ARTICLE
Renal and Cardiovascular Effects of
Angiotensin-Converting Enzyme Inhibitor Plus Angiotensin II
Receptor Antagonist Therapy in Children
With Proteinuria
Riccardo Lubrano, MDa, Francesca Soscia, MDa, Marco Elli, MDb, Flavia Ventriglia, MDa, Claudia Raggi, MDa, Elisabetta Travasso, MDa,
Simona Scateni, MDa, Valeria Di Maio, MDa, Paolo Versacci, MDa, Raffaele Masciangelo, MDc, Stefano Romero, MDa
Departments ofaPediatrics andcExperimental Medicine and Pathology, University of Rome “La Sapienza,” Rome, Italy;bDepartment of Clinical Sciences “Ospedale Luigi
Sacco,” University of Milan, Milan, Italy
The authors have indicated they have no financial relationships relevant to this article to disclose.
ABSTRACT
OBJECTIVE.We investigated whether the combination of an angiotensin-converting
en-zyme inhibitor and an angiotensin II type 1 receptor antagonist offers better control of proteinuria and cardiovascular parameters without causing adverse side effects.
METHODS.We enrolled 10 children (mean age: 12.3⫾4.06 years) with proteinuria
resulting from chronic renal diseases of various causes. The study consisted of 2 phases, 3 months each, for an overall 6-month observation time. During phase 1 (3 months), each child was assigned randomly to treatment with either an angio-tensin-converting enzyme inhibitor or an angiotensin II type 1 receptor antagonist alone. During phase 2, each child was advanced to combination therapy with the addition of an angiotensin II type 1 receptor antagonist or an angiotensin-con-verting enzyme inhibitor, respectively. Renal function tests, echocardiography, and 24-hour ambulatory blood pressure monitoring were performed at the begin-ning of the study (time 0), at 3 months (time 1), and at 6 months (time 2).
RESULTS.At time 2, proteinuria (change: ⫺80.21⫾10.75%), interventricular
sep-tum index (change:⫺13.63⫾18.64%), posterior wall of the left ventricle index (change:⫺30.71⫾20.32%), and left ventricular mass index (change:⫺28.33⫾ 24.44%) were reduced significantly, compared with time 0 and time 1. No unto-ward side effects were detected during the study.
CONCLUSIONS.In the short term, the combination of angiotensin-converting enzyme
inhibitors and angiotensin II type 1 receptor antagonists for children with protein-uria of renal origin reduced proteinprotein-uria significantly, compared with baseline or either drug alone. Furthermore, echocardiographic studies gave evidence of re-duction of left ventricular hypertrophy. Additional studies are needed to evaluate long-term results.
www.pediatrics.org/cgi/doi/10.1542/ peds.2005-2053
doi:10.1542/peds.2005-2053
Key Words
chronic kidney disease, proteinuria, left ventricular hypertrophy, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonist
Abbreviations
ACE—angiotensin-converting enzyme AT1—angiotensin II type 1 IVS—interventricular septum LVM—left ventricular mass PW—posterior wall of the left ventricle GFR— glomerular filtration rate
Accepted for publication Mar 29, 2006
Address correspondence to Riccardo Lubrano, MD, Dipartimento di Clinica Pediatrica, Policlinico “Umberto I,” Universita` degli Studi di Roma “La Sapienza,” Viale Regina Elena 324, 00161, Rome, Italy. E-mail: riccardo.lubrano@ uniroma1.it
B
OTH PROTEINURIA1–4 and hypertension5areconsid-ered among the most significant nonimmunologic causes of progression of renal damage.6,7Therefore, their
treatment is considered the most effective strategy to preserve renal function.8,9
Angiotensin II is recognized presently as one of the most important mediators of progressive renal failure. It is responsible for several hemodynamic effects such as vasoconstriction, proteinuria (secondary to glomerular hyperfiltration attributable to increased intraglomerular pressure), and structural changes to the glomeruli caused by proinflammatory mediators, fibroblast prolif-eration, and production of superoxide free radicals.10The
effects of angiotensin II on the kidney are inhibited by drugs such as angiotensconverting enzyme (ACE) in-hibitors and angiotensin II type 1 (AT1) receptor antag-onists.9The administration of ACE inhibitors reduces the
progression of renal damage from varying causes,11 by
blocking the renin-angiotensin system and therefore lowering proteinuria and/or arterial blood pressure. A similar effect is obtained with AT1 receptor antago-nists.12,13Furthermore, several studies point to increased
efficacy of combination therapy with ACE inhibitors and AT1receptor antagonists.8,14–16
AT1receptor antagonists have been shown to reduce heart hypertrophy secondary to hypertension in rats.17
They are thought to act by blocking angiotensin II pro-fibrotic activity on fibroblasts, which are responsible for increased production of extracellular matrix and the re-sulting cardiac fibrosis.18 In our study, we investigated
whether combined therapy with ACE inhibitors and AT1 receptor antagonists for children with renal diseases and proteinuria could reduce proteinuria and improve he-modynamic features without significant side effects.
