ARTICLE
Placental Growth Factor and Vascular Endothelial
Growth Factor Receptor-2 in Human
Lung Development
Joakim Jane´r, MDa, Sture Andersson, MD, PhDa, Caj Haglund, MD, PhDb, Riitta Karikoski, MDc, Patrik Lassus, MD, PhDd
aHospital for Children and Adolescents and Departments ofbSurgery,cPathology, anddPlastic Surgery, University of Helsinki, Helsinki, Finland
The authors have indicated they have no financial relationships relevant to this article to disclose.
What’s Known on This Subject
The participation of PlGF and VEGFR-2 in human lung development and possibly in the development of BPD is not clear.
What This Study Adds
This study describes the expression of PlGF and VEGFR-2 in the developing human lung and during BPD. Immunohistochemical staining and tracheal aspirate fluid concentra-tions were analyzed.
ABSTRACT
OBJECTIVE.We examined the pulmonary expression of 2 proangiogenic factors, namely, placental growth factor and vascular endothelial growth factor receptor-2, during lung development and acute and chronic lung injury in newborn infants.
METHODS.Six groups were included in an immunohistochemical study of placental growth factor and vascular endothelial growth factor receptor-2, that is, 9 fetuses, 4 preterm and 8 term infants without lung injury who died soon after birth, 5 preterm infants with respiratory distress syndrome of⬍2 days and 7 with respiratory distress syndrome of ⬎10 days, and 6 with bronchopulmonary dysplasia. Placental growth factor concentrations in tracheal aspirate fluid were measured in 70 samples from 20 preterm infants during the first postnatal week.
RESULTS.In immunohistochemical analyses, placental growth factor staining was seen in bronchial epithelium and macrophages in all groups. Distal airway epithelium positivity was observed mostly in fetuses and in preterm infants who died soon after birth. Vascular endothelial growth factor receptor-2 staining was seen in vascular endothelium in all groups and also in lymphatic endothelium in fetuses. Vascular endothelial growth factor receptor-2 staining in arterial endothelium was associated with higher and staining in venous endothelium with lower gestational age. In capillaries, less vascular endothelial growth factor receptor-2 staining was seen in bronchopulmonary dysplasia. The mean placental growth factor protein concentra-tion in tracheal aspirate fluid during the first postnatal week was 0.64⫾0.42 pg/mL per IgA-secretory component unit. Concentrations during the first postnatal week were stable. Lower placental growth factor concentrations correlated with chorio-amnionitis and lactosyl ceramide positivity.
CONCLUSIONS.The vascular endothelial growth factor receptor-2 staining pattern seems to reflect ongoing differentiation and activity of different endothelia. Lower vascular endothelial growth factor receptor-2 expression in capillary endothelium in bronchopulmonary dysplasia might be a reflection of the dysregulation of vascular development that is characteristic of bronchopulmonary dysplasia.Pediatrics2008;122:340–346
H
UMAN PLACENTAL GROWTHfactor (PlGF) initially was located in the human placenta1but also has been located in heart and lung. PlGF mediates its actions through vascular endothelial growth factor receptor (VEGFR)-1.2 VEGFR-2 is a receptor tyrosine kinase3 that binds vascular endothelial growth factor (VEGF)-A, VEGF-C, and VEGF-D and is recognized as the primary receptor transmitting signals in endothelial cells.4,5It has been suggested that PlGF boosts angiogenesis by binding to VEGFR-1, leaving a higher concentration of free VEGF-A that can bind to VEGFR-2.6In addition, PlGF seems to have a distinct angiogenic signaling pathway through VEGFR-1.7–9An infant born early in the third trimester of gestation has poorly developed lungs; the alveoli are just forming, surfactant production has only recently begun, and the capillary bed is poorly developed. Birth at this stage interrupts normal development of the lung in infants of very low birth weight (BW).10The development of bronchopulmonary dysplasia (BPD) may be attributable to disruption of vascular development by premature birth.11,12VEGF-A
partic-www.pediatrics.org/cgi/doi/10.1542/ peds.2007-1941
doi:10.1542/peds.2007-1941
Key Words
lung development, neonatal, respiratory, bronchopulmonary dysplasia
Abbreviations BW— birth weight
BPD— bronchopulmonary dysplasia GA— gestational age
PlGF—placental growth factor RDS—respiratory distress syndrome TAF—tracheal aspirate fluid
VEGF—vascular endothelial growth factor VEGFR—vascular endothelial growth factor receptor
IgA-SC—secretory component of IgA
Accepted for publication Dec 15, 2007
Address correspondence to Joakim Jane´r, MD, Hospital for Children and Adolescents, PO Box 281, 00029 HUS, Helsinki, Finland. E-mail: joakim.janer@helsinki.fi
ipates in alveolarization in animals.13,14 Moreover, in preterm infants, high pulmonary concentrations of VEGF-A during postnatal days 4 to 7 are associated with lower incidence of BPD.15
We wanted to study the roles of these 2 proangio-genic factors, PlGF and VEGFR-2, that participate in VEGF-A-orchestrated angiogenesis during lung develop-ment and in lung injury. Therefore, we evaluated whether postnatal PlGF concentrations in tracheal aspi-rate fluid (TAF) were associated with parameters reflect-ing development of respiratory distress syndrome (RDS) or BPD. We also evaluated whether PlGF and VEGFR-2 expression patterns changed during lung development, with and without lung injury.
