two dimethylsulfoxyde molecules (Fig. 1). Complexes with related structures have been already reported with thiosemicarbazone 2-pyridineformamide (Castiñeiras et al., 2000), and thio-semicarbazone of acenaphthenequinone (Rodriguez-Argüelles et al., 1997).
The structure of (I) is isostructural with the two complexes [Zn(DHA)2(DMSO)2] and [Cd(DHA)2(DMSO)2] (Zucolotto
Chalaça et al., 2002). The O5···Cu bond length for the DMSO in the copper complex, (I) (2.463 Å) is significantly longer than the Zn···O and Cd···O distances of 2.185 and 2.316 Å, respectively, found in the above complexes. This difference may be related to the tendancy of Cu to prefer a square-planar geometry.
The packing of (I) is stabilized by weak intermolecular C—H···O hydrogen bonds (Table 1) which form a three-dimensional network (Fig. 2).
S2. Experimental
A solution of copper acetate monohydrate was added, with stirring, to a solution of dehydroacetic acid in absolute ethanol in a 1:2 stoichiometric ratio. The title complex precipitated after 1 h. The resulting precipitate of (I) was filtered off and recrystallized by slow evaporation of a dimethylsulfoxide solution.
S3. Refinement
supporting information
sup-2
Acta Cryst. (2006). E62, m133–m135 [image:5.610.124.477.79.382.2]1.5Ueq(CH3).
Figure 1
A view of the title compound, with the atomic labelling scheme. Displacement ellipsoids are drawn at the 50% probability level. H atoms have been omitted for clarity. [Symmetry code: (i) −x, −y, −z.]
Figure 2
[image:5.610.130.482.440.660.2]supporting information
sup-3
Acta Cryst. (2006). E62, m133–m135(I)
Crystal data
[Cu(C8H7O4)2(C2H6OS)2] Mr = 554.07
Monoclinic, P21/n
Hall symbol: -P 2yn
a = 11.580 (5) Å
b = 6.320 (5) Å
c = 16.424 (5) Å
β = 92.27 (5)°
V = 1201.1 (11) Å3 Z = 2
F(000) = 574
Dx = 1.532 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 11050 reflections
θ = 2.1–30.1°
µ = 1.14 mm−1 T = 294 K Prism, blue 0.1 × 0.1 × 0.1 mm
Data collection
Nonius KappaCCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ scans, and ω scans with κ offsets 11050 measured reflections 3506 independent reflections
2651 reflections with I > 2σ(I)
Rint = 0.040
θmax = 30.1°, θmin = 2.1° h = −13→16
k = −6→8
l = −22→23
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.035 wR(F2) = 0.093 S = 1.05 3506 reflections 155 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0438P)2 + 0.1877P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001
Δρmax = 0.37 e Å−3
Δρmin = −0.39 e Å−3
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
supporting information
sup-4
