Protective Effect of Losartan in Reducing Microalbuminuria in Type 2 Diabetic Patients with Essential Hypertension
Fadhil Abas Al-Hammami1, Mohammed Najim Abed2, Waleed Zaki Khalil3
1
Department of Pharmacology, College of Medicine, University of Mosul
2Department of Pharmacology, College of Pharmacy, University of Mosul 3Al-Khansaa Teaching Hospital, Ninevah Directorate of Health
ABSTRACT
Background: Patients with type 2 diabetes mellitus have high incidence of developing nephropathy and cardivascular disease, and the presence of hypertension with diabetes mellitus may increase this incidence.
Aim: The aim of the present study was to evaluate the renoprotective and cardiovascular protective effects of losartan in hypertensive patients with type 2 diabetes mellitus.
Patients and Methods: The study was conducted in Al-Wafaa center for diabetes and Endocrinology in Mosul. Fifty three type 2 diabetic patients treated by glibenclamide are newly diagnosed as having hypertension placed on losartan therapy 50mg/day for six weeks and compared with apparently 50 healthy volunteers as control group. Blood pressure and microalbuminuria in hypertensive patients with type 2 diabetes mellitus were measured before and after treatment with losartan and compared with the blood pressure and microalbuminuria of control group by using Z-test.
Results: In the treated group with losartan, blood pressure, urine protein and protein/creatinin ratio were significantly reduced as compared with that before treatment.
Conclusions: Using of losartan is associated with significant reduction in blood pressure (systolic and diastolic), and protein/cretinin ratio (microalbuminurea), however urin creatinin did not changed and this effect is beneficial in protection of patients from renal and cardiovascular complications.
Key Words: diabetic nephropathy, losartan, microalbumiuria
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is increasing in prevalence world wide and is currently estimated to affect more than 6.5% of the populations of the United States (1). Approximately one third of patients with T2DM will eventually develop diabetic nephropathy (DN), which is one of the leading causes of chronic kidney disease. Therefore, the early identification and subsequent renoprotective treatment of all patients at risk is very important. The first sign of renal involvement in DN is microalbuminuria i.e 24 hour urinary albumin of 30-300mg/d1 or urinary microalbumin excretion rate 20-300 mg/min, which can't be detected on routine methods of protein estimation (2).
Microalbuminuria is not only a sign of the progression of renal impairment, but is also an independent risk factor of cardiovascular events (3). Moreover, it is frequently rose as the severity of hypertension increased (4). Therefore, it is recommended that screening for microalbuminuria be done
Strict glycemic and blood pressure control by the inhibition of the renin-angiotensin system (RAS) in the microalbuminuria stage has been suggested in order to inhibit the transition to nephropathy and to normalize microalbuminuria (6).
Therapeutic measures that reduce proteinuria 30% below the baseline value was associated with better preservation of renal function (7). These measurements include achieving a target blood pressure of less than 130/80 mmHg and use of various pharmacological agents like angiotensin receptor blockers (ARBs) and non hydropyridine calcium channel blocker, which have been shown to be successful in reducing albuminuria (8). Recently trial have shown that ARBs were superior in reducing DN as compared with angiotensin converting enzyme inhibitors (ACEIs) by specifically inhibiting angiotensin II-intra-glomerualr pressure with less bradykinin mediated side effect (9).
Previous studies, showed that losartan and irbesartan, respectively, reduce proteinuria and slow the progression of DN in hypertensive patients with T2DM (10,11). Therefore, therapy that interferes with rennin-angiotensin aldosteron system should be initiated when microalbumin uria develops in order to reduce albumin excretion and associated risks for progression to overt nephropaty.
The purpose of this study was to evaluate the renoprotective and cardioprotective effects of losartan in hypertensive patients with T2DM.
PATIENTS AND METHODS
The study was an open, 6-weeks trial conducted to measure the level of microalbuminuria and to evaluate the protective effect of losartan on the microalbuminuria in hypertensive type 2 diabtic patients who were collected from AL-Wafa'a Center for Diabetes Management and Researches in Mosul. The study protocol was approved by the local research ethics committees of the University of Mosul and Ninevah Directorate of Health.
Fifty three newly diagnosed hypertensive patients with T2DM participated in the study, 25 male and 28 female, their mean age were 56.5 8.2 years, the participants were placed on oral losartan (Angizaar) 50 mg as a single daily dose. Another fifty apparently healthy individuals, sex and age matched with the patients, were participated in the study as a control group. They were 23 male and 27 female, and their mean age were 53.3 9.4 years.
Inclusion Criteria
Type 2 diabetic patients with duration of diabetes not less than one year on glibentclamide (5 mg tablet) therapy, these patients are newly diagnosed hypertensive having blood pressure 140/90 mmHg.
Exclusion Criteria
Patients with type 2 diabetes mellitus and having hepatic or renal diseases, myocardial infarction, heart failure or sever hypertension. Pregnant and lactating women were also excluded from the study. Blood pressure was measured at baseline (before drug administration) by standard mercury sphygmomanometer and at the end of 6 weeks-treatment period with losartan. Goal blood pressure after treatment was less than 140/90 mmHg. Antihypertensive efficacy was assessed by finding the difference between blood pressure before and after drug therapy and by determination of the number of patients achieved normal blood pressure after therapy.
