THE EFFECT OF INORGANIC NITROGEN
ON THE RESPIRATION OF EXCISED
WHEAT ROOTS SUPPLIED WITH
ORGANIC CARBON
I. The Effect of Nitrate and Some Reduced Forms of Inorganic Nitrogen on the Endogenous and Exogenous Respiration
BY ANSELM AUSTIN*
Botanisch Laboratorium, University of Utrecht, Utrecht, Holland
THERE is still a good deal of controversy over the mechanism of nitrate reduction in non-green tissues of higher plants. Several authors postu-late a special enzyme, reductase, to be responsible for nitrate reduction. On the other hand, there is strong evidence in higher plants and other organisms that cellular. respiration and nitrate reduction are closely interrelated processes. An increase of respiration after nitrate appli-cation has been found by Hamner (1936) in wheat roots, White and Templeman (1937) in Lemna minor, and Gilbert and Shive (1945) in soybean, oat and tomato roots. An increase in R.Q. in the dark was also reported by Warburg and Negelein (1920) in nitrate-fed Chlorella. Working with Chlorella, Davis (1953) found that three major consti-tuents, namely, glucose, nitrate and phosphate, were necessary for the production of extra carbon dioxide and suggested that the concerned process ultimately was nitrate reduction.
The experiments described in this. work were designed to investi-gate a possible interaction between nitrate and the respiration of excised wheat roots under different conditions of (1) exogenous substrate, (2) substrate concentrations and (3) buffer concentrations. The results of such experiments will be of more theoretical importance if at the same time the effects of NH4+ ion and other intermediates of nitrate reduction on the respiration are known. Experiments with ammo-nium sulphate as nitrogen source were therefore included to study the relative rates of oxygen consumption, carbon dioxide production and the R.Q. The likely intermediates, nitrite and hydroxylamine, were also tested but proved to be toxic at the applied concentration.
Observations of other workers are discussed in relevant places in connection with the results of the present investigation (Part I) and also in the final ·discussion in Part III.
* Present address: Assistant Plant Physiologist, Division of Botany, I.A.R.l.,
140 ANSELM AUSTIN
MATERIALS AND METHODS
Excised roots of two-day old wheat seedlings, var. Starling, were used for the present investigation. The wheat grains were sterilized by first treating them with 10% calcium hypochlorite solution for 15 minutes and then washing them for about 7-8 hours in running tap-water. Then the grains were placed in 0 · 5% potassium permanganate for 5 minutes and finally thoroughly washed with sterile distilled water. The seeds thus sterilized were arranged on moist filter-paper in petri-dishes. The petri-dishes with the filter-paper had been sterilized pre-viously. 90-95% of the sterilized wheat grains germinated quite well within 2-3 days.
These precautions proved to be necessary since in the beginning bacterial infections occurred frequently. The respiration was measured by the Warburg manometric technique. 0·10 gm. fresh weight of roots was suspended in 2 · 0 ml. of M/15 KH2PO 4 buffer contained in each
Warburg vessel. Separate manometers were used for measuring the oxygen uptake and the carbon dioxide production. The vessels were equilibrated in the thermostat at 20° C. for half an hour before the taps were closed. When a substrate was added the basic respiration during the previous hour was noted and after this 0 · 2 ml. of the sub-strate was tipped in from the sidearm. The oxygen uptake and carbon dioxide production were read every hour. The pH of the buffer was 5 ·0. For carbon dioxide absorption 0 · 3 ml. of 10% KOH was put in the central well of the vessel. The manometers were shaken at a rate of 180 oscillations per minute. The gas exchange was always expressed in mm.3 per hour.
The effect of the concentration of phosphate buffer on endogenous respiration was first tested. Two concentrations, namely, M/150 and M/15 KH2P04 of pH 5·0 were used for this purpose. Each flask
con-tained buffer and 0 · 10 gm. root.
The effect of potassium nitrate and ammonium sulphate on tht. endogenous respiration was further studied. To the buffer · solutior. of M/15 KH2P04 , N/50 KN03 or N/100 (NH4hS04 was added as a
source of nitrogen, the amount of nitrogen thus being the same.
