The Vaccine Safety Datalink: A Model for Monitoring
Immunization Safety
abstract
The Vaccine Safety Datalink (VSD) project is a collaborative project between the Centers for Disease Control and Prevention and 8 man-aged care organizations (MCOs) in the United States. Established in 1990 to conduct postmarketing evaluations of vaccine safety, the project has created an infrastructure that allows for high-quality re-search and surveillance. The 8 participating MCOs comprise a large population of 8.8 million members annually (3% of the US population), which enables researchers to conduct studies that assess adverse events after immunization. Each MCO prepares computerized data files by using a standardized data dictionary containing demographic and medical information on its members, such as age and gender, health plan enrollment, vaccinations, hospitalizations, outpatient clinic visits, emergency department visits, urgent care visits, and mortality data, as well as additional birth information (eg, birth weight) when available. Other information sources, such as medical chart review, member surveys, and pharmacy, laboratory, and radiology data, are often used in VSD studies to validate outcomes and vaccination data. Since 2000, the VSD has undergone significant changes including an increase in the number of participating MCOs and enrolled population, changes in data-collection procedures, the creation of near real-time data files, and the development of near real-time postmarketing surveillance for newly licensed vaccines or changes in vaccine recommendations. Rec-ognized as an important resource in vaccine safety, the VSD is working toward increasing transparency through data-sharing and external input. With its recent enhancements, the VSD provides scientific exper-tise, continues to develop innovative approaches for vaccine-safety research, and may serve as a model for other patient safety collabor-ative research projects.Pediatrics2011;127:S45–S53
AUTHORS:James Baggs, PhD,aJulianne Gee, MPH,aEdwin
Lewis, MPH,bGabrielle Fowler, MPH,aPatti Benson, MPH,c
Tracy Lieu, MD, MPH,dAllison Naleway, PhD,eNicola P.
Klein, MD,bRoger Baxter, MD,bEdward Belongia, MD,f
Jason Glanz, PhD,gSimon J. Hambidge, MD, PhD,g,h
Steven J. Jacobsen, MD, PhD,iLisa Jackson, MD, MPH,c
Jim Nordin, MD, MPH,jand Eric Weintraub, MPHa
aImmunization Safety Office, Centers for Disease Control and Prevention, Atlanta, Georgia;bKaiser Permanente Vaccine Study Center, Oakland, California;cGroup Health Center for Health Studies, Seattle, Washington;dDepartment of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts;eKaiser Permanente Northwest, Portland, Oregon;fMarshfield Clinic Research Foundation, Marshfield, Wisconsin;gKaiser Permanente Institute for Health Research, Denver, Colorado;hDenver Health Community Health Services, Denver, Colorado;iKaiser Permanente Southern California, Pasadena, California; and jHealthPartners Research Foundation, Minneapolis, Minnesota
KEY WORDS
vaccine safety, immunization, Vaccine Safety Datalink, postmarketing evaluation, surveillance
ABBREVIATIONS
AEFI—adverse event(s) following immunization CDC—Centers for Disease Control and Prevention VSD—Vaccine Safety Datalink
MCO—managed care organization
VAERS—Vaccine Adverse Event Reporting System DDM—distributed-data model
DDF—dynamic data file RCA—rapid cycle analysis
TIV—trivalent inactivated influenza vaccine OR—odds ratio
CI—confidence interval GBS—Guillain-Barré syndrome RR—rate ratio
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
www.pediatrics.org/cgi/doi/10.1542/peds.2010-1722H doi:10.1542/peds.2010-1722H
Accepted for publication Nov 29, 2010
Address correspondence to James Baggs, PhD, Centers for Disease Control and Prevention, 1600 Clifton Rd, Mail Stop D25, Atlanta, GA 30333. E-mail: jbaggs@cdc.gov
High vaccination coverage has signifi-cantly reduced vaccine-preventable disease morbidity and mortality world-wide, especially among children.1,2
Vaccines are generally regarded as safe and effective; however, serious adverse events following immuniza-tion (AEFI) can occur.3,4Although
vac-cine safety is rigorously assessed dur-ing prelicensdur-ing clinical trials, sample sizes are not adequate to detect rare adverse events, long-term adverse events are not examined, and popula-tions are not heterogenous.3In recent
years, public concerns about the safety of vaccines have grown signifi-cantly.5Immunization-safety programs
are an important component of main-taining the public trust in our national immunization program.5Close
monitor-ing of vaccine safety also protects the public’s health and contributes to safer vaccines and vaccination practices.
In 1990, the Centers for Disease Con-trol and Prevention (CDC) National Im-munization Program created the Vac-cine Safety Datalink (VSD) project to conduct postmarketing evaluations of vaccine safety.6,7 Initially, the project
used medical event and demographic information from⬃6 million children younger than 6 years for VSD research from 4 participating managed care or-ganizations (MCOs): Group Health Co-operative of Puget Sound (GHC) (Seat-tle, WA); Kaiser Permanente Northwest (NWK) (Portland, OR); Kaiser Perma-nente of Northern California (KPNC) (Oakland, CA); and Kaiser Permanente of Southern California (Los Angeles, CA).6–9 Later, vaccine-safety studies
were conducted to include children younger than 18 years at all 4 sites and adults 18 years of age and older at GHC, KPNC, and NWK. In 2001, 4 addi-tional MCOs joined the VSD, and during the last 7 years the VSD has made other changes to enhance its ability to serve as the primary mechanism for population-based evaluations of
vac-cine safety in the United States. The VSD is part of the CDC Immunization Safety Office. The Immunization Safety Office also includes the Vaccine Ad-verse Event Reporting System (VAERS), the Clinical Immunization Safety As-sessment Network, and the Brighton Collaboration.
