http://www.scirp.org/journal/ojf ISSN Online: 2163-0437
ISSN Print: 2163-0429
DOI: 10.4236/ojf.2018.81002 Nov. 30, 2017 15 Open Journal of Forestry
Hemicellulose-Based Hydrogels Present Status
and Application Prospects: A Brief Review
Lisong Hu, Menghao Du, Jinping Zhang
Research Institute of Subtropical Forestry Chinese Academy of Forestry, Fuyang, China
Abstract
Hemicellulose is one of the most abundant natural resources, which exten-sively exist in the pant tissues as the compositions of the cell wall filling be-tween lignin and cellulose. In this review, the hemicellulose based hydrogels was illustrated in details including the hemicellulose derivatives (such as pre-cursor of hemicellulose based hydrogel), hydrogels fabrication methods, origin of hemicellulose, composite hydrogels, and so on. In addition, the authors as well overviewed the new methods for fabrication of hemicellulose based hy-drogels and special functional hemicellulose based hyhy-drogels.
Keywords
Hemicellulose, Hydrogels, Graft polymers, Swelling, Conductivity
1. Introduction
Hydrogels are the mainly three-dimensional, cross-linked networks (forming by chemical and physical method) of hydrophilic polymers that have ability of ad-sorbing up to thousands of times of their dry weight in water (Ahmed, 2015; Se-liktar, 2012). Hydrogels have many excellent properties and extensive applica-tions such as hydrophilicity, soft tissue-mimicking consistency, high permeabil-ity to metabolites, oxygen resilience and environmental sensitivpermeabil-ity (Yamato, Konno, Utsumi, Kikuchi, & Okano, 2002). Hydrogels are wide applied in medi-cal fields such as soft contact lenses (Katsoulos, Karageorgiadis, Vasileiou, Mou-safeiropoulos, & Asimellis, 2009; Yasuda, 2006), tissue engineering (Ma, Tang, Bil-lingham, Armes, & Lewis, 2003; Matricardi, Di Meo, Coviello, Hennink, & Al-haique, 2013), drug delivery (Noro, Hayashi, & Matsushita, 2012), wound dress-ing (Ferreira et al., 2009; Melandri et al., 2006), biosensor (Beines, Klosterkamp, Menges, Jonas, & Knoll, 2007), biomimetic mineralization (Huang et al., 2013), and cancer therapy (Zhang, Mardyani, Chan, & Kumacheva, 2006).
How to cite this paper: Hu, L. S., Du, M. H., & Zhang, J. P. (2018). Hemicellulose-Based Hydrogels Present Status and Application Prospects: A Brief Review. Open Journal of Forestry, 8, 15-28.
https://doi.org/10.4236/ojf.2018.81002
Received: November 9, 2017 Accepted: November 27, 2017 Published: November 30, 2017
Copyright © 2018 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
DOI: 10.4236/ojf.2018.81002 16 Open Journal of Forestry Natural hydrogels such as starch, chitin, cellulose and alginic acid were gradu-ally replaced by synthetic hydrogels which have long service life, high capacity of water absorption, and high gel strength, during last two decades. Synthetic po-lymers usually have well-defined structures that can be modified to yield tailora-ble degradability and functionality (Jagur-Grodzinski, 2009).
Due to the advantage of hydrophilicity, structural versatility, nontoxicity, and broad potential of chemical modification, polysaccharides are highly interesting in the design of hydrogels. Various polysaccharides have been investigated for hydrogel formulations including alginate, dextran, chitosan, starch, and purified hemicellulose (Chang, Duan, & Zhang, 2009; Chen, Jo, & Park, 1995; Hennink, Franssen, van Dijk-Wolthuis, & Talsma, 1997). In the agricultural field, release sys-tems for fertilizers are a long-known opportunity to reduce costs and environ-mental impact as well as increase the profit. Another possible use for release systems in agriculture is a seed treatment that enables so-called relay cropping or cover crops to increase crop yields per area and year as well as protect the land against erosion.
2. Hemicellulose
Hemicellulose as natural polysaccharide is the second most abundant compo-nent of the plant materials. Plant materials main consist of cellulose, hemicellu-lose and lignin. Hemicelluhemicellu-lose is about 20% - 30% of the total weight of the lig-nocellulosic biomass (Anwar, Gulfraz, & Irshad, 2014). Unlike cellulose, hemicel-lulose consists of shorter chains 500 - 3000 sugar units as opposed to 7000 - 15,000 glucose molecules per polymer.
Structure of Hemicellulose
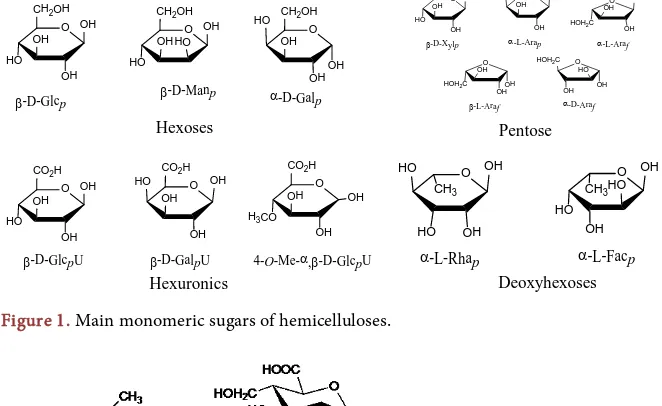
Hemicellulose is hetero polymers (matrix polysaccharides) constructed by differ-ent polysaccharides with differdiffer-ent chemical linkages. Hemicellulose has a random, amorphous structure with little strength. The main unit of the polysaccharides is pentose and hexose. The unit of the pentose and hexose are xylose, mannose, galactose, rhamnose, and arabinose as shown in Figure 1.