Since the purpose of this investigation was to find out whether the oxygen uptake, the carbon dioxide production and consequently the respiratory quotient are influenced when nitrate-fed roots are provided with various respiratory substrates in order to gain further insight in the process of nitrate reduction, the effect of potassium nitrate on the respiration with glucose as substrate was studied. After having measured the rate of the endogenous respiration in M/15 KH2P04 plus
Nj50 KN03 during the first hour, 0·2 ml. of an 11% glucose solution
was tipped in from the sidearm, giving a glucose concentration of I% (8 · 8 mg. C equivalent glucose per flask). The initial and final pH were 5 · 0 and 5 · 2 respectively.
(NH4) 2S04 as the nitrogen source. The initial and final pH were 5 ·0
and 4·9 respectively.
Nitrite ·and hydroxylamine, likely intermediates of nitrate reduc-tion, were further tested as nitrogen source with the same experimental procedure as was applied in the nitrate or ammonium experiment. In these cases N/50 potassium nitrite or hydroxylamine was used to main-tain nitrogen equivalency.
RESULTS
The results of endogenous respiration presented in Table I show that the rate of the process during eight hours was virtually the same in
TABLE 1
Effect of the buffer concentration on the rate of the endogenous respiration
(The gas exchange is expressed in mm.3/hcur)
Treatments
Time
-m
hours
Roots in M/150 KH2P04 Roots in M/ 15 KH2PO 4
02 C02 R.Q. 02 C02 R.Q.
38·3 32·9 39·4 33·9
2 39·8 34·8 39·4 33·0
3 39·2 34·9 40·3 35·0
4 40·0 36·2 38·3 33·7
5 39·1 34·6 39·1 34·4
6 39·8 35·2 39·1 34·4
7 36·5 32·0 38·0 33·4
8 35·6 31·3 35·9 31 ·6
Mean . 38 ·5 33·9 0·88 39 ·1 33·7 0·86
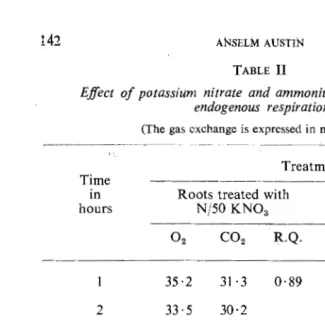
M/15 and M/150 phosphate buffer. The results presented in Table II reveal that during the six-hour experimental period the nature of the endogenous respiration was about the same in both nitrate and ammo-nium treatments. A comparison of the above results with those of the endogenous respiration of the wheat roots in buffer alone shows
142
ANSELM AUSTINTABLE II
Effect of potassium nitrate and ammonium sulphate on the endogenous respiration
Time in hours
2
3
4
5
6
Mean
(The gas exchange is expressed in mm.3jhour)
Treatments
Roots treated with N/50 KN03
R.Q.
35·2 31·3 0·89
33·5 30·2
36·5 32·1
34·5 31 ·4
35·3 31·4
33·5 30·2
34·8 31 ·1 0·89
Roots treated with N/100 (NH4) 2S04
R.Q.
34·0 29·9 0·88
35·5 32·7
34·5 30·7
36·5 32·9
33·5 29·2
33·0 29·4
34·5 30·8 0·89
that the gas exchange was not noticeably affected by the presence of potassium nitrate or ammonium sulphate in the buffer.
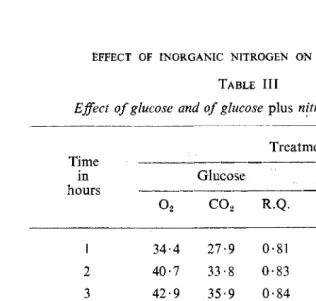
The results of a typical experiment on the effect of potassium nitrate on the respiration with glucose as substrate (exogenous respiration) are presented in Table III. These results show that when glucose was added to the roots, the oxygen uptake and carbon dioxide production increas\!d up to the fourth hour and remained constant until the end of the experiment. The R.Q. increased to nearly one. In the presence of nitrate the oxygen uptake and carbon dioxide production were much higher and the R. Q. increased to I · 13 to 1 · 14.