In this article we provide a review of the VSD and focus on the important modifications and enhancements the project has undertaken since 2001.
THE VSD PROJECT SINCE 2001
Population
The VSD population has increased sub-stantially since 2001, when 4 new MCOs joined the project to provide data on members younger than 18 years: Kai-ser Permanente of Colorado (Denver, CO); Marshfield Clinic Research Foun-dation (Marshfield, WI); Health Part-ners Research Foundation (Minneapo-lis, MN); and Harvard Pilgrim/Harvard Vanguard (Boston, MA). In 2007, the VSD population expanded again as all participating sites except 2 began pro-viding data on members of all ages. Currently, data for ⬎18 million per-sons spanning 16 years are available for VSD research.
Data Sources, Collection, and Confidentiality
Since inception of the VSD, each MCO has prepared annual data files, called cycle files, that contain member infor-mation obtained from administrative files maintained by the individual MCOs. The cycle files include demo-graphic and medical services informa-tion on their members, such as age and gender, health plan enrollment, vaccinations, hospitalizations, outpa-tient clinic visits, emergency depart-ment visits, urgent care visits, mortal-ity data, and additional birth information (eg, birth weight) when available. To ensure confidentiality and comply with federal regulations, each
person within the VSD is assigned a unique, randomized VSD study identifi-cation number that is not linked to their MCO member identification num-ber. VSD study identification numbers can be used to link data on demo-graphics and medical services. A stan-dardized data dictionary, which en-sures data consistency across sites, is updated each year by the CDC and the VSD MCOs. Frequently, medical record data and, occasionally, participant survey or interview data are used to validate clinical diagnosis and vaccina-tion data.
Distributed Data Model
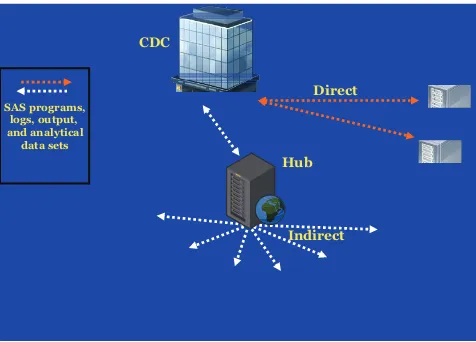
From 1991 through 2000, the VSD used a centralized-data model, which re-quired each MCO to send its cycle files to the CDC annually for merging and analyses. When data were needed for a specific VSD study, the CDC would send a subset of cycle data to the MCO re-sponsible for performing the study analysis. Because of heightened confi-dentiality concerns, the centralized-data model was replaced by a more secure distributed-data model (DDM) in 2001 (see Fig 1).
The DDM allows each MCO to assemble and maintain its computerized data files on a secure server at the site rather than transferring data to the CDC,10and ownership of the data is
plat-form. Each MCO can retrieve and use its own SAS programs but cannot ac-cess programs or information that be-longs to other MCOs. Each MCO sends its SAS logs, output, and analytical data subsets back to the hub for retrieval by CDC researchers. With the direct method, CDC researchers submit SAS programs interactively through a se-cure SAS remote session by using SAS Connect, an Internet communication protocol. Four SAS macros, which are a collection of SAS program state-ments that can be easily recalled, are used to facilitate access of the data and retrieval of SAS logs and output. All data transfers are conducted securely by using encrypted methods.
Dynamic Data Files
Development of the DDM as a secure data-transfer system enabled the VSD to restructure the way data files are collected and used, which led to the creation of dynamic data files (DDFs) in 2005. DDFs permit the ongoing cap-ture of near real-time event-based MCO administrative data, including data on vaccination, hospitalizations, emergency department visits, clinical visits, MCO enrollment, and certain de-mographic characteristics. Most files
are updated weekly with new data from each MCO, although some files are updated monthly or quarterly, de-pending on the capabilities of each site’s data systems. Using the DDM, files are accessed by the CDC on an ongoing basis for analysis and/or ex-traction of necessary data for each on-going study. The DDFs use the same standardized data dictionary as the cy-cle files and continue to provide the flexibility to conduct various types of studies. Additional tracking and data-quality measures were developed to monitor the new DDFs. The sample size of the DDF continuously increases as new data are added to the files, which date back to 1991. The combination of the DDM and DDFs enable the VSD to conduct near real-time postlicensure surveillance, enhance the timeliness of certain studies, and increase effi-ciency in the creation of cycle files.
Research and Surveillance Process
The VSD continues to conduct numer-ous studies on a wide range of immunization-safety topics. Table 1 lists the specific strategic priorities for the VSD. For each study, a team of VSD investigators, comprised of
mem-bers from several of the participating MCOs and the CDC, develops a comhensive study proposal that is pre-sented and reviewed by the members of the project. This scientific proposal includes a detailed description of the hypothesis in question, study design, and analytical plan along with a rigor-ous review of the medical outcomes to be evaluated. All studies meet the nec-essary institutional review board and Health Insurance Portability and Ac-countability Act (HIPAA) requirements. Computerized data often supple-mented with additional medical record or other data are used to create study-specific analytical data files that con-tain only an extremely small portion of the entire VSD data. Analyses and manuscript preparation are typically led by a single MCO or the CDC with input from participating investigators.
