organic papers
Acta Cryst.(2005). E61, o2579–o2581 doi:10.1107/S1600536805022130 Sonaret al. C
20H23NO4
o2579
Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
6
a
-Acetoxy-4,5
a
-epoxy-3-methoxy-17-methyl-morphin-7-ene
Vijayakumar N. Sonar,aSean Parkinband Peter A. Crooksa*
a
Department of Pharmaceutical Sciences, College of Pharmacy, University of Kentucky, Lexington, KY 40536, USA, andbDepartment of
Chemistry, University of Kentucky, Lexington, KY 40506, USA
Correspondence e-mail: pcrooks@uky.edu
Key indicators
Single-crystal X-ray study
T= 90 K
Mean(C–C) = 0.006 A˚
Rfactor = 0.047
wRfactor = 0.112 Data-to-parameter ratio = 7.3
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography
Printed in Great Britain – all rights reserved
Crystals of the title compound, which is also commonly known as 6-O-acetylcodeine, C20H23NO4, were obtained by
acetyl-ation of codeine and subsequent crystallizacetyl-ation of the product from ethyl acetate. The atoms of the benzene ring are nearly coplanar, whereas the five-memberedring is distorted. The ethanamine ring has a typical chair form conformation, while the cyclohexene ring is in a twisted boat form.
Comment
Morphine is the most important component of Papaver somniferumextracts and its semisynthetic analogs, which have been used as pain-alleviating medicines (Brock et al., 1996; Brown et al., 1985). 6-O-Acetylcodeine is a useful inter-mediate for the synthesis of a variety of pharmacologically active morphine and codeine analogs, and is a useful synthon for reactions in which the 6-hydroxy group needs to be protected. Such compounds that are of considerable phar-macological interest are 10-hydroxymorphine, and 10-hydroxycodeine. The title compound, (I), was prepared by the reaction of codeine phosphate with acetic anhydride in the presence of pyridine and dimethylaminopyridine. To obtain more detailed structural information on the conformation of the molecule in the solid state, the X-ray crystal structure determination of (I) has been carried out.
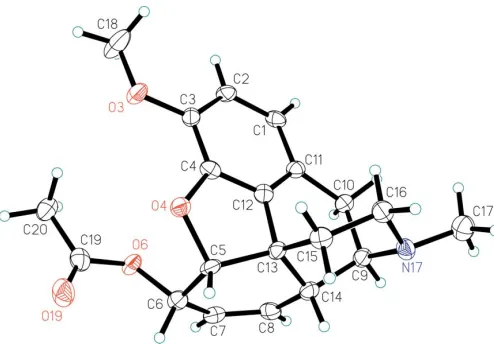
The numbering system of the non-H atoms and the overall configuration of the title compound is shown in Fig. 1, which shows the absolute configuration of the chiral centers in the molecule as identical to that of the starting material, codeine. Selected geometric parameters are presented in Table 1. The atoms of the benzene ring (A) are nearly coplanar, whereas the five membered ring (B) is distorted. The ethanamine ring (E) has a typical chair conformation, while the conformation of ringDis a twist boat. This is caused by the 4,5-ether bridge,
which is also responsible for the overall rigidity of the mol-ecule. The bond lengths and bond angles for the non-H atoms are in agreement with the literature values for codeine hydrobromide (Karthaet al., 1962).
Another structure determination of 6-O-acetylcodeine is reported in the following paper (Kolevet al., 2005).
