organic papers
Acta Cryst.(2006). E62, o811–o813 doi:10.1107/S160053680600273X Duskovaet al. C
13H7D16N2O2
o811
Acta Crystallographica Section EStructure Reports Online
ISSN 1600-5368
The 3,3,5,5[
2H
4]-4-methacrylamido-2,2,6,6-tetra([
2H
3]methyl)piperidin-1-yloxyl radical
Jarmila Duskova,a* Jiri Labsky,a Michal Dusekband Jindrich Haseka
aInstitute of Macromolecular Chemistry,
Academy of Sciences of the Czech Republic, Heyrovskeho nam. 2, Praha 6, Czech Republic, andbInstitute of Physics, Academy of Sciences of
the Czech Republic, Cukrovarnicka 10, Praha 6, Czech Republic
Correspondence e-mail: duskova@imc.cas.cz
Key indicators
Single-crystal X-ray study
T= 150 K
Mean(C–C) = 0.002 A˚
Rfactor = 0.039
wRfactor = 0.109
Data-to-parameter ratio = 11.6
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 10 January 2006 Accepted 23 January 2006
#2006 International Union of Crystallography
All rights reserved
The main features of the crystal structure of the title radical, C13H7D16N2O2, are N—H O and C—H O hydrogen
bonds, which are responsible for the formation of chains of molecules in the [001] direction.
Comment
The title compound, (I), can be assigned to aminoxyl radicals belonging to the group of stable radicals. They have been widely used as spin labels for electron spin resonance measurements (Pilar et al., 1999), or as reagents for redox (Naiket al., 1998) or polymerization reactions (Hawkeret al., 1997).
An overall view of the molecule of (I) is shown in Fig. 1. The piperidine ring adopts a chair conformation. The C2/C3/C5/C6 plane forms angles of 32.1 (1) with the C2/N1/C6 plane and
51.8 (1)with the C3/C4/C5 plane. This geometry fits well with
the average geometry of piperidine rings resulting from 114 hits found in the Cambridge Structural Database (Version 5.26; Allen, 2002).
The second part of the molecule, the short methacrylamide chain, is in an equatorial position with respect to the piper-idine ring. Theoretically, it should be planar. However, stress induced by hydrogen bonds results in a rotation between the amide group and the C13 C14 double bond of 15.3 (1). The
amide group remains planar, the C4—N10—C11—O12 torsion angle being 3.8 (2).
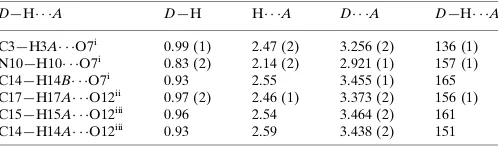
The crystal packing diagram in Fig. 2 shows the network of hydrogen bonds that join molecules of (I) into a chain in the [001] direction. They connect the nitroxyl O atom with three H atoms of a neighbouring molecule. The arrangement of these hydrogen bonds is shown in detail in Fig. 3. The strongest hydrogen bond, with an H O distance of 2.14 (2) A˚ , is directed towards the amino group. Two other hydrogen bonds, towards the methacryloyl group and the CH2 group of the
ring, are significantly longer, with H O = 2.55 and 2.47 (2) A˚ , respectively.
3.438 (2), and 3.464 (2) A˚ , respectively. These bonds connect the [001]-direction chains and give rise to three-dimensional connectivity through the whole crystal structure. Details of all hydrogen bonds are given in Table 1.
Experimental
3,3,5,5[2H4]-4-Methacrylamido-2,2,6,6-tetra([ 2
H3]methyl)piperidine
(1.0 g), sodium ethylendiamintetraacetate (0.1 g) and sodium tung-state(IV) dihydrate (0.1 g) were dissolved in methanol (5 ml) and water (3 ml) with the addition of 30% hydrogen peroxide (1.7 ml).
The mixture was held at laboratory temperature for 4 d. The methanol was removed by evaporation and the product was re-crystallized from dichloromethane–hexane (1:1).