Study Designs
The VSD uses several analytical meth-odologies to evaluate vaccine safety. The calculation of background rates al-lows the VSD to conduct multiple types of observational studies and to calcu-late disease incidence and vaccination coverage. Early VSD studies typically used observational study designs such as retrospective cohort studies or case-control studies. Several recent VSD studies have used self-control case-series designs, which involve an analysis based on a person’s exposure and control time windows, because ap-propriate independent control groups may be unavailable or subject to con-founding.11,12Because a large
percent-age of the VSD population is vacci-CDC
Hub Direct
Indirect SAS programs,
logs, output, and analytical data sets
FIGURE 1
Representation of the VSD DDM. Sites using the indirect method retrieve SAS programs at regular intervals from the hub. Output is then sent back to the hub where it is retrieved by analysts at the CDC. For sites that use the direct method, SAS programs and output are transferred through SAS Connect directly between the sites and the CDC. Both methods use encryption methods to secure data transfers.
TABLE 1 VSD Strategic Priorities Evaluate the safety of newly licensed vaccines Evaluate the safety of new vaccine
recommendations for existing vaccines Evaluate clinical disorders after immunizations Assess vaccine safety in special populations at
high risk
nated, it is difficult to obtain data on unvaccinated persons to be used as a comparison or control group. Self-control case-series designs allow re-searchers to determine if the rate of AEFI is elevated in the hypothesized ex-posure window compared with the other time windows. Additional new case-only methods are being devel-oped and refined to better control for seasonal differences in the uptake of vaccines relevant to studies that in-volve influenza vaccine and vaccines given seasonally in advance of school or college attendance.
Postmarketing Surveillance: Rapid Cycle Analysis
Since 2005, several new vaccines have been licensed: live, oral, human-bovine reassortant rotavirus vaccine (Ro-taTeq [Merck & Co, Inc, Whitehouse Station, NJ]), the tetravalent meningo-coccal polysaccharide-protein conju-gate vaccine (Menactra [Sanofi Pas-teur, Inc, Swiftwater, PA]), 2 tetanus, diphtheria, and acellular pertussis vaccines (Boostrix [GlaxoSmithKline Biologicals, Rixensart, Belgium] and Adacel [Sanofi Pasteur, Toronto, On-tario, Canada]), the human papilloma-virus vaccine (Gardasil [Merck & Co, Inc), and a combination measles-mumps-rubella and varicella vaccine (ProQuad [Merck & Co, Inc]). Using DDFs, the VSD is conducting near real-time postmarketing surveillance for these 6 newly licensed vaccines. The studies, referred to as the rapid cycle analysis (RCA)13,14projects, are an
ac-tive surveillance system that routinely assesses possible associations be-tween vaccines and predefined poten-tial adverse events.
In the RCA, the observed number of suspected adverse events is compared with the expected number of events. The expected number of events may be determined from a variety of sources including previously determined
back-ground rates from the VSD or pub-lished literature, background rates from other data sources, concurrent comparison control groups, or self-control methods. Because the compar-isons are conducted weekly, the esti-mates are adjusted by sequential methods to control for repeated anal-ysis of the data.13,14 When conducting
RCA, researchers are on alert for a “signal,” which is generated if the ex-pected rate of adverse events is signif-icantly greater than the control rate when adjusting for sequential meth-ods and other factors. A VSD data-coordinating center was created to handle the increased demand for data management and analysis generated by these RCA studies.
Postmarketing Surveillance: VSD Collaboration With the VAERS
VSD data are also used in conjunction with information from VAERS to exam-ine AEFI. VAERS is a passive surveil-lance system that receives adverse-event reports from various sources, including vaccine manufacturers, health care providers, immunization programs, and vaccine recipients.15
Possible associations are examined by comparing the number of adverse events reported to VAERS with back-ground rates for these events from VSD data.16–18 Summaries of 2 such
analyses for the newly licensed RotaTeq and Menactra vaccines are included in this article to illustrate this collaboration (see “Postmarketing Monitoring Supplementing VAERS Data With VSD Background Rates”).
Data-Sharing and Oversight
Recognized as an important resource in vaccine safety, the VSD is working toward increasing transparency. In 2002, the VSD established a data-sharing program that allows external researchers to analyze data sets from VSD studies published after August 2002 or to create novel analytical
data sets for analysis by using VSD data through December 31, 2000. This program is administered through the National Center for Health Statistics of the CDC. More in-formation on this program can be found at www.cdc.gov/vaccinesafety/ Activities/VSD/Datasharing.html.
On 2 occasions, the VSD has invited exter-nal experts from a number of scientific disciplines and community members to contribute to the design, implementa-tion, and presentation of high-priority studies. These studies include the study entitled “Early Thimerosal Exposure and Neuropsychological Outcomes at 7 to 10 Years” and the ongoing thimerosal and autism case-control study.19A public-use
data set from the thimerosal and neuro-developmental outcomes study is avail-able at www.cdc.gov/vaccinesafety/ Concerns/Thimerosal/neuropsychological_ Outcomes.html.
EXAMPLE STUDIES
The VSD conducts rigorous epidemio-logic studies primarily on a wide range of vaccine-safety priorities as well as other immunization-related topics, including vaccine coverage, disease incidence, research methodology, cost-effectiveness, and medical infor-matics. Through its innovative ap-proaches, the VSD has proven its abil-ity to adapt and respond to the increasingly complex and controver-sial topics in immunization research. Below are a few examples that illus-trate the capabilities of the VSD.
Hepatitis B Vaccine and Risk of
Autoimmune Thyroid Disease20
data, the VSD was able to investigate this relationship through a multisite case-control study.20Cases were initially
iden-tified through VSD cycle data and vali-dated through medical record review, and then telephone interviews were con-ducted to verify hepatitis B vaccination status. The study analyzed 355 cases of Graves’ disease, 418 cases of Hashimoto thyroiditis, and 1102 frequency-matched controls and revealed that having ever received hepatitis B vaccine did not in-crease the risk of either Graves’ disease or Hashimoto thyroiditis.20 This study’s
results reveal the ability to collect com-prehensive vaccine information and to accurately identify and confirm cases through alternative data-collection methods.