The results presented in Table IV show that the increase in respira-tion after addirespira-tion of glucose was more or less independent of the pre-sence of ammonium sulphate up to the fourth hour. After four hours, however, the roots provided with ammonium showed a definitely higher respiratory rate, rising to about 55% above that of the first hour while
. in
the absence of ammonium the increase was only 37%. Furthermore,IOFFECT OF INORGANIC NITROGEN ON RESPIRATION-I 143
TABLE III
Effect of glucose and of glucose plus nttrate on respiration
-~--·---·
Time
Treatment,s
-in Glucose Glhcose
+
Nitratehours
02 C02 R.Q. 02 C02 R.Q.
I 34·4 27·9 0·81 33·8 28·0 0·83
2 40·7 33·8 0·83 39·2 33·5 0·85
3 42·9 35·9 0·84 41·2 36·9 0·90
4 46·4 41 ·3 0·89 44·4 41·7 0·94
5 48·7 47·9 0·98 47·6 49·5 1·04
6 48·3 47·7 0·99 51·3 56·9 1·10
7 47·1 46·2 0·98 55·9 <'3·5 1 ·14
8 50·0 49·3 0·99 55·5 62·7 1 ·13
______ , _ , ___
TABLE IV
Effect of glucose and of glucose plus ammonium sulphate on respiration
Treatments
Time
---~---...---m Glucose Glucose
+
Ammonium-Nhours
-02 C02 R.Q. 02 C02 R:Q.
l- --- ----1J·2 29·2 0·88 32·4 28 ·8 0·89
2 44·5 40·1 0·90 42·5 38·3 0·90
3 46·9 41·7 0·89 47·6 41 ·9 0·8,8
4 48·2 45·8 0·95 49·2 41· 8 0·85
5 46·5 45·6 0·98 50·1 41·1 0·82
6 ' 49·0 47·5 0·97 54·3 43·4 0·80
7 48·4 48·1 1·00 52·1 41·2 0·79
8 48·2 47·2 0·98 52·5 41·5 0·79
: J
144 ANSE~M AUSTIN
different from those obtained with roots provided with ammonium only -without exogenous glucose supply (cf Table II) where no significant change was observed either in the rate of respiration or in the respiratory
quotient.
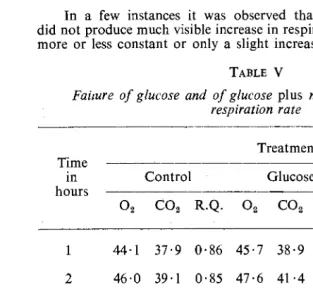
In a few instances it was observed that the addition of glucose did not produce much visible increase in respiration. The rate remained more or less constant or only a slight increase occurred (Table V). In
TABLE V
Faimre of glucose and of glucose plus nitrate to increase the respiration rate
Treatments Time
in Control Glucose Glucose
+
Nitratehours
02 C02 R.Q. 02 C02 R.Q. 02 C02
R.Q.
44·1 37·9 0·86 45·7 38·9 0·85 43·9 36·9 0·84
2 46·0 39 ·1 0·85 47·6 41·4 0·87 43·0 37·9 0·88
3 43·4 37·7 0·87 46·6 40·5 0·87 45·4 41·7 0·92
4 44·6 39·2 0·88 47 ·1 40·5 0·86 47·5 46·5 0·98
5 45·9 39·5 0·86 47 ·1 41·9 0·89 46·2 46·6 1·00
6 43·6 38·8 0·89 48·0 43·2 0·90 46·8 50·1 1·07
7 42·0 36·5 0·87 48·5 44·2 0·91 48·4 52·3 1·08
8 40·2 34·2 0·85 49·0 46·5 0·95 __41-.9._ _52·7 1·10
such cases the respiration of the control (i.e., endogenous respiration) was always falling in the long run so that only near the end of an experi-ment the effect of glucose became apparent on the rate of respiration. The absence of any effect during the first hours will be discussed later.
Potassium nitrite and hydroxylamine (cf Tables VI and VII res-pectively) considerably inhibited respiration and proved to be toxic at the applied concentration. No other concentration was tested. The inhibition due to potassium nitrite was stronger than that due to hydroxylamine.