Experimental
To a stirred mixture of codeine phosphate (1.74 g, 4.29 mmol), DMAP (0.183 g, 1.5 mmol) and pyridine (8 ml) was added acetic anhydride (1.02 g, 10 mmol) at 273 K dropwise. The reaction mixture was stirred at room temperature overnight. The solvent was evapo-rated, then water (10 ml) was added to the residue followed by potassium carbonate solution (20 ml, 2.5%). The resulting solution was extracted with diethyl ether (380 ml), and the ether layers were combined and washed with water, brine and dried over anhy-drous Na2SO4. Evaporation of the solvent gave a pale-yellow solid
which on crystallization from ethyl acetate afforded (I) as colorless flakes suitable for X-ray analysis.1H NMR (300 MHz, CDCl
3):1.86
(d, 1H), 2.05 (td, 1H), 2.16 (s, 3H), 2.33 (td, 2H), 2.44 (s, 3H), 2.59 (dd, 1H), 2.74 (s, 1H), 3.04 (d, 1H), 3.36 (q, 1H), 3.85 (s, 3H), 5.07 (d, 1H), 5.19 (q, 1H), 5.53 (dd, 2H), 6.60 (dd, 2H).
Crystal data
C20H23NO4
Mr= 341.39
Orthorhombic,P212121
a= 8.6588 (4) A˚
b= 12.3368 (7) A˚
c= 15.4441 (9) A˚
V= 1649.77 (15) A˚3
Z= 4
Dx= 1.374 Mg m
3
MoKradiation Cell parameters from 2134
reflections = 1.0–27.5
= 0.10 mm1
T= 90.0 (2) K
Irregular fragment, colorless 0.250.150.10 mm
Data collection
Nonius KappaCCD diffractometer !scans at fixed= 55
Absorption correction: none 2902 measured reflections 1683 independent reflections 1116 reflections withI> 2(I)
Rint= 0.074
max= 25.0
h=10!10
k=14!14
l=18!18
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.048
wR(F2) = 0.113
S= 0.98 1683 reflections 229 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0595P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.002
max= 0.22 e A˚ 3
[image:2.610.46.293.69.241.2]min=0.26 e A˚ 3
Table 1
Selected geometric parameters (A˚ ,).
O3—C3 1.385 (5)
O3—C18 1.413 (5)
O4—C4 1.387 (4)
O4—C5 1.471 (4)
C4—C12 1.374 (6)
C5—C13 1.541 (6)
O6—C19 1.353 (5)
C7—C8 1.331 (6)
C12—C13 1.505 (6)
O19—C19 1.203 (5)
C19—C20 1.492 (6)
C3—O3—C18 116.8 (3)
C2—C3—O3 125.9 (4)
C4—O4—C5 105.0 (3)
C12—C4—O4 111.1 (3) O4—C5—C13 104.1 (3) C19—O6—C6 116.3 (3)
C7—C6—C5 116.2 (3)
C8—C7—C6 125.1 (4)
C7—C8—C14 122.7 (4) C11—C12—C13 127.9 (4) C14—C13—C5 118.5 (3) C14—C13—C15 108.0 (3) C8—C14—C13 112.3 (4) C16—N17—C9 114.2 (3) O19—C19—O6 124.4 (4) O19—C19—C20 126.5 (4)
C18—O3—C3—C2 5.8 (6) C19—O6—C6—C5 74.6 (4)
C6—C7—C8—C14 0.3 (7)
H atoms were found in difference Fourier maps and subsequently placed using riding models in which the H atom coordinates were determined geometrically from their attached parent atom. Bond distances for these H atoms were fixed as follows: aromatic C—H = 0.95 A˚ , CH C—H = 1.00 A˚, CH2C—H = 0.99 A˚ and methyl C—H =
0.9 A˚ . Isotropic displacement parameters for the H atoms were defined as 1.2Ueq(C) for aromatic, CH and CH2, and 1.5Ueq(C) for
the methyl H atoms. The absolute configuration of this compound is known; it was not determined from the X-ray data. Nonetheless, the bulk of the refinement was carried out against data in which the Friedel pairs were kept unmerged. This enabled the Flack (1983) parameter to be determined so as to confirm the expected result that the data do not contain the information required to define x(u). Later, the model was rerefined to convergence with merged data, and it is this model that is contained within this CIF.