Safety of Trivalent Inactivated Influenza Vaccine in Children Aged 6 to 23 Months21
As a result of the decision by the CDC’s Advisory Committee on Immunization Practices to recommend routine vacci-nation of all children aged 6 to 23 months with trivalent inactivated influ-enza vaccine (TIV) in 2004, the VSD proved to be well suited to assess the safety of TIV in this population. In one of the largest population-based TIV stud-ies to date, the VSD conducted a retro-spective cohort study of 45 356 chil-dren who received a total of 69 359 influenza vaccinations between Janu-ary 1, 1991, and May 31, 2003.21
Self-control case-series methods were used for this analysis. Cycle files were analyzed to identify medically attended events seen in clinic, emergency de-partment, or hospital settings after vaccination with TIV. Preliminary anal-yses revealed that gastritis/duodenitis was more likely to occur in the 14 days after TIV (matched odds ratios [ORs]: 5.50 [95% confidence interval (CI): 1.22–24.81] for control period 1 [0 –3 days] and 4.33 [95% CI: 1.23–15.21] for control period 2 [1–14 days]). No other significant associations with medically
attended events were found. Further analysis including chart review and a subanalysis of 28 820 children with no underlying medical conditions that would put them at increased risk of com-plications of influenza vaccination re-vealed that children vaccinated with TIV were not at increased risk of gastritis/ duodenitis compared with the entire study population.21This study supported
the Advisory Committee on Immunization Practices vaccination recommendation by providing reassurance to support the safety of universally immunizing all chil-dren aged 6 to 23 months with influenza vaccination.
Early Thimerosal Exposure and Neuropsychological Outcomes at 7 to 10 Years19
In 1999, the Public Health Service and the American Academy of Pediatrics called on vaccine manufacturers to
remove thimerosal, a
mercury-containing preservative, from vac-cines. The decision to remove thimero-sal was a precautionary measure,22
and subsequent studies have found no significant association between thimerosal and neuropsychological deficits.19,23–31In an effort to better
im-prove on previous studies, the VSD was able to rigorously assess the relation-ship between thimerosal exposure and neuropsychological functioning through a retrospective cohort study with extensive assessments and inter-views among the study population and their mothers. The study population in-cluded 1047 children between the ages of 7 and 10 years who were enrolled in 4 of the VSD MCOs and had received vaccinations as infants when thimero-sal was used as a preservative in many childhood vaccines. For the analysis, the children were grouped according to their level of mercury exposure (low, medium, and high), which was determined from VSD MCO immuniza-tion records, medical records, per-sonal immunization records, and
parent interviews. Information on po-tential confounding factors was ob-tained from the interviews with
moth-ers and from medical records.
Standardized tests that assessed 42 neuropsychological outcomes, includ-ing speech and language measures, verbal memory, fine motor coordina-tion, tics, and behavioral regulacoordina-tion, were administered to the children. Only 5% of the statistical tests (19 of 378) showed significant associations; 12 tests revealed a positive associa-tion, and 7 tests revealed a negative association. The vast majority of the tests revealed no association, and the 5% that showed associations could be explained by chance alone. The results added to accumulated evidence that thimerosal does not cause neuropsy-chological deficits in children.19 This
study’s results further show the ability of the VSD to supplement administra-tive data with data from other sources to conduct rigorous studies and test vaccine-safety hypotheses.
Postmarketing Monitoring Supplementing VAERS Data With VSD Background Rates
The VSD has collaborated with VAERS in conducting postmarketing surveil-lance of potential AEFI for newly licensed vaccines. The potential asso-ciation between RotaTeq and intussus-ception was assessed by comparing the number of VAERS intussusception reports to the number of intussuscep-tion cases expected to occur by chance alone.16,32 To determine the expected
ad-ministered varied substantially ac-cording to age. Using these data, the expected number of background cases was calculated by multiplying VSD background rates for each age group according to the estimated number of vaccine doses administered to that age group. The findings suggested that there is no association between Rota-Teq vaccination and intussusception, because the number of cases of intus-susception reported to the VAERS (32) was not elevated above the expected number of cases (52).16,32Similar
anal-yses were conducted to compare the potential association between Menac-tra and Guillain-Barré syndrome (GBS). For the study time period, the background incidence rate of GBS was determined to be 0.11 per 100 000 person-months in the VSD. By dividing the VAERS reporting rate of GBS (0.20 per 100 000 person-months) by the background incidence rate of GBS (0.11 per 100 000 person-months), the VSD determined the reporting rate
ra-tio (RR) to be 1.77 (95% CI:
0.96 –3.07).17,18Although the data
sug-gest a possible small increased risk of GBS in persons aged 11 to 19 who re-ceived Menactra vaccination, the find-ings should be viewed with caution.17,18
Postmarketing Monitoring Using VSD RCA
In addition to providing background rates to supplement the VAERS, the VSD is also conducting RCA studies to monitor the safety of several vaccines including RotaTeq, Menactra, Gardasil, Adacel and Boostrix, ProQuad, and sea-sonal influenza vaccination. Between May 2006 and May 2008, ⬎205 000 doses of RotaTeq were administered orally to infants at ages 2, 4, and 6 months in VSD-monitored MCOs. Only 5 cases of intussusception within 30 days of vaccination were reported among RotaTeq recipients; in contrast, on the basis of historical background rates, 6.75 cases were expected to