METHODS
Patients
We studied 10 children (8 male patients and 2 female patients; mean age: 12.3 ⫾ 4.06 years; range: 6 –17 years). The primary renal disease was renal dysplasia for 3 patients, focal glomerulosclerosis for 2, reflux ne-phropathy for 2, polycystic renal disease for 1, Alport syndrome for 1, and interstitial nephropathy for 1. For at least 3 months before entering the study, all patients had mean systolic and diastolic arterial blood pressures that were constantly below the 90th percentile for height and gender19(either with or without drug therapy), as
mea-sured with 24-hour ambulatory blood pressure monitor-ing, and overt proteinuria20of⬎150 mg per day.
Study Design
The study was organized over 6 months, divided into 2 subsequent phases of 3 months each. Clinical evalua-tions, consisting of renal function tests, 24-hour
record-ings of blood pressure, and echocardiography, were per-formed at the beginning of the study (time 0), at the end of the first 3-month phase (time 1), and at the end of the second phase (time 2). During the time 0 to time 1 interval, the children were assigned randomly to treat-ment with an ACE inhibitor or an AT1receptor antago-nist. During the time 1 to time 2 interval, all children received combination therapy with both an ACE inhib-itor and an AT1receptor antagonist.
Drugs and Dosages
We used the ACE inhibitor enalapril at 0.1 mg/kg per day (maximum: 10 mg/day) and the AT1receptor an-tagonist losartan at 1 mg/kg per day (maximum: 50 mg/day). During phase 2, the 2 drugs were administered in combination, at the same dosages as reported above.
Biochemical and Instrumental Analyses
A 24-hour urine collection and a 5-mL blood sample, drawn at the end of the urine collection itself, were obtained at time 0, time 1, and time 2. Urinary and blood concentrations of creatinine, sodium, potassium, and proteins were used to calculate the glomerular filtration rate (GFR) through creatinine clearance, fractional ex-cretion of sodium, fractional exex-cretion of potassium, and proteinuria. All analyses were performed with a Beck-man Synchron CX5 Delta Clinical System automatic an-alyzer (Beckman Coulter Inc, Fullerton, CA).
Echocardiography was performed with an Acuson Siemens Aspen system (Siemens Medical Solutions Inc, Malvern, PA) with a 2.5- to 5-MHz probe; we measured the thickness of the interventricular septum (IVS) ex-pressed as the IVS index, the thickness of the posterior wall of the left ventricle (PW) expressed as the PW index, and the left ventricular mass (LVM) expressed as the LVM index. Twenty-four-hour recording of blood pressure was performed with a model N 90207–30 mon-itor (Spacelabs Medical, Richmond, WA).
Statistical Analyses
The differences among 3 related samples in the param-eters under evaluation were first analyzed with the Friedman test. After they proved significant with the Friedman test, the differences among parameters ob-tained at time 0, time 1, and time 2 were analyzed with the nonparametric Wilcoxon signed-rank test. Correla-tions were studied with the R2 test. P values of ⬍.05 were considered significant.
Ethics
RESULTS
Six of 10 patients in the study were assigned initially to receive treatment with an ACE inhibitor during phase 1; the remaining 4 children received an AT1 receptor an-tagonist. During phase 2, all 10 patients received the 2 drugs in combination.