Data collection: COLLECT (Nonius, 1999); cell refinement:
SCALEPACK (Otwinowski & Minor, 1997); data reduction:
DENZO–SMN (Otwinowski & Minor, 1997); program(s) used to solve structure: SHELXS97(Sheldrick, 1997); program(s) used to refine structure:SHELXL97(Sheldrick, 1997); molecular graphics:
XP in SHELXTL/PC (Sheldrick, 1995); software used to prepare material for publication: SHELXL97 (Sheldrick, 1997) and local procedures.
References
Brock, C. P., Kottayil, S., Butterfield, D. A. & Crooks, P. A. (1996).Acta Cryst.
C52, 122–125.
Brown, C. E., Roerig, S. C., Burger, V. T., Cody, R. B. & Fujimoto, J. M. (1985).
J. Pharm. Sci.74, 821–824.
Flack, H. D. (1983).Acta Cryst.A39, 876–881.
Kartha, G., Ahmed, F. R. & Barnes, W. H. (1962).Acta Cryst.15, 326–333. Kolev, T., Bakalska, R., Shivachev, B. & Petrova, R. (2005).Acta Cryst.E61,
o2582–o2584.
organic papers
o2580
Sonaret al. C20H23NO4 Acta Cryst.(2005). E61, o2579–o2581
Figure 1
[image:2.610.311.567.187.361.2]Nonius (1999).COLLECT. Nonius, BV, Delft, The Netherlands.
Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276,
Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
Sheldrick (1995).XP in SHELXTL/PC. Siemens Analytical X-ray Instru-ments Inc., Madison, Wisconsin, USA.
Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
organic papers
Acta Cryst.(2005). E61, o2579–o2581 Sonaret al. C
supporting information
sup-1
Acta Cryst. (2005). E61, o2579–o2581
supporting information
Acta Cryst. (2005). E61, o2579–o2581 [https://doi.org/10.1107/S1600536805022130]
6
α
-Acetoxy-4,5
α
-epoxy-3-methoxy-17-methylmorphin-7-ene
Vijayakumar N. Sonar, Sean Parkin and Peter A. Crooks
6α-Acetoxy-4,5α-epoxy-3-methoxy-17-methyl-morphin-7-ene
Crystal data C20H23NO4
Mr = 341.39
Orthorhombic, P212121
Hall symbol: P 2ac 2ab a = 8.6588 (4) Å b = 12.3368 (7) Å c = 15.4441 (9) Å V = 1649.77 (15) Å3
Z = 4
F(000) = 728 Dx = 1.374 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 2134 reflections θ = 1.0–27.5°
µ = 0.10 mm−1
T = 90 K
Irregular fragment, colorless 0.25 × 0.15 × 0.10 mm
Data collection Nonius KappaCCD
diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
Detector resolution: 18 pixels mm-1
ω scans at fixed χ = 55° 2902 measured reflections
1683 independent reflections 1116 reflections with I > 2σ(I) Rint = 0.074
θmax = 25.0°, θmin = 2.1°
h = −10→10 k = −14→14 l = −18→18