oc-cur by chance alone. Only 2 of the in-tussusception cases were confirmed by medical chart review. This analysis suggested that there was no evidence that RotaTeq vaccine is associated with an increased risk for
intussus-ception or other prespecified
events.32–34 Between March 2005 and
September 2008,⬎570 000 Menactra doses were delivered in participating MCOs, and no cases of GBS after med-ical record review were observed among vaccine recipients aged 11 to 19 years within 6 weeks of vaccination (0.9 cases would be expected during that period). During the same period, 5 unconfirmed cases of GBS were identi-fied among an unvaccinated compari-son group of⬎900 000 persons aged 11 to 19 years (Dr Lieu, update on Men-actra RCA through September 2008, personal communication, October 20, 2008 and refs 14, 17, and 18). The re-sults of neither study suggest an as-sociation of serious AEFI with these vaccines. In February 2008, VSD in-vestigators presented the Advisory Committee on Immunization Practices with preliminary results from an anal-ysis performed after a possible signal of seizure was observed in the VSD ProQuad RCA study.35On the basis of
⬎43 000 administered doses of the combination measles-mumps-rubella and varicella vaccine (MMRV), the at-tributable risk for seizures on days 7 to 10 after MMRV was calculated as 1 per 2000 doses compared with the measles-mumps-rubella and varicella vaccines administered separately but at the same visit.35The VSD continues
to investigate this association and con-tinues to monitor the potential risk of GBS after Menactra. The VSD is now in the process of developing RCA studies for Kinrix (GlaxoSmithKline Biologicals, Rixensart, Belgium), Pentacel (Sanofi Pasteur Ltd, Toronto, Canada), Rotarix (GlaxoSmithKline Biologicals, Rixen-sart, Belgium), and pandemic H1N1 in-fluenza vaccination.
POWER CONSIDERATIONS
With the addition of 4 new MCOS in 2001, the size of the VSD cohort in-creased and, with it, the statistical power available to detect rare vaccine-associated adverse events. Two exam-ples, which illustrate the capacity of the VSD to detect rare events, follow.
The intussusception background rate of infants 6 to 35 weeks old, the age during which RotaTeq vaccine is given, is 32.4 per 100 000 person-years, as es-timated from VSD data. The number of infants aged 6 to 35 weeks in the VSD cohort is⬃95 000. At 90% vaccination coverage and 80% power, it would re-quire 4.3 years to detect an RR equiva-lent to 2.0, 1.4 years to detect an RR of 3.0, and 0.7 years to detect an RR of 4.0.
However, the VSD has limited capabil-ity to detect very rare AEFI in minimal time periods for minimal risk ratios. For example, the background rate of GBS among 11- to 19-year-olds is 1.3 to 1.4 per 100 000 person-years (unpub-lished data). The average monthly co-hort of 11- to 19-year-olds in the VSD
cohort is between 870 000 and
1 000 000 (average monthly 11- to 19-year-old cohort). At 70% vaccination coverage and 80% power, it would re-quire⬃13 years to detect an RR of 2.0, 4 years to detect an RR of 3, and 2 years to detect an RR of 4.
CONCLUSIONS
The VSD has conducted important vaccine-safety research since its in-ception in 1990, and since 2001 it has made changes that have enhanced its ability to answer urgent questions about vaccine safety and other immunization-related issues and to in-form US vaccination policy.
trans-ferring data within the VSD, was devel-oped in response to heightened confi-dentiality concerns. The DDM enabled the development of the DDFs. Together, the DDM and DDFs enabled the imple-mentation of RCA, which is allowing re-searchers to monitor events in near real-time after the introduction of new vaccines and new vaccine recommen-dations. The VSD continues to refine previously used methodologies and develop new methodologies, such as the maximized sequential probability ratio test (maxSPRT), which is applica-ble to the VSD’s RCA.13,14,36With the
re-cent expansion of the VSD, the project is well positioned to conduct RCA stud-ies and traditional VSD analyses on new vaccines being introduced for teenagers and young adults. Although the majority of VSD studies focus on hypotheses related to vaccine safety, the VSD has also demonstrated its ability to conduct studies on vaccine coverage,37–41 disease incidence,42–49
methodology,14,36,50 cost-effectiveness,51–53
and medical informatics.10,54
As described previously, the comput-erized vaccination records have been shown to be of high quality,55whereas
the accuracy of computerized records to determine medical outcomes var-ies.56The VSD recognizes this
limita-tion and uses addilimita-tional data sources, especially medical chart review, to val-idate administrative data. Although not
critical for the strategic priorities of the VSD (listed in Table 1), further stud-ies that compare population charac-teristics and disease incidence rates to additional non-MCO sources char-acterizing the national population could be beneficial for VSD studies, especially those that describe esti-mates of vaccine coverage and dis-ease incidence.
The VSD is one of several worldwide research programs that are able to conduct vaccine-safety studies by us-ing large databases. Other established programs include the US Defense Med-ical Surveillance System,57–59 the UK
General Practice Research Database,60
postlicensure epidemiology of child-hood vaccinations in Denmark,61 and
the Vietnam Vaccine Data Link.62
The VSD provides scientific expertise, continues to develop innovative ap-proaches for vaccine safety, is the pri-mary mechanism for population-based evaluations of vaccine safety in the United States, and may serve as a model for other patient-safety collab-orative research projects.
Information about the VSD project can be obtained at www.cdc.gov/ vaccinesafety/Activities/VSD.html.