METHODS
Ethics Approval
All studies were approved by the ethics committee of the Hospital for Children and Adolescents, University Cen-tral Hospital (Helsinki, Finland).
Patients in the Immunohistochemical Study
Thirty-nine subjects were included in the PlGF and VEGFR-2 immunohistochemical study. Samples were analyzed in 6 groups, as follows: 9 fetal subjects (gesta-tional age [GA]: 18.1⫾2.6 weeks; BW: 194⫾122 g), 4 preterm control subjects (GA: 24.6 ⫾ 2.9 weeks; BW: 788⫾341 g), 8 term control subjects (GA: 38.9 ⫾1.7 weeks; BW: 3411 ⫾608 g), 5 early RDS subjects (GA: 26.8⫾1.9 weeks; BW: 799⫾464 g; age at death: 1.3⫾ 0.7 days), 7 late RDS subjects (GA: 26.8⫾ 1.7 weeks; BW: 692⫾151 g; age at death: 11.3⫾2.8 days), and 6 BPD subjects (GA: 28.2⫾2.3 weeks; BW: 892⫾177 g; age at death: 207 ⫾ 82 days). Samples were collected between March 1991 and June 2000. Infants in the preterm control group did not suffer from RDS and died within 2 hours after birth; causes of premature delivery and death included spontaneous abortion, acute as-phyxia, placental ablation, and feto-fetal transfusion (donor). The early RDS group included preterm infants who died within 2 days after birth. The late RDS group included preterm infants who died⬎10 days after birth but did not meet the criteria for BPD. BPD was diagnosed clinically as a need for supplemental oxygen at the age of 36 gestational weeks, in addition to chest radiographic findings typical of BPD16and postmortem histologic find-ings. The patients in both the RDS and BPD groups died as a result of the lung disease. Fetuses and control sub-jects had macroscopically and microscopically normal
lungs; the microscopic findings did not include hyaline membranes characteristic of lung injury present in RDS. Autopsies were performed within 4 days after death (Table 1).
Patients in the TAF Sample Study
A series of samples were collected from 20 preterm infants. Nine patients who subsequently developed BPD and 11 GA- and BW-matched patients who survived without BPD were selected. BPD was defined as stated above. All of the infants included were intubated at birth because of failure to establish spontaneous ventilation, and none of the patients in the study group received dexamethasone during the study period (Table 2).