Protein was assessed by using protein/creatinin ration (PCR) which is convenient method for estimation of proteinuria (12). The determination of concentration of protein in urine was done by dipstick test.
Creatinin concentration in urine was detected by using a kit (SyrBio-Company/Syria) before and after drug administration.
All values were quoted as the mean SD. Paired Z-test was used to compare the measured parameters for urine between before and after drug therapy. Unpaired Z-test was used to compare between cases before and after drug therapy with control. Level significance was considered significant at p=< 0.05.
RESULTS
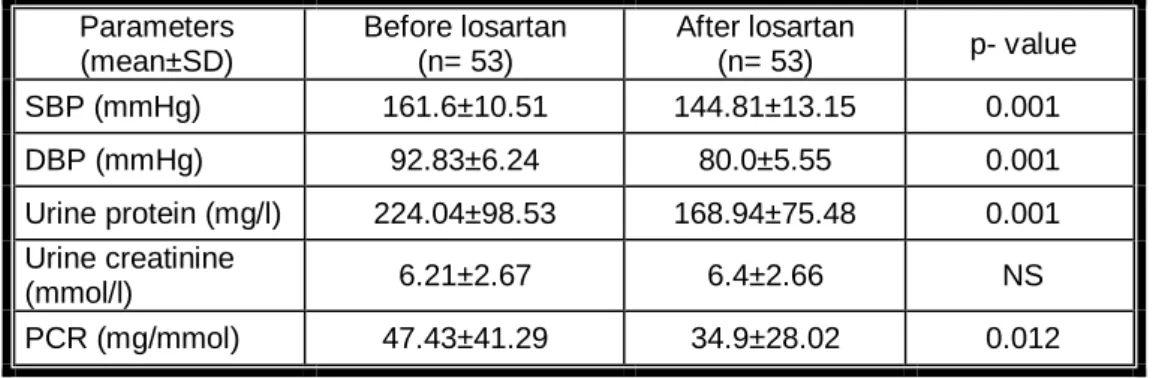
Table 1 shows comparison between patients before and after treatment with losartan reflecting a significant reduction in blood pressure (p = 0.001). The same was shown for urine protein (p = 0.001).Regarding Urine creatinin, non-significant elevation was detected, when statistical comparison between patients before and after treatment with losartan. While PCR, reported a significant reduction (p= 0.012).
Table (1): Study parameters values before and after losartan (50mg) treatment Parameters (mean±SD) Before losartan (n= 53) After losartan (n= 53) p- value SBP (mmHg) 161.6±10.51 144.81±13.15 0.001 DBP (mmHg) 92.83±6.24 80.0±5.55 0.001 Urine protein (mg/l) 224.04±98.53 168.94±75.48 0.001 Urine creatinine (mmol/l) 6.21±2.67 6.4±2.66 NS PCR (mg/mmol) 47.43±41.29 34.9±28.02 0.012
Each value represents mean±SD.
Table 2 demonstrates comparison of blood pressure among patients before and after treatment and control. A significant increase is shown (p=0.001). A significant reduction in urine protein (p=0.001) is depicted, too. Urine creatinin reflected non-significant differences between patients and control before and after treatment (p=0.05). PCR, exhibited a significant increase in comparison between patients before and after treatment and control (p=0.001).
Table (2): Study parameters of patients before and after treatment with losartan (50mg) and control Parameters (mean±SD) Control (n= 50) Before losartan (n= 53) After losartan (n= 53) SBP (mmHg) 126±7.14 161.6±10.51*** 144.81±13.15*** DBP (mmHg) 77.1±6.93 92.83±6.24*** 80.0±5.55* Urine protein (mg/l) 55.32±21.94 224.04±98.53*** 168.94±75.48*** Urine creatinine (mmol/l) 6.85±1.99 6.21±2.67 6.4±2.66 PCR (mg/mmol) 8.12±2.36 47.43±41.29*** 34.9±28.02***
DISCUSSION
The present study has demonstrated that treatment with losartan significantly reduce the rate of progression to clinical albuminuria, the hallmark of overt DN in patients with type 2 diabetes, also the data obtained from the present study showed that antihypertensive treatment has a renoprotective effect in hypertensive patients with T2DM and microalbuminuria, and this is in agreement with many previous studies (13-16).
Diabetic nephropathy (DN) is the single largest cause of end stage renal disease worldwide and account for 20 to 40% of new cases (17). Microalbuminuria is a hallmark of DN (18), and studies have shown that reduction of this parameter can improve cardiovascular and renal outcomes, not only in diabetic but also in non-diabetic patients (19,20).
In this study losartan was chosen to treat patients with hypertensive T2DM, because it has better efficacy in reducing the composite cardiovascular end points and stroke (21).