Different hemicellulose compositions, structures and amounts could be obtained from different source of different biomass. For example, the main hemicellulose in hardwood is O-acetyl-(4-O-methylglucurono) xylan (Figure 2). In the back-bone of the hardwood, approximately 10% of xylopyranose are substituted by 4-o-methyl glucuronic acid.
In contrast, the predominant component of softwood hemicellulose is O-acetyl-galactoglucomannan (AcGGM) (Figure 3). The backbones of softwood hemicellulose are composed by mannose units combining with glucose units randomly (Ayoub, Venditti, Pawlak, Sadeghifar, & Salam, 2013; De & Visser, 2001).
DOI: 10.4236/ojf.2018.81002 17 Open Journal of Forestry
[image:3.595.209.531.377.502.2]Figure 1. Main monomeric sugars of hemicelluloses.
Figure 2. Main hemicellulose of hardwoods: O-acetyl-4-o-methylgucoronoxylan.
Figure 3. Main hemicellulose of softwoods: O-acetyl-galactoglucomannan (AcGGM).
2000), alkaline extraction (Egüés, Sanchez, Mondragon, & Labidi, 2012), liquid hot-water extraction (Hasegawa, Tabata, Okuma, & Mae, 2004), steam treatment (Palm & Zacchi, 2003), ionic liquid extraction (Froschauer et al., 2013) and so on. These methods are available to extract the hemicellulose. However, acid pre-treatment such as sulfuric acid, could obtain more degraded products dissolved sugars. While hot-water method could cleave the acetate group from hemicellu-lose, Ionic liquid method has been used by Froschauer and coworkers to obtain the hemicellulose with high purity. Alkaline method is extensively applied and well-studied in extract hemicellulose. The reason is that alkaline method could furthest retain integrity of hemicellulose the different method employed den-pends on what the product you target to. As for the hydrogel and film, it may be extremely important to use alkaline method for the separation of hemicellulose from biomass. O HO OH OH OH CH2OH
O
HO OH HO
OH CH2OH
O HO
OH
OH OH CH2OH
β-D-Glcp β-D-Manp α-D-Galp
O
HO OH
OH
OH HO O OH
OH OH
O
HOH2C OH
OH OH
O
HOH2C
OH
OH OH
O HOH2C
OH HO
OH
β-D-Xylp α-L-Arap α-L-Araf
β-L-Araf α-D-Araf
O
HO OH
OH OH CO2H
O HO
OH
OH OH CO2H
O
H3CO OH
OH OH CO2H
β-D-GlcpU β-D-GalpU 4-O-Me-α,β-D-GlcpU
O HO HO OH OH CH3 O HO OH HO OH CH3
α-L-Rhap α-L-Facp
Hexoses Pentose
DOI: 10.4236/ojf.2018.81002 18 Open Journal of Forestry
3. Hemicellulose Based Hydrogels
Hemicellulose is one of the most abundant natural resource, which are exten-sively exist in the plant tissue as the compositions of the cell wall filling between lignin and cellulose, with the chemical bond that hemicellulose is combined with lignin and through van der’s force bind with cellulose. Hemicelluloses have tre-mendous potential in various fields. Due to their special properties, unique struc-ture with huge amount of hydroxyl group, which could be modified by many kinds of reactions such as ether, ester, chemical crosslinking, hemicellulose tends to be modified by chemical reactions and physical interactions to manufacture lots of products such as packing films, oxygen barrier and water-resistant coatings (Van Tuil, Fowler, Lawther, & Weber, 2000). Many kinds of hemicellulose from differ-ent of plants are applied to preparation of hydrogel, such as birch wood, aspen wood, spruce, bamboo, straw and so on.