Immunohistochemical Analyses of PlGF and VEGFR-2
Lung samples were obtained as described previously.17 Sections (5 m) were deparaffinized in xylene and re-hydrated through graded concentrations of alcohol and distilled water. The sections were then treated in a 700-W microwave oven for 4⫻5 minutes, in Tris-EDTA solution, and the slides were cooled at room tempera-ture for 20 minutes and washed in 1:10 phosphate-buffered saline/distilled water solution. PlGF-specific an-tibody was used at a 1:100 dilution (anan-tibody ab9542, rabbit polyclonal antibody to human PlGF; Novus Bio-logicals, Littleton, CO). VEGFR-2-specific antibody was used at a 1:60 dilution (antibody AF357, anti-human VEGFR-2 antibody; R&D Systems, Minneapolis, MN), following the instructions provided by the manufac-turer. Bound antibody was visualized by using the avi-din-biotin complex immunoperoxidase technique (Elite ABC kit, Vectastain; Vector, Burlingame, CA). Sections were incubated with the biotinylated second-layer anti-body and peroxidase-labeled avidin-biotin complex for 30 minutes each. All dilutions were made in phosphate-buffered saline (pH 7.2), and all incubations in the avi-din-biotin complex method were conducted in humidi-fied chambers at room temperature. Between each step in the staining process, slides were rinsed in 3 changes of phosphate-buffered saline. Peroxidase staining was visu-alized with 3-amino-9-ethylcarbazole (A-5754; Sigma, St Louis, MO), 0.2 mg/mL in 0.05 mol/L acetate buffer containing 0.03% perhydrol (pH 5.0), at room temper-ature for 15 minutes. Sections were rinsed in tap water for 10 minutes. To complete the process, sections were counterstained in Mayer’s hematoxylin, cleared in tap water, and mounted in aqueous mounting medium (Aquamount; BDH, Poole, England). Negative control
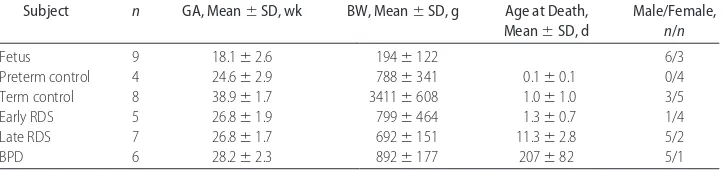
TABLE 1 Patients in Immunohistochemical Study
Subject n GA, Mean⫾SD, wk BW, Mean⫾SD, g Age at Death, Mean⫾SD, d
Male/Female,
n/n
Fetus 9 18.1⫾2.6 194⫾122 6/3
Preterm control 4 24.6⫾2.9 788⫾341 0.1⫾0.1 0/4
Term control 8 38.9⫾1.7 3411⫾608 1.0⫾1.0 3/5
Early RDS 5 26.8⫾1.9 799⫾464 1.3⫾0.7 1/4
Late RDS 7 26.8⫾1.7 692⫾151 11.3⫾2.8 5/2
samples were prepared with omission of the primary antibody, and a known antibody-positive section (fetal liver) was included as a positive control sample.
TAF Sample Collection
Samples of TAF were collected once daily through stan-dardized routine tracheal lavage, as described previous-ly.15 A total of 70 samples collected from 20 patients during the first postnatal week were used for analysis.
Analyses of PlGF and Secretory Component of IgA in TAF PlGF was analyzed with a human PlGF immunoassay kit (R&D Systems). To estimate the in situ pulmonary con-centration of PlGF, a correction for dilution of the TAF sample was calculated by using the concentration of the secretory component of IgA (IgA-SC) in TAF. The con-centration of IgA-SC in lung secretions is independent of capillary leak, and the concentration of IgA-SC in TAF samples is independent of respiratory distress or GA.18 IgA-SC concentrations were determined in direct en-zyme-linked immunosorbent assays, with secretory IgA isolated from human colostrum as a standard. The IgA-SC standards were kindly provided by Dr B. Go¨tze-Speer and Prof C. Go¨tze-Speer (University Children’s Hospital, Wu¨rzburg, Germany).
Statistical Analyses
Nonparametric methods were used in analyses of TAF data, including the Mann-WhitneyU test, the
Kruskal-Wallis test, and simple regression analysis. Values repre-sent mean ⫾ SD for patient data, mean ⫾ SEM for experimental results, and frequencies for categorical variables. Variables with skewed distribution were loga-rithmically transformed before analyses, but values pre-sented in the text and tables are nontransformed. P
values of ⬍.05 were considered statistically significant. In analyses of immunohistochemical data, contingency table tests and t tests were used. All calculations were performed with StatView 5.1 (SAS Institute, Cary, NC).