The Friedman test applied to the groups studied at time 0, time 1, and time 2 for all parameters in the study indicated significant differences among dependent groups (in particular, proteinuria,P⬍.0005; LVM,P⬍ .0006). No statistically significant difference was de-tected in renal function test, echocardiographic, and blood pressure results at time 0 and time 1 between children receiving the ACE inhibitor and children receiv-ing the AT1receptor antagonist.
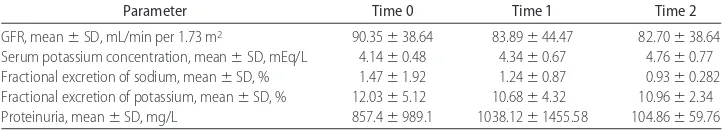
Renal function test results at time 0, time 1, and time 2 are reported in Table 1. GFR, fractional excretion of sodium, fractional excretion of potassium, and serum potassium levels remained constant throughout the en-tire study. Proteinuria remained constant between time 0 and time 1, whereas it was significantly lower at time 2, compared with both time 0 (P⬍.005) and time 1 (P
⬍ .005). Furthermore, a reduction of proteinuria was present, with a decrease equal to⫺80.21⫾10.75%.
Mean arterial blood pressures at time 0, time 1, and
time 2, as recorded with a Holter device for 24-hour ambulatory blood pressure measurements, are plotted in Fig 1. No statistically significant differences were found at any study time.
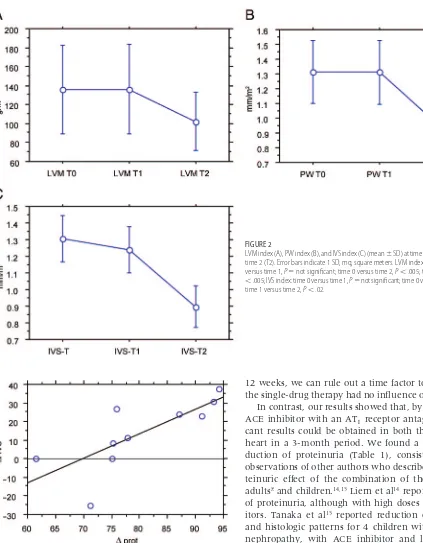
IVS index, PW index, and LVM index are reported in Fig 2. It is of interest that all echocardiographic param-eters were reduced significantly at time 2 (end of phase 2, double-drug treatment), compared with time 0 and time 1. In contrast, no statistically significant differences were found between time 0 and time 1. Between time 0 and time 2, the mean reduction of the IVS index was
⫺13.63⫾18.64%, that of the PW index was⫺30.71⫾ 20.32%, and that of the LVM index was ⫺28.33 ⫾ 24.44%. It is interesting to observe that, between time 0 and time 2, the change in proteinuria was correlated directly with the change in IVS thickness (P⬎.02) but not with the LVM index and PW index (Fig 3).
DISCUSSION
In this study, single-drug therapy with either ACE in-hibitors or AT1 receptor antagonists proved insufficient to reduce proteinuria and LVM significantly. Other au-thors1,13 observed significant reductions with a
single-drug regimen, possibly because of the higher dosages they used. This interpretation is supported by the
find-TABLE 1 Renal Function Tests at the Beginning of the Study (Time 0), at the End of Phase 1 (Single-Drug Therapy) (Time 1), and at the End of Phase 2 (Combination Therapy) (Time 2)
Parameter Time 0 Time 1 Time 2
GFR, mean⫾SD, mL/min per 1.73 m2 90.35⫾38.64 83.89⫾44.47 82.70⫾38.64
Serum potassium concentration, mean⫾SD, mEq/L 4.14⫾0.48 4.34⫾0.67 4.76⫾0.77 Fractional excretion of sodium, mean⫾SD, % 1.47⫾1.92 1.24⫾0.87 0.93⫾0.282 Fractional excretion of potassium, mean⫾SD, % 12.03⫾5.12 10.68⫾4.32 10.96⫾2.34 Proteinuria, mean⫾SD, mg/L 857.4⫾989.1 1038.12⫾1455.58 104.86⫾59.76 GFR, serum potassium concentration, fractional excretion of sodium, and fractional excretion of potassium: time 0 versus time 1, time 0 versus time 2, and time 1 versus time 2,P⫽not significant; proteinuria, time 0 versus time 1,P⫽not significant; time 0 versus time 2,P⬍.005; time 1 versus time 2,P⬍.005.