3.1. Hemicellulose Derivatives
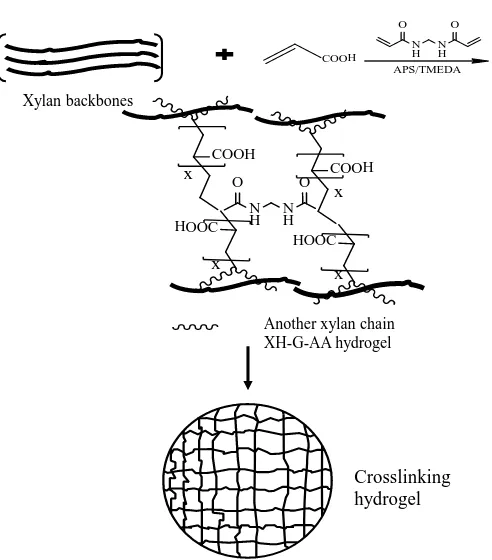
Several monomers have been used to prepare hemicellulose based hydrogels. These monomers include acrylic acid (AA), maleic acid, methacrylic acid and others. Acrylic acid is an important monomer which is widely used for the prep-aration of functional hydrogels. Acrylic acid as monomer is well studied and known as a very effective monomer for forming hydrogel. Indeed, acrylic acid can easily react with hemicellulose extracting from most kinds of plants. In research by Peng and coworkers, hemicellulose which was extracted from bamboo by using alkali method (10% KOH) in the presence of cross-linker copolymerized with acrylic acid as shown in (Figure 4). Different ratio of MBA/XH and AA/XH were employed to investigate the influence of the networks. The results showed as-prepared hy-drogels can absorb 91 to 822 times weight of itself, and were sensitive to ph. The hydrogels also have the feature of reversible on-off switching behavior in acid-ic-basic solutions or water-ethanol or acetone solution (Peng, Ren, Zhong, Peng, & Sun, 2011). In another study, the hydrogel by grafting acrylic acid on the back-bone of hemicellulose possessed honeycomb-like porous structure and had remark-able pH-sensitivity. Meanwhile, the biodegrade ability was conducted as well. In fact, the hydrogel can be degraded, due to their hemicellulose ingredients (Sun, Wang, Jing, & Mohanathas, 2013).
DOI: 10.4236/ojf.2018.81002 19 Open Journal of Forestry
Figure 4. Preparation of xylan-rich hemicellulose based hydrogels with acrylic acid (Peng et al., 2011).
by Maleki and coworkers developed full interpenetrating polymer network (IPN) by using a couple of precursors (Maleki, Edlund, & Albertsson, 2017). The shear storage modulus of IPNs was 2.5 - 5 times higher than that of the corresponding precursor single network. The swelling rate were faster compared the single network gels.
3.2. Synthesis of Methacrylic Hydrogels
Acrylic acid is frequently grafted molecular for preparing hemicellulose-based hydrogel, due to its excellent reaction activity. Researchers studied also studied its analogous such as methacrylic acid to prepare hydrogel. Ferrari and cowork-ers designed renewable hemicellulose based amidoamine hydrogels that prepared through free radical copolymeriaztion of poly (amidoamine) and hemicellulose derivatives. In research, the synthetic approach was viable in order to fabricate the functional hydrogels. The results indicated that as-prepared hydrogels dis-played significant high adsorption capacity for heavy metal ions such as Cu2+, Pb2+, Co2+.
As the reactions list above, the hemicellulose based hydrogels were synthe-sized by grafting the acrylic acid to the backbone of the hemicellulose. The me-chanism of the preparation of hydrogels briefly as follows: sulfate anion radical generated from ammonium persulfate deprives of hydrogen from the hydroxyl group of hemicellulose backbone to form alkoxy radicals, resulting in active centers on the hemicellulose to radically initiate polymerization (Peng et al., 2011; Sun et al., 2015).
Xylan backbones
N
H NH
O O
x COOH
HOOC
x COOH
HOOC
x x
Another xylan chain XH-G-AA hydrogel
COOH N H NH
O O
APS/TMEDA
DOI: 10.4236/ojf.2018.81002 20 Open Journal of Forestry
3.3. Cross-Linking Agent Applied in Fabricating Hemicellulose
Based Hydrogels
The hemicelluloses usually react with acrylic acid through free radical polymeri-zation of using N, N-methylene-bis-acrylamide (MBA) as cross-linker and ammo-nium persulfate N, N, N’, N’-tetramethylethylenediamine (APS/TMEDA) as Re-dox. The agent such as azo-bis-butyrnitrile (AIBN) (Cao, Peng, Zhong, & Sun, 2014) and potassium persulfate (KPS) (Maleki, Edlund, & Albertsson, 2015), sodium sulfite (Maleki et al., 2015), [2-(methacryloyloxy)ethyl] trimethylammonium chlo-ride (MeDMA) (Dax et al., 2014) Sodium Pyrosulfite, ammonium persulfate (Fer-rari, Ranucci, Edlund, & Albertsson, 2014), N-N’-methylenebisacrylamide (Ma-leki, Edlund, & Albertsson, 2016) as initiator, Also have prepared the hydrogel use freeze-thaw methods (Guan, Bian, Peng, Zhang, & Sun, 2014; Guan, Zhang, Bian, Peng, & Sun, 2014).
3.4. Click Reaction of Hemicellulose and Thiol-Ene
The click chemistry introduced by Sharpless, has attracted a lot of attention in many fields of chemistry. a click reaction should have these features such as highly efficient, no byproducts, be stereospecific, use readily available starting materials, use benign or no solvent, and require simple purification technique (Kolb, Finn, & Sharpless, 2001). One of the most widely employed click reactions: thiol-ene has attracted attention in the last ten years due to the commercial availability of a wide range of thiols (Hoyle, Lowe, & Bowman, 2010; Kade, Burke, & Hawker, 2010). The versatility of thiol click reactions has been demonstrated by preparing tailor-made macromolecular architectures varying from telechelic homopoly-mers to highly complex dendritic structures (Jones et al., 2009; Nurmi, Lindqv-ist, Randev, Syrett, & Haddleton, 2009).