---TABLE VI
Influence of nitrite on the exogenous respiration ~~----·--- --- ··--- - - - -·----~"
Treatments Time
in Glucose Glucose + Nitrite hours
02 C02 R.Q. 02 C02 R.Q.
28·8 20·9 0·73 27·1 19·8 0·73
- - -
-2 33·0 25·0 14·8 9·2
3 34·8 27·7 13·2 ll. 3
4 37·8 34·0 8·8 7·8
5 41·9 39·8 11 ·I 18·9
6 38·2 38·3 8·4 6·9
Total 185·7 164·8 0·89 56·3 54· I 0·96
TABLE VII
Effect of hydroxylamine on the exogenous respiration
--·-·---~"-Treatments
Time
-m Glucose Glucose+ Hydroxylamine hours
02 C02 R.Q. 02 C02 R.Q.
36·9 31·2 0·85 32·9 30·6 0·93
- - - -
-2 37·3 35·3 22·3 25·5
3 39·9 35·9 23·5 25·3
4 41 ·9 42·6 24·6 27·9
5 44·2 43·9 22·5 25· I
6 45·1 44·5 23·0 24·7
146 ANSELM AUSTIN
DISCUSSION
The increase in the rate of respiration of the roots after the supply of glucose indicates that the enzyme system responsible for carbohydrate utilization was not fully saturated with the endogenous substrate. In the case of a full saturation of the enzyme system with endogenous sub-strate, external application of respirable substrate will not produce any visible effect. This offers a likely explanation for the failure to observe any significant increase in respiration in a few experiments.
It is quite possible that the roots used in those experiments were not carbohydrate deficient during the first hours, so that the exogenous substrate pr<;>dvced an increase in respiration as compared with the control (no glucose) during the later hours only.
In the present investigation the endogenous respiration was not influenced by the presence of inorganic nitrogen compounds available to the roots. However in the presence of glucose both ammonium and nitrate nitrogen increased the rate of respiration. This is in accordance with the observations of several workers (Hamner, .1936; White and Templeman, 1937; McLean and Fisher, 1947; Willis, 1951; Burris and Wilson, 1952; Syrett, 1953; Yemm and Folkes, 1954). A general conclusion which can be made from the above-mentioned investigations is that the application of nitrates or ammonium salts to a tissue will be followed by an increase in the rate of respiration provided some carbon compounds are available in excess. Gilbert and Shive (1945) reported that soybean, oat and tomato roots grown in nitrate had a higher rate of carbon dioxide production than those grown in ammo-nium salts or without nitrogen, which is in line with the present results. The experiments with glucose supply· show that the influence exerted by nitrate and ammonium salts on the exogenous respiration is the same as far as the oxygen uptake is concerned. Nitrate, however, causes much higher carbon dioxide production than ammonium salts and consequently a proportional increase of the R.Q. This would indicate that nitrate was reduced by acting as a hydrogen acceptor in the respira-tion in competirespira-tion with the oxygen, in this way causing an extra de-carboxylation of intermediate metabolites of the respiratory cycle.
Nitrite and hydroxylamine are likely intermediates in nitrate reduc-tion, but both of them were found to be poorly assimilated. It may be that the particular concentration used in the present investigation was toxic. Should nitrite and hydroxylamine be formed in nitrate reduction, it may be that their production is so controlled that they never accumulate to any extent and hence may not reach the toxic level. The presence of these two substances in plants has been observed by several workers (Woods, 1938 ; Lascelles and Still, 1946; Eckerson, 1924; Anderson, 1924; Virtanen and Arhimo, 1939). Enzymes which reduce nitrate, nitrite and hydroxylamine have also been isolated from micro-organisms and higher plants ( cf Nason and Evans, 1953 ; Evans and Nason, 1953; Taniguchi et a!., 1953).