ACKNOWLEDGMENTS
This study was supported, in part, by the VSD contract with America’s
Health Insurance Plans, funded by
the CDC.
We thank all the organizations and
their staff for participating in the VSD project, including the following key members: Rich Platt, MD, MS, Kather-ine Yih, PhD, Virginia Hinrichsen, MPH, Rich Fox, MPH, Renny Li, MS, RuiHua
Yin, MS, Sharon Greene, PhD, and Irene Shui, MPH (Harvard Pilgrim/Harvard Vanguard), Leslie Kuckler and Amy Bu-tani (Health Partners Research
Foun-dation), Jim Donahue, PhD, DVM, Jer-emy McCauley, MBA, and Stephanie Irving, MS (Marshfield Clinic
Re-search Foundation), Christina
Clarke, Matthew Daley, MD, Dave Mc-Clure, PhD, Jo Ann Shoup, MS, and Stan Xu, PhD (Kaiser Permanente of Colorado), Y. Craig Cheetham, PharmD, Wansu Chen, MS, Marlene
Lugg, DrPH, Mike Marcy, MD, and Lina
Sy, MPH (Kaiser Permanente of
Southern California), Paula Ray, MPH (Kaiser Permanente of Northern
Cal-ifornia), Rachel Gold, PhD, MPH, John Mullooly, PhD, Lois Drew, BA, and Karen Riedlinger, MPH (Kaiser Per-manente Northwest), Jennifer
Nel-son, PhD, Darren Malais, and Onchee Yu, MS (Group Health Cooperative), and Karen Broder, MD, MPH, Sophia Greer, MPH, Paul Gargiullo, PhD, John
Iskander, MD, MPH, and William Thompson, PhD (CDC).
REFERENCES
1. Roush SW, Murphy TV. Historical compari-sons of morbidity and mortality for vaccine-preventable diseases in the United States.
JAMA. 2007;298(18):2155–2163
2. Centers for Disease Control and Prevention. Impact of vaccines universally recom-mended for children: United States, 1990 –1998.MMWR Morb Mortal Wkly Rep. 1999;48(12):243–248
3. Chen RT, Mootrey G, DeStefano F. Safety of routine childhood vaccinations: an epidemi-ological review.Paediatr Drugs. 2000;2(4): 273–290
4. Bohlke K, Davis RL, Marcy SM, et al; Vaccine
Safety Datalink Team. Risk of anaphylaxis after vaccination of children and adoles-cents.Pediatrics. 2003;112(4):815– 820 5. Cooper LZ, Larson HJ, Katz SL. Protecting
public trust in immunization.Pediatrics. 2008;122(1):149 –153
6. Chen RT, DeStefano F, Davis RL, et al. The Vaccine Safety Datalink: immunization re-search in health maintenance organiza-tions in the USA.Bull World Health Organ. 2000;78(2):186 –194
7. DeStefano F. The Vaccine Safety Datalink project. Pharmacoepidemiol Drug Saf. 2001;10(5):403– 406
8. Chen RT, Glasser JW, Rhodes PH, et al. Vaccine Safety Datalink project: a new tool for improving vaccine safety moni-toring in the United States. The Vaccine Safety Datalink Team. Pediatrics. 1997; 99(6):765–773
9. Wassilak SG, Glasser JW, Chen RT, Hadler SC. Utility of large-linked databases in vac-cine safety, particularly in distinguishing in-dependent and synergistic effects. The Vac-cine Safety Datalink Investigators.Ann N Y Acad Sci. 1995;754:377–382
HMO Research Network Conference. May 5, 2004; Dearborn, MI
11. Farrington CP, Nash J, Miller E. Case series analysis of adverse reactions to vaccines: a comparative evaluation.Am J Epidemiol. 1996;143(11):1165–1173
12. Farrington CP. Control without separate controls: evaluation of vaccine safety using c a s e - o n l y m e t h o d s . V a c c i n e. 2 0 0 4 ; 22(15–16):2064 –2070
13. Davis RL, Kolczak M, Lewis E, et al. Active surveillance of vaccine safety: a system to detect early signs of adverse events. Epide-miology. 2005;16(3):336 –341
14. Lieu TA, Kulldorff M, Davis RL, et al; Vaccine Safety Datalink Rapid Cycle Analysis Team. Real-time vaccine safety surveillance for the early detection of adverse events.Med Care. 2007;45(10 suppl 2):S89 –S95 15. Varricchio F, Iskander J, DeStefano F, et al.
Understanding vaccine safety information from the Vaccine Adverse Event Reporting System.Pediatr Infect Dis J. 2004;23(4): 287–294
16. Centers for Disease Control and Prevention. Postmarketing monitoring of intussuscep-tion after RotaTeq vaccinaintussuscep-tion: United States, February 1, 2006 –February 15, 2007.
MMWR Morb Mortal Wkly Rep. 2007;56(10): 218 –222
17. Centers for Disease Control and Prevention. Update: Guillain-Barré syndrome among re-cipients of Menactra meningococcal conju-g a t e v a c c i n e — U n i t e d S t a t e s , J u n e 2005–September 2006 [published correc-tion appears inMMWR Morb Mortal Wkly Rep. 2006;55(43):1177].MMWR Morb Mortal Wkly Rep. 2006;55(41):1120 –1124 18. Centers for Disease Control and Prevention.
Update: Guillain-Barré syndrome among re-cipients of Menactra meningococcal conju-gate vaccine—United States, October 2005–February 2006.MMWR Morb Mortal Wkly Rep. 2006;55(13):364 –366
19. Thompson WW, Price C, Goodson B, et al; Vaccine Safety Datalink Team. Early thimer-osal exposure and neuropsychological out-comes at 7 to 10 years.N Engl J Med. 2007; 357(13):1281–1292
20. Yu O, Bohlke K, Hanson CA, et al. Hepatitis B vaccine and risk of autoimmune thyroid disease: a Vaccine Safety Datalink study.