Crystal data
C13H7D16N2O2
Mr= 255.44
Monoclinic,P21=c
a= 12.0838 (5) A˚
b= 11.6346 (6) A˚
c= 11.5420 (6) A˚
= 116.522 (4)
V= 1451.92 (13) A˚3
Z= 4
Dx= 1.169 Mg m
3
MoKradiation
Cell parameters from 16217 reflections
= 1.0–29.5 = 0.07 mm1
T= 150 K
Irregular fragment, yellow 0.270.180.09 mm
Data collection
Oxford Sapphire 2 CCD area-detector diffractometer
!scans
Absorption correction: none 16217 measured reflections 2632 independent reflections
2147 reflections withI> 2(I)
Rint= 0.038
max= 25.4
h=14!14
k=14!14
l=13!13
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.039
wR(F2) = 0.109
S= 1.12 2632 reflections 226 parameters
H atoms treated by a mixture of independent and constrained refinement
w= 1/[2(F
o2) + (0.0616P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.001
max= 0.24 e A˚
3
min=0.18 e A˚
3
organic papers
o812
Duskovaet al. C [image:2.610.47.296.66.254.2]13H7D16N2O2 Acta Cryst.(2006). E62, o811–o813
Figure 1
[image:2.610.337.532.73.354.2]The structure of the title compound, showing the atom-numbering scheme and the chair conformation of the ring of the piperidine derivative. Displacement ellipsoids are drawn at the 50% probability level.
Figure 2
Part of the crystal structure of (I), showing the molecular packing and formation of a chain of molecules along the [001] direction. Dashed lines indicate hydrogen bonds.
Figure 3
Two neighbouring molecules related by symmetry code (x,1 2y,z
1 2),
[image:2.610.46.296.321.576.2]Table 1
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
C3—H3A O7i 0.99 (1) 2.47 (2) 3.256 (2) 136 (1) N10—H10 O7i
0.83 (2) 2.14 (2) 2.921 (1) 157 (1) C14—H14B O7i
0.93 2.55 3.455 (1) 165 C17—H17A O12ii
0.97 (2) 2.46 (1) 3.373 (2) 156 (1) C15—H15A O12iii 0.96 2.54 3.464 (2) 161 C14—H14A O12iii
0.93 2.59 3.438 (2) 151
Symmetry codes: (i)x;yþ1 2;z
1
2; (ii)xþ1;y;zþ1; (iii)x;y 1 2;z
1 2.
All H atoms were found in a difference Fourier map. The H atoms of the more flexible methacryloyl part were refined as riding, with C—H = 0.96 A˚ for –CH3 or 0.93 A˚ for CH2 groups, and with Uiso(H) = 1.5Ueq(C). The deuterium atoms of the methyl groups of
the piperidine ring were considered as H atoms and were stable during the refinement procedure, as well as the remaining H atoms attached to C3 and N2; accordingly, they were refined freely and isotropically.
Data collection: CrysAlis CCD (Oxford Diffraction, 2004); cell refinement: CrysAlis RED (Oxford Diffraction, 2004); data reduc-tion:CrysAlis RED; program(s) used to solve structure:SHELXS97
(Sheldrick, 1997); program(s) used to refine structure:SHELXL97
(Sheldrick, 1997); molecular graphics:DIAMOND(Brandenburg & Putz, 2005); software used to prepare material for publication:
SHELXL97.
This research was supported by the Grant Agency of the Czech Republic (project No. T400500402).
References
Allen, F. H. (2002).Acta Cryst.B58, 380–388.
Brandenburg, K. & Putz, H. (2005).DIAMOND. Release 3.0c. Crystal Impact GbR, Bonn, Germany.
Hawker, C. J. (1997).Acc. Chem. Res.30, 373–382. Naik, N. & Braslau, R. (1998).Tetrahedron,54, 667–696.
Oxford Diffraction (2004). CrysAlis CCD and CrysAlis RED. Oxford Diffraction Ltd., Abingdon, Oxford, England.
Pilar, J., Labsky, J., Marek, A., Konak, C. & Schlick, S. (1999).Macromolecules, 32, 8230–8233.