SUMMARY
The influence of nitrate and ammonium on the respiration of excised wheat roots was studied by means of the Warburg manometric technique using phosphate buffer (pH 5) as medium. The rate of the endogenous respiration and the value of the R.Q. were not influenced by either ammonium sulphate or potassium nitrate. The R.Q. of the endo-genous respiration was sl~ghtly lower than one (0 · 90). The addition of glucose was followed by a marked increase in the rate of oxygen uptake and by a gradual rise of the R.Q. to one. A further increase of the oxygen uptake was found when glucose was added in combina-tion with either nitrate or ammonium salt. When nitrite or hydroxyl-amine in concentrations equivalent to the nitrate was added to roots supplied with glucose, the oxygen uptake was considerably depressed. Only a slight increase of the R.Q. was observable. When nitrate was present the R.Q. rose to 1 ·I 3 whereas in the presence of ammonium sulphate it was 0 · 80 to 0 · 85. This rise of the R.Q. can be explained in terms of nitrate reduction. It appeared that an exogenous supply of carbon (i.e., glucose) was necessary to bring about a rise of the R.Q.
in the presence of nitrate.
REFERENCES
ANDERSON, V. L. (1924). Some observations on the nitrate-reducing properties of plants. Ann. Bot., 38, 699.
BURRIS, R. H. AND WILSON, P. W. (1952). Effect of hremoglobin and other nitro-genous compounds on the respiration of the Rhizobia. Biochem. J., 51, 90.
DAVIS, E. A. (1953). Nitrate reduction by Chlore!la. Plant Physiol., 28, 539.
ECKERSON, S. (1924). Protein synthesis by plants. I. Nitrr.tc reduction. Bot. Gaz., 77, 377.
EvANS, H. J. AND NASON, A. (1953). Pyridine nucleotide-nitrate reduct~se from extracts of higher plants. Plant Physiol., 28, 233.
GILBERT, S. G. AND SHIVE, G. W. (1945). The importance of oxygen in tr.c nutrient substrate for plants. Relation of the nitrat·~ ion to respiration. Soil Sci., 59, 453.
HAMNER, K. C. (1936). Effects of nitrogen supply on rates of photcsyntl".esis and respiration in plants. Bot. Gaz., 97, 744.
LASCELLES, J. AND STILL, J. L. (1946). The reduction of nitrate, nitrite ard r.ydroxyl-amine by E. coli. Aust. J. Exp. Bioi. med. Sci., 24, 159.
McLEAN, D. J. AND FISHER, K. C. (1947). The relation between oxygen cor.sumption and the utilization of ammonia for growth in Serratia marcescens. J. Bact., 54, 599.
NASON, A. AND EVANS, H. J. (1953). Triphosphopyridine nuckotide-r.itrate recuc-tase in Neurospora. J. Bioi. Chem., 202, 655.
SYRETT P. J. (1953 a). The assimilation of ammonia by nitrogen-starved cells of Chiarella vulgaris. Part l. The correlation of assimilation with respiration. Ann. Bot., N.S., 17, l.
_ _ _ (1953 b). The assimilation o~ :;tmi?onia by nitr?gen-starved cells of Chiarella vlilgmis. Part 11. The assimilatiOn of ammoma to other compounds,
148 ANSELM AUSTIN
fANIGUCAI, S., MITSU, H., ToYODA, J., YAMADA, T. AND EoAMI, F. (1953). The successive reduction from nitrate to ammonia by cell-free bacterial enzyme sys-tems. J. Biochem. (Japan), 40, 175.
VIRTANEN, A. I. AND ARHIMO, A. A. (1939). Formation of amino-acids in green plants with nitrate as nitrogen source. Suomen Kemistilehtil, 12 B, 24.
WARBURG, 0. AND NEGELEIN, E. (1920). Ober die Reduktion der Salpetersaure in griinen Zellen. Biochem. Z., 110, 66.
WHITE, H. L. AND TEMPLEMAN, W. G. (1937). The interaction of factors in the growth of Lemna. X. The interaction of nitrogen and light intensity in rela-tion to respirarela-tion. Ann. Bot., N.S., 1, 191.
WILLIS, A. J. (1951). Synthesis of amfno-acids in young roots of barley. Biochem. J., 49, (Proceedings of the Biochemical Society), xxvii-xxviii.
WooDs, D. D. (1938). The reduction of nitrate to ammonia by Clostridium we/chii. Ibid., 32, 2000.