RESULTS
Immunohistochemical Analysis of PlGF
Positive staining for PlGF was observed in macrophages in 34 (87%) of the 39 cases. Macrophages were stained throughout the samples in most cases. Bronchial epithe-lial positivity was found in 24 (62%) of the 39 cases. Staining was visible in large bronchi and in small bron-chioli. Staining was observed both apically and more uniformly within epithelial cells. No differences existed between groups in bronchial epithelial staining. Distal airway epithelial positivity was observed in 11 (28%) of the 39 cases. Distal airway positivity was seen almost exclusively in fetuses and in preterm infants who died soon after birth, with or without lung injury. Epithelial cell staining was observed apically in fetuses, whereas staining was distributed more uniformly within the ep-ithelial cells in preterm infants. Positive PlGF staining in
TABLE 2 Patient Data and Results in TAF Study
Parameters Patient Data PlGF/IgA-SC, Mean⫾SD, pg/mL per IgA-SC Unit
Prenatal parameters
Male vs female,n 10 vs 10 NS
Prenatal betamethasone treatment, yes vs no,n 14 vs 6 NS Chorioamnionitis, yes vs no,na 7 vs 13 0.48⫾0.06 vs 0.83⫾0.19
(P⫽.015)
Preeclampsia, yes vs no,nb 4 vs 16 NS
Parameters reflecting maturity
GA, mean⫾SD, wk 27.7⫾2.3 NS
BW, mean⫾SD, g 1088⫾409 NS
Lecithin/sphingomyelin ratio (0–10), mean⫾SDc 7⫾3 NS
Postnatal parameters
Indomethacin treatment, yes vs no,n 14 vs 6 NS
Infection, yes vs no,nd 4 vs 16 NS
Lactosyl ceramide, yes vs no,nc 5 vs 5 0.46⫾0.08 vs 1.0⫾0.27
(P⫽.027) Respiratory distress parameters
Initial arteriolar/alveolar ratio, mean⫾SD 0.16⫾0.14 NS
Surfactant treatment, yes vs no,n 19 vs 1 NS
Dexamethasone treatment, yes vs no,n 5 vs 15 NS
Extubation, mean⫾SD (range), d 16⫾13 (5–44) NS
Development of BPD, yes vs no 9 vs 11 NS
Fraction of inspired oxygen, mean⫾SD, %e 38⫾18 NS NS indicates not significant.
aChorioamnionitis was diagnosed on the basis of clinical signs, leukocytosis (B leukocyte count:⬎14⫻109cells per L), or plasma C-reactive
protein concentration of⬎50 mg/L.
bPreeclampsia was diagnosed on the basis of maternal elevated blood pressure and proteinuria. cFetal lung maturity was measured in a TAF sample within 3 hours after birth.
dInfection was diagnosed on the basis of clinical signs, leukocytosis (B leukocyte count:⬎14⫻109cells per L), or plasma C-reactive protein
concentration of⬎50 mg/L.
distal airway epithelium was associated with lower GA (20.9⫾4.4 weeks vs 29.7⫾6.7 weeks;P⫽.0003) and with lower BW or weight at the time of abortion (361⫾ 250 g vs 1515 ⫾1219 g; P⫽ .0060). Gender or post-mortem time to autopsy did not correlate with positive PlGF staining (Figs 1 and 2).
Immunohistochemical Analysis of VEGFR-2
Positive staining for VEGFR-2 was seen in vascular en-dothelium in 32 (82%) of the 39 cases. Positive staining in arterial endothelium was visible in 9 (23%) of the 39 cases, and it associated with higher GA (32.1 ⫾ 6.9 weeks vs 25.8⫾6.9 weeks;P⫽.021) and with higher BW or weight at the time of abortion (2008⫾1383 g vs 943 ⫾ 1069 g; P ⫽ .019). Positive staining in venous endothelium was visible in 27 (69%) of the 39 cases, and it was associated with lower GA (25.5 ⫾6.5 weeks vs 31.1 ⫾ 7.8 weeks; P ⫽ .024) and with lower BW or weight at the time of abortion (900⫾963 g vs 1839⫾ 1502 g;P⫽.024). Positive staining of VEGFR-2 in cap-illary endothelium was seen in 29 (74%) of the 39 cases. Between groups, capillary positivity was ⬎60% except in the BPD group, where positivity was 33%. Capillary VEGFR-2 positivity was associated with lower postnatal age of death (12⫾41 days vs 96⫾127 days;P⫽.0034). VEGFR-2 staining was also seen in lymphatic vessel-resembling structures in 7 (18%) of the 39 cases. Of the 9 fetuses, positivity in lymphatic structures was seen in 5 (55%). All fetuses that exhibited positive lymphatic en-dothelial staining were at⬍21 weeks of gestation (GA: 17.0⫾2.7 weeks). Gender or postmortem time to au-topsy did not correlate with positive VEGFR-2 staining (Figs 1 and 3).