Pharmacoepidemiol Drug Saf. 2007;16(7): 736 –745
21. Hambidge SJ, Glanz JM, France EK, et al; Vac-cine Safety Datalink Team. Safety of triva-lent inactivated influenza vaccine in chil-dren 6 to 23 months old. JAMA. 2006; 296(16):1990 –1997
22. Centers for Disease Control and Prevention.
Recommendations regarding the use of vaccines that contain thimerosal as a pre-servative.MMWR Morb Mortal Wkly Rep. 1999;48(43):996 –998
23. Andrews N, Miller E, Grant A, Stowe J, Os-borne V, Taylor B. Thimerosal exposure in infants and developmental disorders: a ret-rospective cohort study in the United king-dom does not support a causal association.
Pediatrics. 2004;114(3):584 –591
24. DeStefano F. Vaccines and autism: evidence does not support a causal association.Clin Pharmacol Ther. 2007;82(6):756 –759 25. Heron J, Golding J; ALSPAC Study Team.
Thimerosal exposure in infants and devel-opmental disorders: a prospective cohort study in the United kingdom does not sup-port a causal association.Pediatrics. 2004; 114(3):577–583
26. Madsen KM, Lauritsen MB, Pedersen CB, et al. Thimerosal and the occurrence of autism: negative ecological evidence from Danish population-based data. Pediatrics. 2003;112(3 pt 1):604 – 606
27. Meadows M. IOM report: no link between vaccines and autism. FDA Consum. 2004; 38(5):18 –19
28. Parker SK, Schwartz B, Todd J, Pickering LK. Thimerosal-containing vaccines and autis-tic spectrum disorder: a criautis-tical review of published original data [published correc-tion appears in Pediatrics. 2005;115(1): 200].Pediatrics. 2004;114(3):793– 804 29. Stehr-Green P, Tull P, Stellfeld M, Mortenson
PB, Simpson D. Autism and thimerosal-containing vaccines: lack of consistent evi-dence for an association.Am J Prev Med. 2003;25(2):101–106
30. Taylor B. Vaccines and the changing epide-miology of autism.Child Care Health Dev. 2006;32(5):511–519
31. Verstraeten T, Davis RL, DeStefano F, et al; Vaccine Safety Datalink Team. Safety of thimerosal-containing vaccines: a two-phased study of computerized health main-tenance organization databases [published correction appears in Pediatrics. 2004; 113(1):184]. Pediatrics. 2003;112(5): 1039 –1048
32. Haber P, Patel M, Izurieta HS, et al. Postlicen-sure monitoring of intussusception after RotaTeq vaccination in the United States, February 1, 2006, to September 25, 2007. Pe-diatrics. 2008;121(6):1206 –1212
33. Belongia E, Irving S, Shui I, et al. Rapid cycle analysis of pentavalent rotavirus (RotaTeq) vaccine safety in the Vaccine Safety Datalink population: preliminary results. Presented at: Advisory Committee on Immunization
Practices meeting; June 25, 2008; Atlanta, GA
34. Irving SA, Belongia EA, Shui IM, et al. Rapid cycle analysis of pentavalent rotavirus vac-cine safety in the Vacvac-cine Safety Datalink Population. Presented at: the joint 46th Annual Infectious Diseases Society of America Conference and 48th Annual In-terscience Conference on Antimicrobial Agents and Chemotherapy. October 25, 2008; Washington, DC
35. Klein N. Vaccine Safety Datalink project: evaluation of MMRV and febrile seizures. Presented at: Advisory Committee on Immu-nization Practices February 2008 meeting; February 27, 2008; Atlanta, GA
36. Glanz JM, McClure DL, Xu S, et al. Four differ-ent study designs to evaluate vaccine safety were equally validated with contrasting lim-itations. J Clin Epidemiol. 2006;59(8): 808 – 818
37. Centers for Disease Control and Prevention. Rapid assessment of influenza vaccination coverage among HMO members: northern California influenza seasons, 2001– 02 through 2004 – 05.MMWR Morb Mortal Wkly Rep. 2005;54(27):676 – 678
38. Jackson LA, Neuzil KM, Baggs J, et al. Com-pliance with the recommendations for 2 doses of trivalent inactivated influenza vac-cine in children less than 9 years of age receiving influenza vaccine for the first time: a Vaccine Safety Datalink study. Pedi-atrics. 2006;118(5):2032–2037
39. Centers for Disease Control and Prevention; Advisory Committee on Immunization Prac-tices. Rotavirus vaccination coverage and adherence to the Advisory Committee on Im-munization Practices (ACIP)-recommended vaccination schedule: United States, Febru-ary 2006 –May 2007.MMWR Morb Mortal Wkly Rep. 2008;57(15):398 – 401
40. Lin ND, Kleinman K, Chan KA, et al; Vaccine Safety Datalink Team. Variation in hepatitis B immunization coverage rates associated with provider practices after the temporary suspension of the birth dose.BMC Pediatr. 2006;6:31
41. Lin ND, Kleinman K, Chan KA, et al; Vaccine Safety Datalink Group. Impact of the intro-duction of pneumococcal conjugate vaccine on immunization coverage among infants.
BMC Pediatr. 2005;5:43
42. Belay ED, Holman RC, Clarke MJ, et al. The incidence of Kawasaki syndrome in West Coast health maintenance organizations.