AcGGM was materials for thiolation through one-pot synthetic methodology, this reaction realized by the AcGGM-mediated nucleophilic ring-opening reaction of thiobutyrolactone via the activation of the polysaccharide pendant hydroxyl groups. The first step is to synthesis thiolate acetyl galactoglucomannan, previous study was applied pendant amine or carboxylic acid via transesterfication to complete the objective products for several steps (Lin et al., 2010). However, in the reac-tion of thiol-ene click chemistry, it just needed one step. And the solvent used water as solvent, use thioacetic acid-ene approach; they successfully introduced thiol- amine- or amino acid functional xylan. First of all, the allyl chloride reacted with hydroxyl on xylan through esterification, secondly, via click chemistry; the thiol was introduced on the backbone of the xylan. The thiol-containing xylan was used to modify filter paper surface by a simple dipping method, which pro-vides a novel and convenient (Pahimanolis, Kilpeläinen, Master, Ilvesniemi, & Seppälä, 2015).
3.5. Hemicellulose-Polymer Composite Hydrogels
DOI: 10.4236/ojf.2018.81002 21 Open Journal of Forestry the free radical polymerization and click chemistry for fabrication of which has functional hydrogels. However, these hydrogels displayed many drawbacks such as weak thermostability, brittle properties and weak mechanical properties. To overcome these problems, plenty of methods were applied. Among of them, em-ploying compatible ingredients such as nanowhiskers chitin, chitosan, polyvinyl alcohol, oat spelt and so, for enhancing their properties (Gabrielii & Gatenholm, 2015; Guan, Bian, et al., 2014; Guan, Zhang, et al., 2014; Meena, Lehnen, Schmitt, & Saake, 2011). Guan and coworkers (Guan, Zhang, et al., 2014) studied prepar-ing hemicellulose based hydrogels through freeze-thaw treatment. In this study, the hydrogels were prepared from hemicelluloses, polyvinyl alcohol and nanow-hiskers. With increasing the portion of chitin whiskers, the mechanical of the hydrogels were significantly improved, with the highest stress of 9.6 MPa. In another study (Gabrielii & Gatenholm, 2015), the hydrogel complexing between glucu-ronic acid and amino acids indicated porous structure, sensitive to pH.
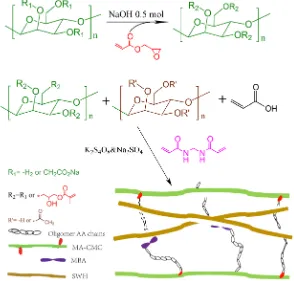
IPNs are ‘‘alloys’’ of cross-linked polymers, at least one of them being synthe-sized and/or cross-linked within the immediate presence of the other, without any covalent bonds between them, which cannot be separated unless chemical bonds are broken (Dragan, 2014; Myung et al., 2008). IPN is a multi-component network made by molecular scale penetration of a linear or branched polymer into a cross-linked network that offer superior control and versatility over single network hydrogels with possibilities to tailor-make products with desired prop-erties by mixing countless combinations of polymers with complementing cha-racteristics (Matricardi et al., 2013; Myung et al., 2008; Reddy, Rao, Vani, Rao, & Lee, 2016). In study by Malaki et al. employed the abundant hemicellulose by green method to fabricate the semi-IPN hydrogels. The hydrogel was directly produced by a facile and green synthetic pathway through cross-linking mod-ified. A category of said composite networks can be developed by the simulta-neous or sequential cross-linking of at least two polymer networks in juxtaposi-tion. In another study of Maleki and coworkers (Maleki et al., 2017), AcGGM was employed to prepare IPNs-hydrogels via free radical polymerization and “click chemistry reaction” (Figure 5). As-prepared IPNs hydrogels indicated faster swelling rates, highly porous structure and significantly improved shear storage modulus than the corresponding precursor single networks of AcGGM (Maleki et al., 2017).
3.6. Conductivity Hemicellulose Based Hydrogels
DOI: 10.4236/ojf.2018.81002 22 Open Journal of Forestry
Figure 5. Synthetic pathway of preparing semi-IPN hydrogels (Maleki, Ed-lund, & Albertsson, 2016).
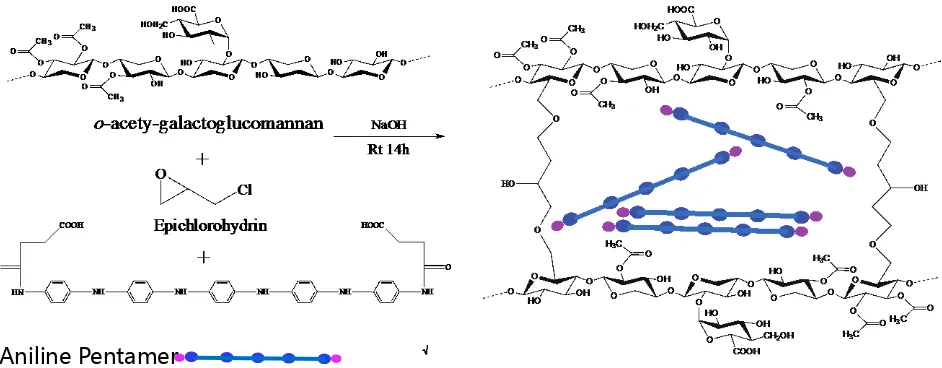
field. However, they are not environmentally friend (Guo, Glavas, & Albertsson, 2013). Zhao et al. synthesized electrically conductive hemicellulose hydrogels via one-pot reaction. In this study, o-acety-galactoglucomannan reacts with aniline pentamer. The reactions were green and ambient (water and room temperature). The author used aniline oligomers with carboxyl group to enhance the solubility in water, where previous studies that aniline with poor solubility in water (Mey-bodi, Imani, & Atai, 2013). The hemicellulose based hydrogel were prepared through cross-linking reaction of AcGGM with epichlorohydrin in the presence of ani-line pentama and sodium hydroxide (Figure 6).