Sheldrick G. M. (1997).SHELXS97andSHELXL97. University of Go¨ttingen, Germany.
organic papers
Acta Cryst.(2006). E62, o811–o813 Duskovaet al. C
supporting information
sup-1
Acta Cryst. (2006). E62, o811–o813
supporting information
Acta Cryst. (2006). E62, o811–o813 [https://doi.org/10.1107/S160053680600273X]
The 3,3,5,5[
2H
4]-4-methacrylamido-2,2,6,6-tetra([
2H
3]methyl)piperidin-1-yloxyl
radical
Jarmila Duskova, Jiri Labsky, Michal Dusek and Jindrich Hasek
3,3,5,5[2H
4]-4-Methacrylamido-2,2,6,6-tetra([2H3]methyl)piperidin- 1-yloxyl
Crystal data
C13H7D16N2O2
Mr = 255.44
Monoclinic, P21/c
Hall symbol: -P 2ybc a = 12.0838 (5) Å b = 11.6346 (6) Å c = 11.5420 (6) Å β = 116.522 (4)° V = 1451.92 (13) Å3
Z = 4
F(000) = 524 Dx = 1.169 Mg m−3
Mo Kα radiation, λ = 0.7107 Å
Cell parameters from 16217 reflections θ = 1.0–29.5°
µ = 0.07 mm−1
T = 150 K Irregular, yellow 0.27 × 0.18 × 0.09 mm
Data collection
Oxford Sapphire 2 CCD area-detector diffractometer
Radiation source: sealed tube Graphite monochromator
Detector resolution: 8.3438 pixels mm-1
ω scans
16217 measured reflections
2632 independent reflections 2147 reflections with I > 2σ(I) Rint = 0.038
θmax = 25.4°, θmin = 3.5°
h = −14→14 k = −14→14 l = −13→13
Refinement
Refinement on F2
Least-squares matrix: full R[F2 > 2σ(F2)] = 0.039
wR(F2) = 0.109
S = 1.12 2632 reflections 226 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(F
o2) + (0.0616P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 0.24 e Å−3
Δρmin = −0.18 e Å−3
Special details
supporting information
sup-2
Acta Cryst. (2006). E62, o811–o813
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
N1 0.19148 (10) 0.25664 (9) 0.41034 (10) 0.0213 (3) C2 0.07348 (12) 0.20618 (11) 0.30986 (12) 0.0228 (3) C3 0.10042 (13) 0.13321 (12) 0.21510 (13) 0.0227 (3) H3A 0.1152 (13) 0.1852 (12) 0.1556 (13) 0.025 (4)* H3B 0.0260 (13) 0.0871 (12) 0.1627 (13) 0.025 (4)* C4 0.21217 (12) 0.05503 (11) 0.27942 (12) 0.0214 (3) H4A 0.2005 (11) −0.0022 (11) 0.3360 (11) 0.013 (3)* C5 0.32586 (13) 0.12779 (12) 0.35805 (13) 0.0234 (3) H5A 0.3985 (13) 0.0769 (11) 0.4001 (13) 0.021 (3)* H5B 0.3395 (13) 0.1805 (12) 0.2988 (13) 0.025 (4)* C6 0.31554 (12) 0.19942 (11) 0.46433 (12) 0.0237 (3) O7 0.17835 (9) 0.33633 (8) 0.48079 (9) 0.0307 (3) C8 0.01282 (16) 0.13525 (14) 0.37832 (16) 0.0321 (4) H8A 0.0608 (14) 0.0651 (13) 0.4244 (14) 0.031 (4)* H8B −0.0667 (16) 0.1093 (14) 0.3180 (15) 0.042 (5)* H8C 0.0018 (14) 0.1826 (13) 0.4454 (15) 0.036 (4)* C9 −0.01029 (15) 0.30642 (13) 0.23774 (16) 0.0328 (4) H9A −0.0859 (16) 0.2743 (13) 0.1631 (16) 0.041 (4)* H9B 0.0321 (14) 0.3573 (13) 0.2027 (14) 0.033 (4)* H9C −0.0302 (14) 0.3522 (13) 0.2956 (15) 0.036 (4)* N10 0.22804 (11) −0.01080 (9) 0.17994 (11) 0.0259 (3) H10 0.2151 (14) 0.0225 (13) 0.1112 (15) 0.036 (4)* C11 0.28168 (11) −0.11508 (11) 0.20321 (12) 0.0195 (3) O12 0.31476 (9) −0.16342 (8) 0.30842 (9) 0.0280 (3) C13 0.29670 (12) −0.17229 (11) 0.09358 (12) 0.0204 (3) C14 0.28749 (13) −0.11195 (12) −0.01252 (13) 0.0283 (3) H14A 0.2990 −0.1493 −0.0776 0.042* H14B 0.2698 −0.0337 −0.0194 0.042* C15 0.32418 (15) −0.29466 (12) 0.10982 (14) 0.0321 (4) H15A 0.3284 −0.3241 0.0342 0.048* H15B 0.2602 −0.3340 0.1218 0.048* H15C 0.4021 −0.3066 0.1843 0.048* C16 0.33133 (17) 0.12413 (14) 0.57972 (15) 0.0339 (4) H16A 0.2732 (14) 0.0562 (13) 0.5517 (14) 0.035 (4)* H16B 0.4186 (17) 0.0988 (14) 0.6224 (16) 0.046 (5)* H16C 0.3134 (16) 0.1669 (14) 0.6408 (16) 0.046 (5)* C17 0.41298 (15) 0.29402 (14) 0.50904 (17) 0.0338 (4) H17A 0.4935 (16) 0.2575 (13) 0.5372 (15) 0.039 (4)* H17B 0.3977 (13) 0.3451 (12) 0.4338 (14) 0.027 (4)* H17C 0.4050 (14) 0.3405 (13) 0.5777 (15) 0.035 (4)*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-3
Acta Cryst. (2006). E62, o811–o813
C2 0.0232 (7) 0.0204 (7) 0.0254 (7) −0.0018 (5) 0.0115 (6) −0.0046 (5) C3 0.0279 (8) 0.0194 (7) 0.0207 (7) 0.0008 (6) 0.0109 (6) −0.0019 (6) C4 0.0332 (8) 0.0167 (7) 0.0187 (7) 0.0031 (6) 0.0156 (6) 0.0008 (5) C5 0.0276 (8) 0.0210 (7) 0.0245 (7) 0.0051 (6) 0.0143 (6) 0.0024 (6) C6 0.0255 (8) 0.0205 (7) 0.0229 (7) 0.0020 (5) 0.0087 (6) −0.0017 (5) O7 0.0372 (6) 0.0235 (5) 0.0379 (6) −0.0041 (4) 0.0225 (5) −0.0149 (4) C8 0.0350 (9) 0.0320 (9) 0.0376 (9) −0.0099 (7) 0.0235 (8) −0.0108 (7) C9 0.0305 (9) 0.0278 (8) 0.0376 (9) 0.0060 (7) 0.0129 (8) −0.0053 (7) N10 0.0460 (8) 0.0176 (6) 0.0190 (6) 0.0079 (5) 0.0189 (5) 0.0036 (5) C11 0.0217 (7) 0.0169 (7) 0.0211 (7) −0.0002 (5) 0.0108 (6) 0.0003 (5) O12 0.0402 (6) 0.0248 (5) 0.0224 (5) 0.0106 (4) 0.0169 (4) 0.0056 (4) C13 0.0218 (7) 0.0190 (7) 0.0213 (7) −0.0002 (5) 0.0105 (6) −0.0025 (5) C14 0.0401 (9) 0.0245 (7) 0.0255 (7) 0.0043 (6) 0.0193 (7) −0.0037 (6) C15 0.0434 (9) 0.0260 (8) 0.0316 (8) 0.0065 (6) 0.0210 (7) −0.0021 (6) C16 0.0496 (11) 0.0277 (8) 0.0228 (8) 0.0039 (8) 0.0148 (8) −0.0011 (6) C17 0.0266 (9) 0.0290 (8) 0.0402 (9) −0.0013 (6) 0.0098 (7) −0.0051 (7)