PlGF Protein Levels in TAF
The mean PlGF protein concentration in TAF during the first postnatal week was 0.64⫾0.42 pg/mL per IgA-SC unit; the mean PlGF levels during the first postnatal week were used in statistical analyses (Fig 4). Of the 20 infants, 7 were born from pregnancies complicated by chorioamnionitis or premature rupture of the mem-branes. Those infants had lower mean PlGF levels in TAF than did infants with no maternal infections or pre-eclampsia (P⫽.015).
Lactosyl ceramide has been demonstrated in large amounts in granulocytes and inflamed fetal membranes. It was measured in 10 infants with thin layer chroma-tography, as described previously.19 Lactosyl ceramide levels correlated positivity with lower PlGF levels in TAF (P⫽.027). No correlations were found between BW or
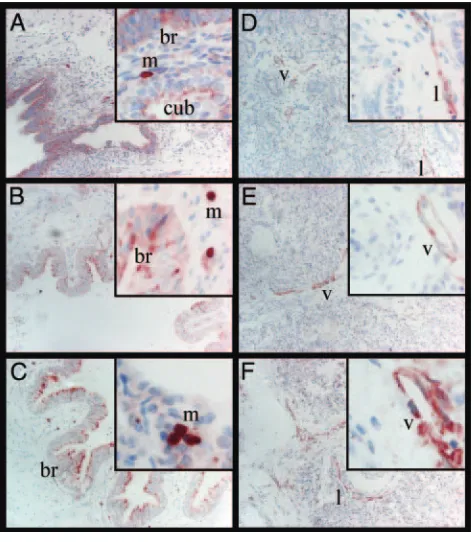
FIGURE 1
A, PlGF staining in a fetus (GA: 18.0 weeks; BW: 160 g), with positive staining in macro-phages, bronchial epithelium, and cuboidal epithelium. B, PlGF staining in a subject with RDS (GA: 25.7 weeks; BW: 500 g), with positive staining in macrophages and bronchial epithelium. C, PlGF staining in a control subject (GA: 36.0 weeks; BW: 2900 g), with positive staining in macrophages and bronchial epithelium. D, VEGFR-2 staining in a fetus (GA: 17.0 weeks; BW: 160 g), with positive staining in lymphatic and venous endothelia. E, VEGFR-2 staining in a subject with RDS (GA: 26.0 weeks; BW: 825 g), with positive staining in venous endothelium. F, VEGFR-2 staining in a control subject (GA: 39.1 weeks; BW: 4190 g), with positive staining in lymphatic and venous endothelia. m indicates macro-phage; br, bronchial epithelium; cub, cuboidal epithelium; l, lymphatic endothelium; v, venous endothelium. (Magnification of panels: 40⫻, insets: 100⫻. Insets were chosen to illustrate and highlight positively staining structures in respective groups.)
FIGURE 2
GA or between pH or base excess in cord artery blood and PlGF levels in TAF. Duration of mechanical ventila-tion, mean concentration of inspired oxygen during the first postnatal week, and subsequent development of BPD did not correlate with PlGF concentrations in TAF. PlGF concentrations in TAF did not correlate with pre-natal betamethasone treatment, indomethacin treat-ment, or subsequent need for dexamethasone therapy (Table 2).
DISCUSSION
In this study, we showed that PlGF protein is expressed consistently in airway epithelium during lung develop-ment. Expression was seen throughout lung epithelium from large proximal bronchi to cuboidal and alveolar epithelia. PlGF protein expression was detected in the fetal lung as early as 14 weeks of gestation. In addition, a significant amount of PlGF protein was detected in TAF from human preterm infants during the first postnatal week. PlGF⫺/⫺mice were shown to exhibit normal
vas-cular development.20In our study, PlGF protein
expres-sion in distal airway epithelium was restricted to fetuses and preterm infants, and there were correlations be-tween PlGF expression in alveolar and cuboidal epithelia and lower BW and GA. However, levels of PlGF in TAF did not correlate with GA or BW. PlGF mediates its effects mainly through VEGFR-1.2Interestingly, positive VEGFR-1 staining noted previously resembles the PlGF staining seen in this study; positive staining could be seen throughout the developing lung in fetuses and preterm infants, whereas staining was not seen adjacent to distal epithelium in term infants.17We conclude that, in the developing human lung, PlGF is expressed con-tinuously throughout the fetal period. There is, how-ever, a change in PlGF expression patterns from distal epithelium to more-proximal epithelium during devel-opment, which suggests that, at least during the later stages of development, PlGF asserts its effects mainly on conducting airways.