Refinement Refinement on F2
Least-squares matrix: full R[F2 > 2σ(F2)] = 0.048
wR(F2) = 0.113
S = 0.98 1683 reflections 229 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[σ2(F
o2) + (0.0595P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.002
Δρmax = 0.22 e Å−3
Δρmin = −0.26 e Å−3
Absolute structure: Flack (1983) Absolute structure parameter: 2 (2)
Special details
supporting information
sup-2
Acta Cryst. (2005). E61, o2579–o2581
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
C1 0.6277 (5) 0.3238 (3) 0.6390 (3) 0.0209 (10)
H1 0.5549 0.3212 0.5930 0.025*
C2 0.7851 (5) 0.3297 (3) 0.6203 (3) 0.0217 (10)
H2 0.8176 0.3286 0.5616 0.026*
O3 1.0536 (3) 0.3441 (2) 0.67238 (16) 0.0240 (8) C3 0.8955 (5) 0.3373 (3) 0.6849 (3) 0.0195 (10) O4 0.9288 (3) 0.3454 (2) 0.84450 (17) 0.0210 (7) C4 0.8426 (5) 0.3361 (3) 0.7692 (2) 0.0184 (10) C5 0.8166 (4) 0.3746 (3) 0.9121 (3) 0.0201 (11)
H5 0.8516 0.3462 0.9694 0.024*
O6 0.8980 (3) 0.5506 (2) 0.85046 (17) 0.0233 (7) C6 0.8021 (4) 0.5005 (4) 0.9158 (3) 0.0203 (11)
H6 0.8389 0.5253 0.9739 0.024*
C7 0.6444 (5) 0.5460 (4) 0.9010 (3) 0.0235 (11)
H7 0.6359 0.6222 0.8937 0.028*
C8 0.5148 (5) 0.4882 (4) 0.8973 (3) 0.0226 (11)
H8 0.4200 0.5250 0.8878 0.027*
C9 0.3861 (5) 0.3107 (3) 0.8539 (3) 0.0209 (10)
H9 0.2850 0.3448 0.8692 0.025*
C10 0.4096 (4) 0.3249 (3) 0.7545 (2) 0.0214 (10)
H10A 0.3517 0.2669 0.7242 0.026*
H10B 0.3641 0.3951 0.7368 0.026*
C11 0.5761 (4) 0.3215 (3) 0.7243 (3) 0.0197 (10) C12 0.6882 (5) 0.3243 (3) 0.7876 (3) 0.0191 (10) C13 0.6669 (4) 0.3169 (3) 0.8841 (2) 0.0180 (10) C14 0.5125 (5) 0.3686 (3) 0.9074 (3) 0.0216 (11)
H14 0.4922 0.3526 0.9698 0.026*
C15 0.6617 (5) 0.1971 (3) 0.9123 (3) 0.0220 (11)
H15A 0.7590 0.1609 0.8950 0.026*
H15B 0.6531 0.1931 0.9761 0.026*
C16 0.5261 (5) 0.1383 (4) 0.8715 (3) 0.0229 (11)
H16A 0.5452 0.1293 0.8087 0.028*
H16B 0.5166 0.0653 0.8975 0.028*
N17 0.3815 (4) 0.1979 (3) 0.8842 (2) 0.0220 (9) C17 0.2487 (4) 0.1377 (4) 0.8515 (3) 0.0302 (12)
H17A 0.1548 0.1809 0.8595 0.045*
H17B 0.2388 0.0693 0.8833 0.045*
H17C 0.2632 0.1224 0.7898 0.045*
C18 1.1055 (5) 0.3535 (5) 0.5859 (3) 0.0436 (14)
supporting information
sup-3
Acta Cryst. (2005). E61, o2579–o2581
H18B 1.2180 0.3619 0.5853 0.065*
H18C 1.0575 0.4170 0.5589 0.065*
O19 1.1091 (3) 0.5338 (3) 0.9356 (2) 0.0337 (8) C19 1.0517 (5) 0.5514 (3) 0.8661 (3) 0.0252 (11) C20 1.1357 (5) 0.5753 (3) 0.7839 (3) 0.0273 (11)