The hydrogels without AP were transparent; however, due to dark of AP, ECHHs were opaque. And the FT-IR shows the structure of the ECHHs exist the carbonyl group and hemicellulose successfully incorporated to AP at 1568−1 peak. The smelling behavior affect a lot by cross-linker agent, and the thermal stability of ECHHs was enhanced, and the conductivity increased by 3 orders of magnitude.
4. Applications of Hemicellulose Based Hydrogels
DOI: 10.4236/ojf.2018.81002 23 Open Journal of Forestry
Figure 6. The synthetic pathway of conduct polymers (Zhao, Glavas, Odelius, Edlund, & Albertsson, 2014).
Nowadays, as the development of economy and industrialization, environment was heavily polluted through water, atmosphere and soil. Among of them, the heavy metal pollution is harmful to human beings and animals, due to the heavy metal can penetrate in soil and water. Animals and mankind once take in the body; the heavy metal could accumulate in body hurt organs. In recent years, environmen-tally sensitive hydrogels have been widely applied as adsorbents for the removal of hazardous substances from waste water.
Sun and coworkers (Sun et al., 2015), using CaCO3 as the porogen, prepared the wheat straw hemicellulose based hydrogels, which had high adsorption ca-pacity for methylene blue and smart swelling property. These properties were very significant for wastewater treatment application. In another research, ma-cro porous xylan-rich hemicellulose based hydrogels were fabricated via free radical copolymerization of xylan and acrylic acid. In study, the results indicated that adsorption capacities of as-prepared hydrogels for up-taking Pb2+, Cd2+, and Zn2+ were 635, 448, 158 mg/g respectively. While, as the pH value increasing, due to the proton depriving by base, the adsorption capacity significantly in-creased. A novel hemicellulose based hydrogels were designed by Ferrari and coworkers (Ferrari et al., 2014). The amidoamine was introduced into the back-bone of the hemicellulose, through water based free radical graft copolymeriza-tion and cross-linking. And the hydrogel displayed a significantly high adsorp-tion capacity for Cu2+, Cd2+, Pb2+, Zn2+, Ni2+, Co2+, and 2
4
CrO −.
5. Summary and Overview
The hemicelluloses based hydrogels as renewable and biodegradable materials, have a lot of favorable properties such as hydrophilicity, biodegradability, bio-compatibility, transparency, low cost, and non-toxicity. Therefore, hemicellulose based hydrogel was widely applied in contact lenses, tissue engineering, and so on. Hemicellulose based hydrogels can be prepared via free radical pathway and click reaction of thiol directives. Theses hydrogels have been studied in drug
DOI: 10.4236/ojf.2018.81002 24 Open Journal of Forestry livery, super adsorbent of heavy metal for environment treatment and adsorp-tion of water. Recent researches into hemicellulose based hydrogels have com-bined hemicellulose with chitosan, polyvinyl alcohol to create hybrid hydrogels with anti-bacterial function.
However, hemicellulose based hydrogels are usually brittle and weak-strength, which restrict their application in material field. In future, preparing hemicellu-lose based hydrogels such as semi and full IPN hydrogels that have high strength and swelling capacity will have promising prospect. In addition, special func-tional hydrogels such as conductible, magnetic, and hybrid composites will at-tract lots of attention. In a word, hemicellulose is environmental friendly and low-cost hydrogels, which will form a viable substitute for fossil-based materials in near future.
Acknowledgements
This work was financial supported by the National Key R&D Program of China (2016YFD0600803)
References
Ahmed, E. M. (2015). Hydrogel: Preparation, Characterization, and Applications: A Re-view. Journal of Advanced Research, 6, 105-121.
https://doi.org/10.1016/j.jare.2013.07.006
Anwar, Z., Gulfraz, M., & Irshad, M. (2014). Agro-Industrial Lignocellulosic Biomass a Key to Unlock the Future Bio-Energy: A Brief Review. Journal of Radiation Research and Applied Sciences, 7, 163-173. https://doi.org/10.1016/j.jrras.2014.02.003
Ayoub, A., Venditti, R. A., Pawlak, J. J., Sadeghifar, H., & Salam, A. (2013). Development of an Acetylation Reaction of Switchgrass Hemicellulose in Ionic Liquid without Cata-lyst. Industrial Crops and Products, 44, 306-314.
https://doi.org/10.1016/j.indcrop.2012.10.036
Beines, P. W., Klosterkamp, I., Menges, B., Jonas, U., & Knoll, W. (2007). Responsive Thin Hydrogel Layers from Photo-Cross-Linkable Poly (N-isopropylacrylamide) Ter-polymers. Langmuir, 23, 2231-2238. https://doi.org/10.1021/la063264t
Cao, X., Peng, X., Zhong, L., & Sun, R. (2014). Multiresponsive Hydrogels Based on Xy-lan-Type Hemicelluloses and Photoisomerized Azobenzene Copolymer as Drug Deli-very Carrier. Journal of Agricultural and Food Chemistry, 62, 10000-10007.