Acta Cryst. (2006). E62, m133–m135O4 0.58586 (10) 0.62317 (18) 0.41185 (7) 0.0380 (3) O5 0.67750 (12) 0.3121 (2) 0.54540 (8) 0.0490 (3) C1 0.46505 (13) 0.2507 (2) 0.35549 (10) 0.0300 (3) C2 0.41204 (15) 0.0666 (3) 0.31725 (11) 0.0352 (4) H2 0.3803 −0.0375 0.3496 0.042* C3 0.40800 (15) 0.0437 (3) 0.23720 (12) 0.0376 (4) C4 0.51841 (15) 0.3628 (3) 0.21731 (11) 0.0372 (4) C5 0.52246 (13) 0.3973 (2) 0.30415 (10) 0.0303 (3) C6 0.58663 (14) 0.5735 (3) 0.33815 (10) 0.0315 (3) C7 0.66346 (17) 0.7094 (3) 0.28811 (12) 0.0462 (4) H7A 0.6168 0.7918 0.2503 0.069* H7B 0.7147 0.6209 0.2586 0.069* H7C 0.7079 0.8023 0.3234 0.069* C8 0.35032 (19) −0.1283 (3) 0.18953 (13) 0.0540 (5) H8A 0.3185 −0.2294 0.2261 0.081* H8B 0.4057 −0.1971 0.1565 0.081* H8C 0.2894 −0.0698 0.1551 0.081* C9 0.8695 (2) 0.3578 (3) 0.46739 (15) 0.0630 (6) H9A 0.8531 0.4998 0.4497 0.095* H9B 0.9170 0.2892 0.4287 0.095* H9C 0.9095 0.3611 0.5197 0.095* C10 0.7993 (2) −0.0257 (3) 0.51126 (15) 0.0553 (5) H10A 0.8419 −0.0004 0.5616 0.083* H10B 0.8503 −0.0806 0.4717 0.083* H10C 0.7389 −0.1264 0.5201 0.083*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-5
Acta Cryst. (2006). E62, m133–m135Geometric parameters (Å, º)
Cu1—O1 1.9091 (15) C3—C8 1.483 (3) Cu1—O1i 1.9091 (15) C4—C5 1.442 (2)
Cu1—O4i 1.9506 (14) C5—C6 1.439 (3)
Cu1—O4 1.9506 (14) C6—C7 1.504 (2) S1—O5 1.5040 (15) C7—H7A 0.9600 S1—C10 1.776 (2) C7—H7B 0.9600 S1—C9 1.782 (2) C7—H7C 0.9600 O1—C1 1.264 (2) C8—H8A 0.9600 O2—C3 1.367 (2) C8—H8B 0.9600 O2—C4 1.394 (2) C8—H8C 0.9600 O3—C4 1.210 (2) C9—H9A 0.9600 O4—C6 1.2508 (19) C9—H9B 0.9600 C1—C5 1.434 (2) C9—H9C 0.9600 C1—C2 1.447 (2) C10—H10A 0.9600 C2—C3 1.322 (3) C10—H10B 0.9600 C2—H2 0.9300 C10—H10C 0.9600
O1—Cu1—O1i 180.0 O4—C6—C5 123.12 (15)
O1—Cu1—O4i 89.78 (6) O4—C6—C7 114.35 (15)
O1i—Cu1—O4i 90.22 (6) C5—C6—C7 122.50 (15)
O1—Cu1—O4 90.22 (6) C6—C7—H7A 109.5 O1i—Cu1—O4 89.78 (6) C6—C7—H7B 109.5
O4i—Cu1—O4 180.0 H7A—C7—H7B 109.5
O5—S1—C10 106.36 (10) C6—C7—H7C 109.5 O5—S1—C9 105.76 (10) H7A—C7—H7C 109.5 C10—S1—C9 96.98 (13) H7B—C7—H7C 109.5 C1—O1—Cu1 127.85 (10) C3—C8—H8A 109.5 C3—O2—C4 122.20 (14) C3—C8—H8B 109.5 C6—O4—Cu1 129.90 (11) H8A—C8—H8B 109.5 O1—C1—C5 126.05 (15) C3—C8—H8C 109.5 O1—C1—C2 116.37 (15) H8A—C8—H8C 109.5 C5—C1—C2 117.58 (15) H8B—C8—H8C 109.5 C3—C2—C1 121.14 (16) S1—C9—H9A 109.5 C3—C2—H2 119.4 S1—C9—H9B 109.5 C1—C2—H2 119.4 H9A—C9—H9B 109.5 C2—C3—O2 121.79 (16) S1—C9—H9C 109.5 C2—C3—C8 127.07 (18) H9A—C9—H9C 109.5 O2—C3—C8 111.14 (17) H9B—C9—H9C 109.5 O3—C4—O2 113.46 (16) S1—C10—H10A 109.5 O3—C4—C5 128.58 (17) S1—C10—H10B 109.5 O2—C4—C5 117.94 (15) H10A—C10—H10B 109.5 C1—C5—C6 121.08 (15) S1—C10—H10C 109.5 C1—C5—C4 119.11 (15) H10A—C10—H10C 109.5 C6—C5—C4 119.79 (14) H10B—C10—H10C 109.5
supporting information
sup-6
Acta Cryst. (2006). E62, m133–m135Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
C2—H2···O5ii 0.93 2.55 3.475 (3) 170
C9—H9B···O3iii 0.96 2.55 3.394 (3) 146
C10—H10B···O3iii 0.96 2.53 3.379 (3) 147
C10—H10C···O1ii 0.96 2.58 3.486 (3) 158