FIGURE 1
ings of Liern et al,14who observed reduced proteinuria
only when high dosages of ACE inhibitors were used. Other authors reported that the antiproteinuric effect of ACE inhibitors was strictly dose dependent.8Because the
antiproteinuric effect of these drugs goes into effect after an average 4 weeks of treatment22and our study lasted
12 weeks, we can rule out a time factor to explain why the single-drug therapy had no influence on proteinuria. In contrast, our results showed that, by combining an ACE inhibitor with an AT1 receptor antagonist, signifi-cant results could be obtained in both the kidney and heart in a 3-month period. We found a significant re-duction of proteinuria (Table 1), consistent with the observations of other authors who described an antipro-teinuric effect of the combination of the 2 drugs for adults8and children.14,15Liern et al14reported reduction
of proteinuria, although with high doses of ACE inhib-itors. Tanaka et al15 reported reduction of proteinuria
and histologic patterns for 4 children with IgA-related nephropathy, with ACE inhibitor and losartan doses comparable to ours but after a 12-month treatment. It is noteworthy that, with our protocol for therapy, the re-duction of proteinuria between time 0 and time 2 was greater than that observed for children by White et al13
with the 2 drug families in single-drug therapy. To our knowledge, the efficacy of 2-drug therapy at low doses had not been proved for children; however, it was stud-ied in adults.16,23 Those authors reported
more-pro-nounced reduction of proteinuria and a lack of un-wanted side effects on tubules and on GFR, compared with the use of a single drug at higher dosages. This FIGURE 2
LVM index (A), PW index (B), and IVS index (C) (mean⫾SD) at time 0 (T0), time 1 (T1), and time 2 (T2). Error bars indicate 1 SD; mq, square meters. LVM index and PW index: time 0 versus time 1,P⫽not significant; time 0 versus time 2,P⬍.005; time 1 versus time 2,P
⬍.005; IVS index: time 0 versus time 1,P⫽not significant; time 0 versus time 2,P⬍.035; time 1 versus time 2,P⬍.02.
FIGURE 3
better clinical outcome is particularly desirable for ne-phropathic patients and is in full agreement with our present observations for children with low doses of an ACE inhibitor and an AT1receptor antagonist in associ-ation.
We also observed reductions in the LVM index, PW index, and IVS index (Fig 2). He et al24 reported a
sig-nificant reduction of the LVM in hypertensive rats after 8- to 16-week treatment with losartan or fosinopril as a single drug. Similar findings were reported by Asai et al,17 who described LVM reduction after treatment of
hypertensive rats with losartan for 6 weeks. For adult hypertensive human patients, Devereux et al25reported
reduction of the LVM after 12 and 24 months of treat-ment with losartan. Suzuki et al26 reported that, for
diabetic adults with end-stage renal disease undergoing hemodialysis, combined therapy with ACE inhibitors and AT1receptor antagonists was more effective in re-ducing the LVM after 6 and 12 months of treatment than was single therapy with ACE inhibitors or AT1receptor antagonists alone. It is noteworthy that the experimental studies with rats suggested that the heart mass reduction could become evident much earlier than commonly de-scribed, which supports our observation.16,17
GFR, fractional excretion of sodium, fractional excre-tion of potassium, and serum potassium levels remained constant throughout the entire study, showing that the chosen drugs at the dosages used, both alone and in combination, did not produce adverse effects on renal function and potassium levels. During the study, we did not observe any significant reduction in blood pressure at time 1 and time 2, compared with the starting values at time 0. Similarly, we observed no difference in blood pressure readings between time 1 and time 2 (Fig 1). There is no general agreement on the action of these families of drugs on blood pressure. Some studies re-ported that ACE inhibitors and AT1receptor antagonists, used in single-drug therapy for control of proteinuria, had no effect on blood pressure,4,27whereas some studies
observed that ACE inhibitors caused a reduction of dia-stolic blood pressure only28and others reported a
reduc-tion of arterial blood pressure with administrareduc-tion of AT1 receptor antagonists forⱖ1 year.23Finally, Liern et al,14
using a combination of an ACE inhibitor and a AT1 receptor antagonist, but with higher doses of the ACE inhibitor, reported a reduction of arterial blood pressure; the latter finding was correlated significantly with the reduction of proteinuria. In our study, proteinuria de-creased significantly (Table 1) without a concomitant reduction in blood pressure readings at time 0, a likely demonstration that the 2 effects are unrelated, as sug-gested also by Viberti and Wheeldon.27
Furthermore, our findings showed a correlation be-tween the reduction of proteinuria and the reduction of IVS thickness. This points to direct actions of the 2 drugs
on the kidney and heart, not mediated by systemic blood pressure decreases (Fig 3).