https://doi.org/10.1021/jf504040s
Chang, C., Duan, B., & Zhang, L. (2009). Fabrication and Characterization of Novel Ma-croporous Cellulose-Alginate Hydrogels. Polymer, 50, 5467-5473.
https://doi.org/10.1016/j.polymer.2009.06.001
Chen, J., Jo, S., & Park, K. (1995). Polysaccharide Hydrogels for Protein Drug Delivery. Carbohydrate Polymers, 28, 69-76. https://doi.org/10.1016/0144-8617(95)00080-1
Dax, D., Chávez, M. S., Xu, C., Willför, S., Mendonça, R. T., & Sánchez, J. (2014). Catio-nic Hemicellulose-Based Hydrogels for ArseCatio-nic and Chromium Removal from Aqueous Solutions. Carbohydrate Polymers, 111, 797-805.
https://doi.org/10.1016/j.carbpol.2014.05.045
De, R. V., & Visser, J. (2001). Aspergillus Enzymes Involved in Degradation of Plant Cell Wall Polysaccharides. Microbiology and Molecular Biology Reviews: MMBR, 65, 497-522.
DOI: 10.4236/ojf.2018.81002 25 Open Journal of Forestry Dragan, E. S. (2014). Design and Applications of Interpenetrating Polymer Network
Hy-drogels. A Review. Chemical Engineering Journal, 243, 572-590.
https://doi.org/10.1016/j.cej.2014.01.065
Egüés, I., Sanchez, C., Mondragon, I., & Labidi, J. (2012). Effect of Alkaline and Autohy-drolysis Processes on the Purity of Obtained Hemicelluloses from Corn Stalks. Biore-source Technology, 103, 239-248. https://doi.org/10.1016/j.biortech.2011.09.139
Ferrari, E., Ranucci, E., Edlund, U., & Albertsson, A.-C. (2014). Design of Renewable Poly(Amidoamine)/Hemicellulose Hydrogels for Heavy Metal Adsorption. Journal of Applied Polymer Science, 132, 41695 p. https://doi.org/10.1002/app.41695
Ferreira, L. M., Blanes, L., Gragnani, A., Veiga, D. F., Veiga, F. P., Nery, G. B., & Okamoto, R. (2009). Hemicellulose Dressing versus Rayon Dressing in the Re-Epithelialization of Split-Thickness Skin Graft Donor Sites: A Multicenter Study. Journal of Tissue Viability, 18, 88-94. https://doi.org/10.1016/j.jtv.2009.06.001
Froschauer, C., Hummel, M., Iakovlev, M., Roselli, A., Schottenberger, H., & Sixta, H. (2013). Separation of Hemicellulose and Cellulose from Wood Pulp by Means of Ionic Liquid/Cosolvent Systems. Biomacromolecules, 14, 1741-1750.
https://doi.org/10.1021/bm400106h
Gabrielii, I., & Gatenholm, P. (2015). Preparation and Properties of Hydrogels Based on Hemicellulose. Journal of Applied Polymer Science, 69, 1661-1667.
https://doi.org/10.1002/(SICI)1097-4628(19980822)69:8<1661::AID-APP19>3.0.CO;2-X
Green, R. A., Lovell, N. H., & Poole-Warren, L. A. (2009). Cell Attachment Functionality of Bioactive Conducting Polymers for Neural Interfaces. Biomaterials, 30, 3637-3644.
https://doi.org/10.1016/j.biomaterials.2009.03.043
Guan, Y., Bian, J., Peng, F., Zhang, X.-M., & Sun, R.-C. (2014). High Strength of Hemi-celluloses Based Hydrogels by Freeze/Thaw Technique. Carbohydrate Polymers, 101, 272-280. https://doi.org/10.1016/j.carbpol.2013.08.085
Guan, Y., Zhang, B., Bian, J., Peng, F., & Sun, R.-C. (2014). Nanoreinforced Hemicellu-lose-Based Hydrogels Prepared by Freeze-Thaw Treatment. Cellulose, 21, 1709-1721.
https://doi.org/10.1007/s10570-014-0211-9
Guo, B., Glavas, L., & Albertsson, A.-C. (2013). Biodegradable and Electrically Conduct-ing Polymers for Biomedical Applications. Progress in Polymer Science, 38, 1263-1286.
https://doi.org/10.1016/j.progpolymsci.2013.06.003
Hasegawa, I., Tabata, K., Okuma, O., & Mae, K. (2004). New Pretreatment Methods Combining a Hot Water Treatment and Water/Acetone Extraction for Ther-mo-Chemical Conversion of Biomass. Energy & Fuels, 18, 755-760.
https://doi.org/10.1021/ef030148e
Hennink, W. E., Franssen, O., van Dijk-Wolthuis, W. N. E., & Talsma, H. (1997). Dextran Hydrogels for the Controlled Release of Proteins. Journal of Controlled Release, 48, 107-114. https://doi.org/10.1016/S0168-3659(97)00047-3
Hoyle, C. E., Lowe, A. B., & Bowman, C. N. (2010). Thiol-Click Chemistry: A Multifa-ceted Toolbox for Small Molecule and Polymer Synthesis. Chemical Society Reviews, 39, 1355-1387. https://doi.org/10.1039/b901979k
Huang, J., Ten, E., Liu, G., Finzen, M., Yu, W., Lee, J. S., Tomsia, A. P. et al. (2013). Bio-composites of pHEMA with HA/β-TCP (60/40) for Bone Tissue Engineering: Swelling, Hydrolytic Degradation, and in Vitro Behavior. Polymer, 54, 1197-1207.
https://doi.org/10.1016/j.polymer.2012.12.045
Jagur-Grodzinski, J. (2009). Polymeric Gels and Hydrogels for Biomedical and Pharma-ceutical Applications. Polymers for Advanced Technologies.