Pediatr Infect Dis J. 2000;19(9):828 – 832 43. Parashar UD, Holman RC, Bresee JS, et al.
Datalink Team.Pediatr Infect Dis J. 1998; 17(7):605– 611
44. Averhoff F, Zucker J, Vellozzi C, et al. Ade-quacy of surveillance to detect endemic ru-bella transmission in the United States.Clin Infect Dis. 2006;43(suppl 3):S151–S157 45. Mullooly JP, Bridges CB, Thompson WW, et
al; Vaccine Safety Datalink Adult Working Group. Influenza- and RSV-associated hospi-talizations among adults. Vaccine. 2007; 25(5):846 – 855
46. Bohlke K, Davis RL, DeStefano F, Marcy SM, Braun MM, Thompson RS. Epidemiology of anaphylaxis among children and adoles-cents enrolled in a health maintenance or-ganization.J Allergy Clin Immunol. 2004; 113(3):536 –542
47. Mullooly JP, Riedlinger K, Chun C, Weinmann S, Houston H. Incidence of herpes zoster, 1997–2002. Epidemiol Infect. 2005;133(2): 245–253
48. Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children.N Engl J Med. 2000;342(4): 232–239
49. O’Brien MA, Uyeki TM, Shay DK, et al. Inci-dence of outpatient visits and hospitaliza-tions related to influenza in infants and young children.Pediatrics. 2004;113(3 pt 1): 585–593
50. McClure DL, Glanz JM, Xu S, Hambidge SJ, Mullooly JP, Baggs J. Comparison of epide-miologic methods for active surveillance of vaccine safety. Vaccine. 2008;26(26): 3341–3345
51. Lieu TA, Black SB, Ray GT, Martin KE, Shine-field HR, Weniger BG. The hidden costs of infant vaccination. Vaccine. 2000;19(1): 33– 41
52. Prosser LA, Bridges CB, Uyeki TM, et al. Val-ues for preventing influenza-related mor-bidity and vaccine adverse events in chil-dren.Health Qual Life Outcomes. 2005;3:18 53. Prosser LA, Bridges CB, Uyeki TM, et al.
H e a l t h b e n e fi t s , r i s k s , a n d c o s t -effectiveness of influenza vaccination of children. Emerg Infect Dis. 2006;12(10): 1548 –1558
54. Hinrichsen VL, Kruskal B, O’Brien MA, Lieu TA, Platt R. Using electronic medical records to enhance detection and reporting of vac-cine adverse events.J Am Med Inform As-soc. 2007;14(6):731–735
55. Mullooly J, Drew L, DeStefano F, et al. Quality of HMO vaccination databases used to mon-itor childhood vaccine safety. Vaccine Safety Datalink Team.Am J Epidemiol. 1999; 149(2):186 –194
56. Mullooly J, Drew L, DeStefano F, et al. Quality assessments of HMO diagnosis databases
used to monitor childhood vaccine safety.
Methods Inf Med. 2004;43(2):163–170 57. Davila JC, Payne DC, Zhang Y, et al. Quality
assessment of nonanthrax vaccination data in the Defense Medical Surveillance System (DMSS), 1998 –2004.Vaccine. 2008;26(12): 1577–1584
58. Payne DC, Rose CE Jr, Aranas A, et al. Assess-ment of anthrax vaccination data in the De-fense Medical Surveillance System, 1998 –2004.Pharmacoepidemiol Drug Saf. 2007;16(6):605– 611
59. Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the De-partment of Defense serum repository: glimpses of the future of public health sur-veillance.Am J Public Health. 2002;92(12): 1900 –1904
60. Wong IC, Murray ML. The potential of UK clin-ical databases in enhancing paediatric medication research.Br J Clin Pharmacol. 2005;59(6):750 –755
61. Hviid A. Postlicensure epidemiology of child-hood vaccination: the Danish experience.
Expert Rev Vaccines. 2006;5(5):641– 649 62. Ali M, Canh GD, Clemens JD, et al; Vaccine
Safety Datalink Group. The use of a comput-erized database to monitor vaccine safety in Viet Nam.Bull World Health Organ. 2005; 83(8):604 – 610
(Continued from first page)
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275). Copyright © 2011 by the American Academy of Pediatrics
DOI: 10.1542/peds.2010-1722H originally published online April 18, 2011;
2011;127;S45
Pediatrics
Simon J. Hambidge, Steven J. Jacobsen, Lisa Jackson, Jim Nordin and Eric Weintraub
Allison Naleway, Nicola P. Klein, Roger Baxter, Edward Belongia, Jason Glanz,
James Baggs, Julianne Gee, Edwin Lewis, Gabrielle Fowler, Patti Benson, Tracy Lieu,
The Vaccine Safety Datalink: A Model for Monitoring Immunization Safety
Services
Updated Information &
http://pediatrics.aappublications.org/content/127/Supplement_1/S45 including high resolution figures, can be found at:
References
BIBL
http://pediatrics.aappublications.org/content/127/Supplement_1/S45# This article cites 58 articles, 11 of which you can access for free at:
Subspecialty Collections
b
http://www.aappublications.org/cgi/collection/infectious_diseases_su Infectious Disease
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2010-1722H originally published online April 18, 2011;
2011;127;S45
Pediatrics
Simon J. Hambidge, Steven J. Jacobsen, Lisa Jackson, Jim Nordin and Eric Weintraub
Allison Naleway, Nicola P. Klein, Roger Baxter, Edward Belongia, Jason Glanz,
http://pediatrics.aappublications.org/content/127/Supplement_1/S45
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.