In our opinion, more than the reduction of protein-uria, the most important finding in the study was the reduction of the cardiac mass, which should decrease cardiovascular risk and improve heart function. Like proteinuria, the IVS hypertrophy found in chronic renal diseases is likely to result from high blood levels of angiotensin II.18
Definitive assessment of the beneficial effects of the 2-drug combination therapy on preservation of renal and cardiac function requires collaborative studies with much longer follow-up periods. It could also be impor-tant to compare the effects (including possible adverse effects) of the combined therapy with those of a single-drug treatment at a double dose. Similarly, larger studies with longer follow-up periods are needed to assess pos-sible side effects such as hyperkalemia and excessive decreases in blood pressure, which are linked frequently to the use of these drugs.
REFERENCES
1. Ellis D, Vats A, Morits ML, et al. Long-term antiproteinuric and renoprotective efficacy and safety of losartan in children with proteinuria.J Pediatr.2003;143:89 –97
2. Burton MB, Harris KGP. The role of proteinuria in the progres-sion of chronic renal disease.Am J Kidney Dis.1996;27:765–775 3. Remuzzi G, Bertani T. Pathophysiology of progressive
ne-phropathies.N Engl J Med.1998;339:1448 –1456
4. GISEN Group. Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropa-thy.Lancet.1997;349:1857–1863
5. Soergel M, Schaefer F. Effect of hypertension on the progres-sion of chronic renal failure in children.Am J Hypertens.2002; 15(suppl 1):53–56
6. Klahr S, Schreiner G, Ichikawa I. The progression of renal disease.N Engl J Med.1998;318:1657–1666
7. Campbell RC, Ruggenti P, Remuzzi G. Halting the progression of chronic nephropathy.J Am Soc Nephrol.2002;13(suppl 3): S190 –S195
8. Laverman GD, Navis G, Henning RH, et al. Dual renin-angiotensin system blockade at optimal doses for proteinuria. Kidney Int.2002;62:1020 –1025
9. Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney Int. 2005;67:799 – 812
10. Wolf G, Butzmann U, Wenzel UO. The renin-angiotensin sys-tem and progression of renal disease: from hemodynamics to cell biology.Nephron Physiol.2003;93:P3–P13
11. Ruggenti P, Perna A, Benini R, et al. In chronic nephropathies prolonged ACE inhibition can induce remission: dynamics of time-dependent changes in GFR: investigators of the GISEN Group.J Am Soc Nephrol.1999;10:997–1006
12. Tikkanen I, Omvik O, Jensen HE. Comparison of the angio-tensin II antagonist losartan with the angioangio-tensin-converting enzyme inhibitor enalapril in patients with essential hyperten-sion.J Hypertens.1995;13:1343–1351
14. Liern M, Dieguez SM, De Reyes V, et al. The additive antipro-teinuric effect of enalapril and losartan to normotensive pa-tients with pathology proteinuria [in Spanish]. Nefrologia. 2004;24:553–558
15. Tanaka H, Suzuki K, Nakahata T, et al. Combined therapy of enalapril and losartan attenuates histologic progression in im-munoglobulin A nephropathy.Pediatr Int.2004;46:576 –579 16. Tylicki L, Rutkowski P, Renke M, et al. Renoprotective effect of
small doses of losartan and enalapril in patients with primary glomerulonephritis: short term observation. Am J Nephrol. 2002;22:356 –362