DOI: 10.4236/ojf.2018.81002 26 Open Journal of Forestry Jones, M. W., Mantovani, G., Ryan, S. M., Wang, X., Brayden, D. J., & Haddleton, D. M.
(2009). Phosphine-Mediated One-Pot Thiol-Ene “Click” Approach to Polymer-Protein Conjugates. Chemical Communications, 35, 5272-5274.
https://doi.org/10.1039/b906865a
Kade, M. J., Burke, D. J., & Hawker, C. J. (2010). The Power of Thiol-Ene Chemistry. Journal of Polymer Science Part A: Polymer Chemistry, 48, 743-750.
https://doi.org/10.1002/pola.23824
Katsoulos, C., Karageorgiadis, L., Vasileiou, N., Mousafeiropoulos, T., & Asimellis, G. (2009). Customized Hydrogel Contact Lenses for Keratoconus Incorporating Correc-tion for Vertical Coma AberraCorrec-tion. Ophthalmic & Physiological Optics: The Journal of the British College of Ophthalmic Opticians (Optometrists), 29, 321-329.
https://doi.org/10.1111/j.1475-1313.2009.00645.x
Kolb, H. C., Finn, M. G., & Sharpless, K. B. (2001). Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angewandte Chemie International Edition, 40, 2004-2021.
https://doi.org/10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
Lin, C., Zhao, P., Li, F., Guo, F., Li, Z., & Wen, X. (2010). Thermosensitive in Situ Form-ing Dextran-Pluronic Hydrogels through Michael Addition. Materials Science and En-gineering: C, 30, 1236-1244. https://doi.org/10.1016/j.msec.2010.07.004
Ma, Y., Tang, Y., Billingham, N. C., Armes, S. P., & Lewis, A. L. (2003). Synthesis of Bio-compatible, Stimuli-Responsive, Physical Gels Based on ABA Triblock Copolymers. Biomacromolecules, 4, 864-868. https://doi.org/10.1021/bm034118u
Maleki, L., Edlund, U., & Albertsson, A.-C. (2015). Thiolated Hemicellulose as a Versatile Platform for One-Pot Click-Type Hydrogel Synthesis. Biomacromolecules, 16, 667-674.
https://doi.org/10.1021/bm5018468
Maleki, L., Edlund, U., & Albertsson, A.-C. (2016). Green Semi-IPN Hydrogels by Direct Utilization of Crude Wood Hydrolysates. ACS Sustainable Chemistry & Engineering, 4, 4370-4377. https://doi.org/10.1021/acssuschemeng.6b00938
Maleki, L., Edlund, U., & Albertsson, A.-C. (2017). Synthesis of Full Interpenetrating Hemicellulose Hydrogel Networks. Carbohydrate Polymers, 170, 254-263.
https://doi.org/10.1016/j.carbpol.2017.04.091
Matricardi, P., Di Meo, C., Coviello, T., Hennink, W. E., & Alhaique, F. (2013). Interpe-netrating Polymer Networks Polysaccharide Hydrogels for Drug Delivery and Tissue Engineering. Advanced Drug Delivery Reviews, 65, 1172-1187.
https://doi.org/10.1016/j.addr.2013.04.002
Meena, R., Lehnen, R., Schmitt, U., & Saake, B. (2011). Effect of Oat Spelt and Beech Xy-lan on the Gelling Properties of Kappa-Carrageenan Hydrogels. Carbohydrate Poly-mers, 85, 529-540. https://doi.org/10.1016/j.carbpol.2011.03.002
Melandri, D., Angelis, A. D., Orioli, R., Ponzielli, G., Lualdi, P., Giarratana, N., & Reiner, V. (2006). Use of a New Hemicellulose Dressing (Veloderm) for the Treatment of Split-Thickness Skin Graft Donor Sites: A Within-Patient Controlled Study. Burns, 32, 964-972. https://doi.org/10.1016/j.burns.2006.03.013
Meybodi, Z. E., Imani, M., & Atai, M. (2013). Kinetics of Dextran Crosslinking by Epi-chlorohydrin: A Rheometry and Equilibrium Swelling Study. Carbohydrate Polymers, 92, 1792-1798. https://doi.org/10.1016/j.carbpol.2012.11.030
Myung, D., Waters, D., Wiseman, M., Duhamel, P. E., Noolandi, J., Ta, C. N., & Frank, C. W. (2008). Progress in the Development of Interpenetrating Polymer Network Hydro-gels. Polymers for Advanced Technologies, 19, 647-657.