The development of BPD has been suggested to result from disruption of vascular development, which leads to an arrest of lung alveolarization.11,12Preterm infants who develop BPD have lower levels of VEGF-A than do those who do not develop BPD.15 PlGF boosts angiogenesis through binding of VEGFR-1 and subsequent activation of VEGFR-2 by VEGF-A.6It has been shown that PlGF is induced to assist VEGF-A in pathologic angiogenesis in adults.20Pathologic angiogenesis is often associated with inflammation, and PlGF itself is an attractant of inflam-matory cells.21 Preterm birth is often associated with maternal chorioamnionitis; histologic chorioamnionitis is related inversely to GA, being well above 50% at GA of 20 to 24 weeks.22In addition, mechanical ventilation elicits an inflammatory state in the preterm lung. The injury caused by ventilation leads to the release of proin-flammatory cytokines, which in turn activate and attract neutrophils and phagocytes to the site of injury.23,24In view of this, it was reasonable to expect a postpartum elevation of PlGF protein levels. Interestingly, in our patients, the concentrations of PlGF in TAF during the first postnatal week were stable (Fig 4). Moreover, PlGF protein concentrations in TAF were not elevated in con-junction with parameters reflecting RDS or BPD. In-stead, infants born from pregnancies complicated by chorioamnionitis or premature rupture of the
mem-FIGURE 4
Daily mean PlGF concentrations in TAF in preterm infants (n⫽20; GA: 27.7⫾2.3 weeks) during the first postnatal week (samples,n⫽70; daily values represent mean⫾SEM). Numbers in graph represent sample sizes for each respective day.
FIGURE 3
branes and those who tested positive for lactosyl cer-amide had lower PlGF levels in TAF during the first postnatal week. We showed previously that lower VEGF concentrations in TAF after birth correlated with the development of BPD.15In view of our finding, it is pos-sible to draw the conclusion that inflammation in the lung perinatally hinders alveolarization through de-creased angiogenesis, mirrored by the decrease in PlGF concentrations in TAF, and inflammation could be an important factor in the development of BPD. Therefore, PlGF seems to have a constitutional role in the develop-ing human lung, various states of inflammation perina-tally lead to decreased levels of PlGF, and inflammation could contribute to the pathogenesis of BPD.
We found constant VEGFR-2 staining in airway dothelium. However, staining in different types of en-dothelium varied during development. We detected some VEGFR-2 expression in lymphatic endothelium, mostly in fetuses. In addition to vascular endothelial development, VEGFR-2 has been shown to have other roles. In mice, VEGFR-2 is essential for the development of hematopoietic and endothelial cells.25 In early stages of development, there seems to be some overlapping of vasculogenesis, angiogenesis, and lymphangiogenesis; VEGF-C binds VEGFR-2, in addition to VEGFR-3, in early stages of lung development,26 and VEGF-C may play a role in the development of the vascular tree.27 Also, VEGFR-3, which is strictly lymphangiogenic later in development, is vital to venous development in mouse lung.27VEGFR-2 was detected previously on lym-phatic endothelial cells.28In our study, we found more staining for VEGFR-2 in venous endothelium in more-immature lungs, whereas more staining in arterial en-dothelium was seen in more-mature lungs (Fig 3). VEGFR-2 expression is downregulated in mature vascu-lar endothelium and is upregulated again in angiogenic vessels.29 The VEGFR-2 staining in lymphatic and ve-nous endothelia during the early stages of lung devel-opment and arterial staining during later develdevel-opment could reflect the ongoing differentiation and activity of different endothelia.