17. Asai T, Kushiro T, Fujita H, Kammatsuse K. Different effects on cardiac hypertrophy in spontaneously hypertensive rats by monotherapy and combination therapy of adrenergic receptor antagonists and/or the angiotensin II type 1 receptor blocker under comparable blood pressure reduction. Hypetens Res. 2005;28:79 – 87
18. Stawowy P, Margeta C, Blaschke F, et al. Protein kinase C⑀ mediates angiotensin II-induced activation of1-integrins in cardiac fibroblasts.Cardiovasc Res.2005;67:50 –59
19. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents.Pediatrics. 2004;114:555–576
20. Bergstein JM. A practical approach to proteinuria.Pediatr Neph-rol.1999;13:697–700
21. World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA.2000;284:3043–3045
22. Ganservoort RT, De Zeeuw D, De Jong PE. Is the antiprotein-uric effect of ACE inhibition mediated by interference in the renin-angiotensin system?Kidney Int.1989;36:272–279 23. Renke M, Tylicki L, Rutkowski P, et al. Low-dose blockade of
renin-angiotensin system improves tubular status in non-diabetic proteinuric patients. Scand J Urol Nephrol. 2005;6: 511–517
24. He BX, Yu GL, Liang XQ. Effects of losartan, fosinopril on myocardial fibrosis, angiotensin II and cardiac remolding in hypertensive rats [in Chinese].Hunan Yi Ke Da Xue Xue Bao. 2001;26:118 –120
25. Devereux RB, Palmieri V, Liu JE, et al. Progressive hypertrophy regression with sustained pressure reduction in hypertension: the Losartan Intervention For Endpoint Reduction Study.J Hy-pertens.2002;20:1445–1450
26. Suzuki H, Kanno Y, Kaneko K, et al. Comparison of the effects of angiotensin receptor antagonist, angiotensin-converting enzyme inhibitor, and their combination on re-gression of left ventricular hypertrophy of diabetes type 2 patients on recent onset hemodialysis therapy. Ther Apher Dial.2004;8:320 –327
27. Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure independent effect.Circulation.2002;106:672– 678 28. Maschio G, Alberti D, Janin G, et al. Effect of the
DOI: 10.1542/peds.2005-2053 originally published online August 21, 2006;
2006;118;e833
Pediatrics
Masciangelo and Stefano Romero
Elisabetta Travasso, Simona Scateni, Valeria Di Maio, Paolo Versacci, Raffaele
Riccardo Lubrano, Francesca Soscia, Marco Elli, Flavia Ventriglia, Claudia Raggi,
Plus Angiotensin II Receptor Antagonist Therapy in Children With Proteinuria
Renal and Cardiovascular Effects of Angiotensin-Converting Enzyme Inhibitor
Services
Updated Information &
http://pediatrics.aappublications.org/content/118/3/e833
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/118/3/e833#BIBL
This article cites 27 articles, 2 of which you can access for free at:
Subspecialty Collections
atistics_sub
http://www.aappublications.org/cgi/collection/research_methods_-_st
Research Methods & Statistics
b
http://www.aappublications.org/cgi/collection/medical_education_su
Medical Education following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2005-2053 originally published online August 21, 2006;
2006;118;e833
Pediatrics
Masciangelo and Stefano Romero
Elisabetta Travasso, Simona Scateni, Valeria Di Maio, Paolo Versacci, Raffaele
Riccardo Lubrano, Francesca Soscia, Marco Elli, Flavia Ventriglia, Claudia Raggi,
Plus Angiotensin II Receptor Antagonist Therapy in Children With Proteinuria
Renal and Cardiovascular Effects of Angiotensin-Converting Enzyme Inhibitor
http://pediatrics.aappublications.org/content/118/3/e833
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.