DOI: 10.4236/ojf.2018.81002 27 Open Journal of Forestry Nguyen, Q. A., Tucker, M. P., Keller, F. A., & Eddy, F. P. (2000). Two-Stage Dilute-Acid Pretreatment of Softwoods. Applied Biochemistry and Biotechnology, 84-86, 561-576.
https://doi.org/10.1385/ABAB:84-86:1-9:561
Noro, A., Hayashi, M., & Matsushita, Y. (2012). Design and Properties of Supramolecular Polymer Gels. Soft Matter, 8, 6416-6429. https://doi.org/10.1039/c2sm25144b
Nurmi, L., Lindqvist, J., Randev, R., Syrett, J., & Haddleton, D. M. (2009). Glycopolymers via Catalytic Chain Transfer Polymerisation (CCTP), Huisgens Cycloaddition and Thiol-Ene Double Click Reactions. Chemical Communications (Cambridge, England), No. 19, 2727-2729. https://doi.org/10.1039/b904249k
Pahimanolis, N., Kilpeläinen, P., Master, E., Ilvesniemi, H., & Seppälä, J. (2015). Novel Thiol-Amine and Amino Acid Functional Xylan Derivatives Synthesized by Thiol-Ene Reaction. Carbohydrate Polymers, 131, 392-398.
https://doi.org/10.1016/j.carbpol.2015.06.007
Palm, M., & Zacchi, G. (2003). Extraction of Hemicellulosic Oligosaccharides from Spruce using Microwave Oven or Steam Treatment. Biomacromolecules, 4, 617-623.
https://doi.org/10.1021/bm020112d
Peng, X.-W., Ren, J.-L., Zhong, L.-X., Peng, F., & Sun, R.-C. (2011). Xylan-Rich Hemicel-luloses-Graft-Acrylic Acid Ionic Hydrogels with Rapid Responses to pH, Salt, and Or-ganic Solvents. Journal of Agricultural and Food Chemistry, 59, 8208-8215.
https://doi.org/10.1021/jf201589y
Reddy, N. S., Rao, K. M., Vani, T. J. S., Rao, K. S. V. K., & Lee, Y. I. (2016). Pec-tin/poly(acrylamide-co-acrylamidoglycolic acid) pH Sensitive Semi-IPN Hydrogels: Selective Removal of Cu2+ and Ni2+, Modeling, and Kinetic Studies. Desalination and Water Treatment, 57, 6503-6514. https://doi.org/10.1080/19443994.2015.1008053
Seliktar, D. (2012). Designing Cell-Compatible Hydrogels for Biomedical Applications. Science, 336, 1124-1128. https://doi.org/10.1126/science.1214804
Sun, X.-F., Gan, Z., Jing, Z., Wang, H., Wang, D., & Jin, Y. (2015). Adsorption of Methy-lene Blue on Hemicellulose-Based Stimuli-Responsive Porous Hydrogel. Journal of Applied Polymer Science, 132. https://doi.org/10.1002/app.41606
Sun, X.-F., Wang, H., Jing, Z., & Mohanathas, R. (2013). Hemicellulose-Based pH-Sensitive and Biodegradable Hydrogel for Controlled Drug Delivery. Carbohydrate Polymers, 92, 1357-1366. https://doi.org/10.1016/j.carbpol.2012.10.032
Van Tuil, R., Fowler, P., Lawther, M., & Weber, C. J. (2000). Biobased Packaging Mate-rials for the Food Industry. Journal of Food Science and Technology, 5.
Voepel, J., Sjöberg, J., Reif, M., Albertsson, A.-C., Hultin, U.-K., & Gasslander, U. (2009). Drug Diffusion in Neutral and Ionic Hydrogels Assembled from Acetylated Galactog-lucomannan. Journal of Applied Polymer Science, 112, 2401-2412.
https://doi.org/10.1002/app.29878
Yamato, M., Konno, C., Utsumi, M., Kikuchi, A., & Okano, T. (2002). Thermally Respon-sive Polymer-Grafted Surfaces Facilitate Patterned Cell Seeding and Co-Culture. Bio-materials, 23, 561. https://doi.org/10.1016/S0142-9612(01)00138-7
Yasuda, H. (2006). Biocompatibility of Nanofilm-Encapsulated Silicone and Sili-cone-Hydrogel Contact Lenses. Macromolecular Bioscience, 6, 121-138.
https://doi.org/10.1002/mabi.200500153
Yousaf, M. N., Houseman, B. T., & Mrksich, M. (2001). Turning on Cell Migration with Electroactive Substrates. Angewandte Chemie International Edition, 40, 1093-1096.
https://doi.org/10.1002/1521-3773(20010316)40:6<1093::AID-ANIE10930>3.0.CO;2-Q
Biocom-DOI: 10.4236/ojf.2018.81002 28 Open Journal of Forestry patible Chitosan Microgels for Targeted pH-Mediated Intracellular Release of Cancer Therapeutics. Biomacromolecules, 7, 1568-1572. https://doi.org/10.1021/bm050912z
Zhao, W., Glavas, L., Odelius, K., Edlund, U., & Albertsson, A. C. (2014). Facile and Green Approach towards Electrically Conductive Hemicellulose Hydrogels with Tuna-ble Conductivity and Swelling Behavior. Chemistry of Materials, 26, 4265-4273.