Capillary endothelial staining was fairly constant, the only exception being that staining of capillary and septal endothelia was decreased in BPD. This could be ex-plained by the vascular hypothesis of BPD,11,12according to which deranged angiogenesis leads to dilated distal airspaces and decreased alveolar surface area attribut-able to alveolar and septal apoptosis. VEGFR-2 expres-sion has been shown to be guided by VEGF-A.30,31We showed previously that lower VEGF-A concentrations in TAF correlated with the development of BPD.15 Accord-ing to Shalaby et al,25 VEGFR-2-deficient mice fail to develop blood islands, and vasculogenesis is seriously impaired. This mutation leads to death in utero between postcoital days 8.5 and 9.5. More recently, Hosford et al32 demonstrated that hyperoxia (⬎95%) administered postnatally in neonatal rats led to decreased mRNA ex-pression of VEGF, as well as both VEGFR-1 and VEGFR-2, from postnatal day 6 onward, compared with rats raised in a normoxic (21%) environment. These changes in mRNA expression then led to decreased
VEGF, VEGFR-1, and VEGFR-2 protein expressions seen on postnatal days 12 and 14, inhibiting angiogenesis and subsequently alveolarization.32Levels of angiopoietin-2, which has antiangiogenic effects by destabilizing vessel walls, inducing endothelial cell apoptosis, and increasing vascular permeability, have been shown to increase in TAF in infants who develop BPD.33 According to views on BPD, it is thought that impaired angiogenesis at crit-ical stages of lung development hinders lung alveolar-ization and is central in the pathophysiologic processes of BPD.12Evidence also suggests that impaired angiogen-esis does not contribute to the pathogenangiogen-esis of BPD.34,35 De Paepe et al36 also concluded that no mismatch be-tween alveolar epithelium and vascular endothelium existed in BPD; however, that study was quantitative rather than qualitative in its approach and did not pro-vide an opportunity to study vascular structures in greater detail. Additional work is needed for better un-derstanding of the many aspects of BPD and the roles of the different proangiogenic and antiangiogenic growth factors. However, we think that decreased VEGFR-2 ex-pression in septal capillaries is a sign of dysregulation of vascular development characteristic of BPD.
CONCLUSIONS
PlGF is expressed constantly throughout lung develop-ment. Staining during earlier stages of development is seen throughout the lung epithelium, whereas staining in the later stages of development is restricted to proxi-mal airway epithelium, which suggests that, at least during the later stages of development, PlGF asserts its effects mainly on conducting airways. Various states of inflammation perinatally lead to decreased levels of PlGF; this may be one of the mechanisms through which inflammation contributes to the pathogenesis of BPD. VEGFR-2 also is expressed constantly throughout hu-man lung development. The VEGFR-2 expression profile changes with lung maturation; staining in immature lung is seen in lymphatic and venous endothelia, whereas staining in more-mature lung is seen mostly in arterial endothelium. This is seen as a reflection of the ongoing differentiation and activity of different endothe-lia during development. Expression in septal and capil-lary endothelium is decreased in BPD, which may illus-trate an aspect of the pathogenesis of BPD.
ACKNOWLEDGMENTS
This work was supported by the Sigrid Juse´lius Founda-tion, Finska La¨karesa¨llskapet, Nylands NaFounda-tion, Helsinki University Central Hospital Research Fund, and Foun-dation for Pediatric Research.
REFERENCES
1. Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein re-lated to the vascular permeability factor.Proc Natl Acad Sci USA.
1991;88(20):9267–9271
2. Yla¨-Herttuala S, Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth.Nat Med.2003;9(6):694 –701 3. Terman BI, Carrion ME, Kovacs E, Rasmussen BA, Eddy RL,
Shows TB. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene. 1991;6(9): 1677–1683
4. Wise LM, Veikkola T, Mercer AA, et al. Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1.Proc Natl Acad Sci USA.1999;96(6): 3071–3076
5. Zachary I, Gliki G. Signaling transduction mechanisms medi-ating biological actions of the vascular endothelial growth fac-tor family.Cardiovasc Res.2001;49(3):568 –581
6. Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor potentiation of vascular endothelial growth fac-tor bioactivity, in vitro and in vivo, and high affinity binding to flt-1 but not to flk-1/KDR. J Biol Chem. 1994;269(41): 25646 –25654
7. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors.FASEB J.
1999;13(1):9 –22
8. Shibuya M, Ito N, Claesson-Welsh L. Structure and function of vascular endothelial growth factor receptor-1 and -2.Curr Top Microbiol Immunol.1999;237:59 – 83
9. Cao Y, Linden P, Shima D, Browne F, Folkman J. In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor.
J Clin Invest.1996;98(11):2507–2511
10. Zoban P, Cerny M. Immature lung and acute lung injury.
Physiol Res.2003;52(5):507–516
11. Jobe AJ. The new BPD: an arrest of lung development.Pediatr Res.1999;46(6):641– 643
12. Abman SH. Bronchopulmonary dysplasia: “a vascular hypoth-esis.”Am J Respir Crit Care Med.2001;164(10):1755–1756 13. Akeson AL, Cameron JE, Le Cras TD, Whitsett JA, Greenberg
JM. Vascular endothelial growth factor-A induces prenatal neovascularization and alters bronchial development in mice.