Geometric parameters (Å, º)
N1—O7 1.289 (1) C9—H9A 1.007 (17) N1—C2 1.499 (2) C9—H9B 0.981 (16) N1—C6 1.498 (2) C9—H9C 0.965 (16) C2—C9 1.525 (2) N10—C11 1.345 (2) C2—C3 1.530 (2) N10—H10 0.833 (16) C2—C8 1.536 (2) C11—O12 1.232 (1) C3—C4 1.519 (2) C11—C13 1.511 (2) C3—H3A 0.989 (14) C13—C14 1.372 (2) C3—H3B 0.988 (14) C13—C15 1.455 (1) C4—N10 1.462 (2) C14—H14A 0.9300 C4—C5 1.521 (2) C14—H14B 0.9300 C4—H4A 0.986 (12) C15—H15A 0.9600 C5—C6 1.533 (2) C15—H15B 0.9600 C5—H5A 0.989 (19) C15—H15C 0.9600 C5—H5B 0.986 (14) C16—H16A 1.010 (16) C6—C17 1.524 (2) C16—H16B 0.989 (18) C6—C16 1.533 (2) C16—H16C 0.963 (17) C8—H8A 1.005 (15) C17—H17A 0.975 (17) C8—H8B 0.949 (17) C17—H17B 0.999 (14) C8—H8C 1.006 (16) C17—H17C 0.999 (16)
supporting information
sup-4
Acta Cryst. (2006). E62, o811–o813
C3—C2—C8 111.9 (1) C11—N10—H10 118.6 (11) C4—C3—C2 114.2 (1) C4—N10—H10 118.0 (11) C4—C3—H3A 108.0 (8) O12—C11—N10 121.6 (1) C2—C3—H3A 108.6 (8) O12—C11—C13 121.1 (1) C4—C3—H3B 110.0 (8) N10—C11—C13 117.2 (1) C2—C3—H3B 108.2 (8) C14—C13—C15 122.6 (1) H3A—C3—H3B 107.7 (11) C14—C13—C11 122.0 (1) N10—C4—C3 109.2 (1) C15—C13—C11 115.4 (1) N10—C4—C5 111.1 (1) C13—C14—H14A 120.0 C3—C4—C5 109.1 (1) C13—C14—H14B 120.0 N10—C4—H4A 105.9 (7) H14A—C14—H14B 120.0 C3—C4—H4A 111.7 (7) C13—C15—H15A 109.5 C5—C4—H4A 109.8 (7) C13—C15—H15B 109.5 C4—C5—C6 113.91 (11) H15A—C15—H15B 109.5 C4—C5—H5A 109.1 (8) C13—C15—H15C 109.5 C6—C5—H5A 107.8 (8) H15A—C15—H15C 109.5 C4—C5—H5B 108.6 (8) H15B—C15—H15C 109.5 C6—C5—H5B 108.4 (8) C6—C16—H16A 111.7 (8) H5A—C5—H5B 109.0 (11) C6—C16—H16B 107.1 (9) N1—C6—C17 107.4 (1) H16A—C16—H16B 111.2 (13) N1—C6—C5 109.4 (1) C6—C16—H16C 111.1 (10) C17—C6—C5 109.7 (1) H16A—C16—H16C 106.7 (13) N1—C6—C16 109.2 (1) H16B—C16—H16C 109.1 (14) C17—C6—C16 109.9 (1) C6—C17—H17A 107.7 (9) C5—C6—C16 111.2 (1) C6—C17—H17B 108.7 (8) C2—C8—H8A 114.4 (8) H17A—C17—H17B 108.5 (12) C2—C8—H8B 110.6 (10) C6—C17—H17C 109.0 (9) H8A—C8—H8B 106.6 (13) H17A—C17—H17C 114.0 (12) C2—C8—H8C 111.1 (9) H17B—C17—H17C 108.8 (11) H8A—C8—H8C 106.3 (12)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
C3—H3A···O7i 0.99 (1) 2.47 (2) 3.256 (2) 136 (1)
N10—H10···O7i 0.83 (2) 2.14 (2) 2.921 (1) 157 (1)
C14—H14B···O7i 0.93 2.55 3.455 (1) 165
C17—H17A···O12ii 0.97 (2) 2.46 (1) 3.373 (2) 156 (1)
C15—H15A···O12iii 0.96 2.54 3.464 (2) 161
C14—H14A···O12iii 0.93 2.59 3.438 (2) 151