H20A 1.2317 0.6140 0.7970 0.041*
H20B 1.0705 0.6203 0.7465 0.041*
H20C 1.1599 0.5072 0.7542 0.041*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C1 0.020 (2) 0.025 (3) 0.017 (2) 0.005 (2) −0.006 (2) −0.005 (2) C2 0.029 (2) 0.023 (3) 0.013 (2) −0.001 (2) 0.004 (2) −0.001 (2) O3 0.0168 (16) 0.0364 (19) 0.0189 (16) 0.0001 (15) 0.0032 (14) 0.0026 (15) C3 0.016 (2) 0.016 (2) 0.026 (2) 0.000 (2) 0.002 (2) −0.003 (2) O4 0.0177 (16) 0.0267 (17) 0.0185 (15) −0.0015 (14) 0.0007 (14) −0.0015 (14) C4 0.024 (2) 0.017 (2) 0.014 (2) 0.000 (2) −0.004 (2) 0.001 (2) C5 0.018 (2) 0.028 (3) 0.015 (2) 0.002 (2) 0.004 (2) −0.002 (2) O6 0.0202 (16) 0.0280 (17) 0.0216 (16) −0.0014 (15) 0.0041 (14) 0.0042 (15) C6 0.018 (2) 0.027 (3) 0.016 (2) 0.002 (2) 0.000 (2) 0.003 (2) C7 0.027 (2) 0.025 (3) 0.018 (2) 0.002 (2) 0.003 (2) 0.000 (2) C8 0.021 (2) 0.031 (3) 0.016 (2) 0.004 (2) −0.004 (2) −0.003 (2) C9 0.019 (2) 0.023 (2) 0.020 (2) −0.001 (2) −0.001 (2) 0.000 (2) C10 0.019 (2) 0.023 (2) 0.023 (2) 0.000 (2) 0.003 (2) −0.001 (2) C11 0.022 (2) 0.017 (2) 0.020 (2) 0.001 (2) 0.001 (2) 0.000 (2) C12 0.019 (2) 0.015 (2) 0.024 (2) 0.003 (2) 0.001 (2) 0.000 (2) C13 0.014 (2) 0.024 (3) 0.016 (2) 0.001 (2) 0.0002 (19) −0.001 (2) C14 0.024 (2) 0.025 (3) 0.016 (2) −0.003 (2) −0.001 (2) 0.002 (2) C15 0.023 (2) 0.023 (2) 0.020 (2) 0.001 (2) −0.002 (2) 0.001 (2) C16 0.026 (2) 0.024 (2) 0.019 (2) −0.002 (2) −0.001 (2) 0.002 (2) N17 0.020 (2) 0.023 (2) 0.023 (2) −0.0041 (18) −0.0034 (18) 0.0004 (17) C17 0.028 (3) 0.034 (3) 0.029 (3) −0.002 (2) −0.004 (2) 0.003 (2) C18 0.024 (3) 0.081 (4) 0.026 (3) 0.002 (3) 0.009 (2) 0.009 (3) O19 0.0246 (17) 0.047 (2) 0.0296 (18) −0.0058 (17) −0.0085 (16) 0.0053 (16) C19 0.023 (3) 0.016 (2) 0.037 (3) −0.001 (2) 0.000 (2) −0.004 (2) C20 0.022 (2) 0.026 (3) 0.034 (3) 0.001 (2) 0.006 (2) 0.002 (2)
Geometric parameters (Å, º)
C1—C11 1.392 (6) C10—C11 1.516 (5)
C1—C2 1.396 (5) C10—H10A 0.9900
C1—H1 0.9500 C10—H10B 0.9900
C2—C3 1.385 (5) C11—C12 1.377 (5)
C2—H2 0.9500 C12—C13 1.505 (6)
O3—C3 1.385 (5) C13—C14 1.524 (6)
O3—C18 1.413 (5) C13—C15 1.542 (5)
supporting information
sup-4
Acta Cryst. (2005). E61, o2579–o2581
O4—C4 1.387 (4) C15—C16 1.516 (5)
O4—C5 1.471 (4) C15—H15A 0.9900
C4—C12 1.374 (6) C15—H15B 0.9900
C5—C13 1.541 (6) C16—N17 1.465 (5)
C5—C6 1.558 (6) C16—H16A 0.9900
C5—H5 1.0000 C16—H16B 0.9900