Pediatr Res.2005;57(1):82– 88
14. Compernolle V, Brusselmans K, Acker T, et al. Loss of HIF-2␣ and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in pre-mature mice.Nat Med.2002;8(7):702–710
15. Lassus P, Ristima¨ki A, Ylikorkala O, Viinikka L, Andersson S. Vascular endothelial growth factor in the human preterm lung.
Am J Respir Crit Care Med.1999;159(5):1429 –1433
16. Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: predic-tion from oxygen requirement in the neonatal period. Pediat-rics.1988;82(4):527–532
17. Lassus P, Turanlahti M, Heikkila¨ P, et al. Pulmonary endo-thelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hyperten-sion of the newborn. Am J Respir Crit Care Med. 2001; 164(10):1981–1987
18. Watts CL, Fanaroff AA, Bruce MC. Elevation of fibronectin levels in lung secretions of infants with respiratory distress
syndrome and development of bronchopulmonary dysplasia.
J Pediatr.1992;120(4):614 – 620
19. Hallman M, Bry K, Pitkanen O. Ceramide lactoside in amniotic fluid: high concentration in chorioamnionitis and in preterm labor.Am J Obstet Gynecol.1989;161(2):313–318
20. Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in patho-logical conditions.Nat Med.2001;7(5):575–583
21. Carmeliet P. Mechanisms of angiogenesis and arteriogenesis.
Nat Med.2000;6(4):389 –395
22. Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth.Am J Obstet Gynecol.2004;190(1):147–151
23. Jobe AH. Glucocorticoids, inflammation and the perinatal lung.Semin Neonatol.2001;6(4):331–342
24. Turunen R, Nupponen I, Siitonen S, Repo H, Andersson S. Onset of mechanical ventilation is associated with rapid acti-vation of circulating phagocytes in preterm infants.Pediatrics.
2006;117(2):448 – 454
25. Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice.
Nature.1995;376(6535):62– 66
26. Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases.
EMBO J.1996;15(2):290 –298
27. Dumont DJ, Jussila L, Taipale J, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998; 282(5390):946 –949
28. Ma¨kinen T, Veikkola T, Mustjoki S, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory sig-nals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001; 20(17):4762– 4773
29. Partanen TA, Alitalo K, Miettinen M. Lack of lymphatic vas-cular specificity of vasvas-cular endothelial growth factor receptor 3 in 185 vascular tumors.Cancer.1999;86(11):2406 –2412 30. Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides
angio-genic sprouting utilizing endothelial tip cell filopodia. J Cell Biol.2003;161(6):1163–1177
31. Hiratsuka S, Kataoka Y, Nakao K, et al. Vascular endothelial growth factor A (VEGF-A) is involved in guidance of VEGF receptor-positive cells to the anterior portion of early embryos.
Mol Cell Biol.2005;25(1):355–363
32. Hosford GE, Olson DM. Effects of hyperoxia on VEGF, its receptors, and HIF-2␣in the newborn rat lung.Am J Physiol Lung Cell Mol Physiol.2003;285(1):L161–L168
33. Bhandari V, Choo-Wing R, Lee CG, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death.Nat Med.2006;12(11):1286 –1293
34. Ambalavanan N, Novak ZE. Peptide growth factors in tracheal aspirates of mechanically ventilated preterm neonates.Pediatr Res.2003;53(2):240 –244
35. Asikainen TM, Ahmad A, Schneider BK, White CW. Effect of preterm birth on hypoxia-inducible factors and vascular endo-thelial growth factor in primate lungs.Pediatr Pulmonol.2005; 40(6):538 –546
DOI: 10.1542/peds.2007-1941
2008;122;340
Pediatrics
Joakim Janér, Sture Andersson, Caj Haglund, Riitta Karikoski and Patrik Lassus
Human Lung Development
Placental Growth Factor and Vascular Endothelial Growth Factor Receptor-2 in
Services
Updated Information &
http://pediatrics.aappublications.org/content/122/2/340
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/122/2/340#BIBL
This article cites 34 articles, 9 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/neonatology_sub
Neonatology
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_
Fetus/Newborn Infant following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2007-1941
2008;122;340
Pediatrics
Joakim Janér, Sture Andersson, Caj Haglund, Riitta Karikoski and Patrik Lassus
Human Lung Development
Placental Growth Factor and Vascular Endothelial Growth Factor Receptor-2 in
http://pediatrics.aappublications.org/content/122/2/340
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.