O6—C19 1.353 (5) N17—C17 1.460 (5)
O6—C6 1.446 (4) C17—H17A 0.9800
C6—C7 1.495 (6) C17—H17B 0.9800
C6—H6 1.0000 C17—H17C 0.9800
C7—C8 1.331 (6) C18—H18A 0.9800
C7—H7 0.9500 C18—H18B 0.9800
C8—C14 1.484 (6) C18—H18C 0.9800
C8—H8 0.9500 O19—C19 1.203 (5)
C9—N17 1.469 (5) C19—C20 1.492 (6)
C9—C14 1.546 (6) C20—H20A 0.9800
C9—C10 1.559 (5) C20—H20B 0.9800
C9—H9 1.0000 C20—H20C 0.9800
C11—C1—C2 120.7 (4) C11—C12—C13 127.9 (4)
C11—C1—H1 119.7 C12—C13—C14 108.4 (3)
C2—C1—H1 119.7 C12—C13—C5 98.4 (3)
C3—C2—C1 122.0 (4) C14—C13—C5 118.5 (3)
C3—C2—H2 119.0 C12—C13—C15 110.0 (3)
C1—C2—H2 119.0 C14—C13—C15 108.0 (3)
C3—O3—C18 116.8 (3) C5—C13—C15 112.9 (3)
C4—C3—C2 116.7 (4) C8—C14—C13 112.3 (4)
C4—C3—O3 117.4 (4) C8—C14—C9 114.4 (4)
C2—C3—O3 125.9 (4) C13—C14—C9 107.6 (3)
C4—O4—C5 105.0 (3) C8—C14—H14 107.4
C12—C4—C3 121.2 (4) C13—C14—H14 107.4
C12—C4—O4 111.1 (3) C9—C14—H14 107.4
C3—C4—O4 127.6 (4) C16—C15—C13 111.3 (3)
O4—C5—C13 104.1 (3) C16—C15—H15A 109.4
O4—C5—C6 108.9 (3) C13—C15—H15A 109.4
C13—C5—C6 113.8 (3) C16—C15—H15B 109.4
O4—C5—H5 110.0 C13—C15—H15B 109.4
C13—C5—H5 110.0 H15A—C15—H15B 108.0
C6—C5—H5 110.0 N17—C16—C15 111.5 (3)
C19—O6—C6 116.3 (3) N17—C16—H16A 109.3
O6—C6—C7 104.9 (3) C15—C16—H16A 109.3
O6—C6—C5 110.8 (3) N17—C16—H16B 109.3
C7—C6—C5 116.2 (3) C15—C16—H16B 109.3
O6—C6—H6 108.2 H16A—C16—H16B 108.0
C7—C6—H6 108.2 C17—N17—C16 111.8 (3)
C5—C6—H6 108.2 C17—N17—C9 113.1 (3)
C8—C7—C6 125.1 (4) C16—N17—C9 114.2 (3)
supporting information
sup-5
Acta Cryst. (2005). E61, o2579–o2581
C6—C7—H7 117.4 N17—C17—H17B 109.5
C7—C8—C14 122.7 (4) H17A—C17—H17B 109.5
C7—C8—H8 118.6 N17—C17—H17C 109.5
C14—C8—H8 118.6 H17A—C17—H17C 109.5
N17—C9—C14 106.6 (3) H17B—C17—H17C 109.5
N17—C9—C10 115.1 (3) O3—C18—H18A 109.5
C14—C9—C10 112.5 (3) O3—C18—H18B 109.5
N17—C9—H9 107.4 H18A—C18—H18B 109.5
C14—C9—H9 107.4 O3—C18—H18C 109.5
C10—C9—H9 107.4 H18A—C18—H18C 109.5
C11—C10—C9 115.0 (3) H18B—C18—H18C 109.5
C11—C10—H10A 108.5 O19—C19—O6 124.4 (4)
C9—C10—H10A 108.5 O19—C19—C20 126.5 (4)
C11—C10—H10B 108.5 O6—C19—C20 109.2 (4)
C9—C10—H10B 108.5 C19—C20—H20A 109.5
H10A—C10—H10B 107.5 C19—C20—H20B 109.5
C12—C11—C1 116.4 (4) H20A—C20—H20B 109.5
C12—C11—C10 116.9 (3) C19—C20—H20C 109.5
C1—C11—C10 126.5 (4) H20A—C20—H20C 109.5
C4—C12—C11 122.8 (4) H20B—C20—H20C 109.5
C4—C12—C13 109.3 (4)
supporting information
sup-6
Acta Cryst. (2005). E61, o2579–o2581