A comparative study between losartan and captopril, showed that patients randomized to losartan had a 46 % reduction in all cases of mortality in comparison to captopril treated patients which has primary due to decrease in sudden cardiac death in losartan group (22). Dovovisik et al. (23) compared between losartan and metoprolol, and showed that a significant reduction in blood pressure versus baseline after 6 weeks therapy with the two drugs but with a lower incidence of adverse effects in losartan treated group. The effect of ACEIs on renal hemodynamics could be limited by the non ACE dependent generation on Angiotensin II that has been documented in patients in the hyperglycemic state (24).
American Diabetes Association (ADA) in 2002 recommended Angiotensin II receptor antagonists as first choice for the management of type 2 diabetic hypertensive patients with myocardial infarction or clinical nephropathy (25). Brenner et al. (26) compared losartan with placebo in 1513 hypertensive type 2 diabetic patients and showed that blood pressure in losartan group decreased from 152/82 mmHg to 140/74 mmHg. Also a comparative study between losartan and enalapril on 92 hypertensive type 2 diabetic patients showed that losartan significantly decrease blood pressure, and this reduction is equal to that done by enalapril (27).
All of the above studies indicated that losartan is an effective and safe antihypertensive drug and these results are in consistent with the results obtained from the present work.
Losartan has a role in renal protection and reduction of proteinuria, which is an independent risk factor for the progression of renal disease and its reduction, is an important therapeutic goal in T2DM patients (28). The results of the present study conferred beneficial renal protective effect of losartan, and cause a significant reduction of proteinuria (measured as PCR) occurred after using 50 mg losartan single daily dose for 6 weeks.
The cause of reduction in proteinuria may be due to blocking the effect of Angiotensin II by losartan, Angiotensin II is believed to play a role in the progression of renal damage not only through hemodynamic effect, but also through the stimulation of growth factors and cytokines and alteration in extracellular matrix metabolism (29). Angiotensin II also increases intraglomerular pressure and alter the filtration properties of the glomerular basement membrane leading to proteinuria (30). Angiotensin II produces glomerular sclerosis via stimulation of transforming growth factor B (TGF-B), endothelial and vascular matrix, resulting in increased urinary albumin excretion rate as well as with increased risk of cardiovascular diseases in type 2 diabetic patients (29,31). The reduction of the urinary albumin excretion (UAE) may reflect recovery of endothelial function, leading to a reduction in the risk of the complications (31).
The results of the present study were in agreement with the results obtained from other studies. Brenner et al.(26) reported that losartan was well tolerated and conferred renal beneficial effects in type 2 diabetic patients and led to an average reduction in the level of PCR by 35% and also it reduce the risk of end stage renal disease by 28% when compared with placebo. Delik et al. (32) reported that
significant reduction in proteinuria after one month's therapy. Steen et al. (33) studied the effect of losartan on PCR and concluded that it caused a significant reduction by 20% from baseline levels. All of the above studies indicated that losartan has a valuable renal protective effect by reducing proteinuria and this reduction can reduce the risk of progressive renal disease, which is in agreement with the present study.
The present study concluded that losartan therapy in hypertensive type 2 diabetic patients were associated with significant reduction in microalbuminuria. This suggesting a possible renoprotective effect in addition to its blood pressure lowering effect, thus losartan may be regarded as the drug of choice in the treatment of hypertensive diabetic patients.
REFERENCES
1. Mokdad AH, Ford ES, Bowman BA. Diabetes trends in the U.S., Diabet Care 2000; 23:1278-83.
2. Remmuzz G, Schipath A, Rugenetiti P. Nephropathy in patients with type 2 diabetes mellitus. NEMJ 2000;346:1145-1151.
3. Hillege HL, Fidler V, Diercks GF, Van Gilst WH, Dee Zeeuw D, Van Veldhuisen DJ, et al,. For the prevention of renal and vascular end stage disease (PREVEND) study group: urinary albumin excretion predicts cardiovascular and non cardiovascular mortality in the general population. Circulation 2002;106:1777-82.
4. Agrawi B, Berger A, Wolf K, Luis F. Microalbuminuria screening by reagent strip predicts cardiovascular risk in hypertension. Hypertension 1999;14:223-228.
5. American Diabetes Association (ADA). Diabetic nephropathy. Diabetes Care 2004; 27 (Suppl1):S69-S83.
6. Gaede P, Tarnow L, Vedel P, Parving HH, Pedersen O. Remission to normoalbuminuria during multifactorial treatment preserve kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant 2004;9(11):2784-8.
7. Onuigbo M, Weir MR. Evidence- based treatment of hypertension in patients with type 2 diabetes mellitus, Diabetes 2003;5:13-26.
8. Ruggenenti P, Fossi A, Ilieva AP, Bergamo. Nephrologic diabetes complications trial (BENEDICT) investigation preventing microalbuminuria in type 2 diabetes. NEMJ 2004;351:1941-51.
9. Parvanova A, Chiurchiu C, Ruggenenti P, Remuzzi G. Inhibition of the renin- angiotensin system and cardio-renal protection: focus on losartan and angiotensin receptor blockade. Exper Opin Pharmaco Ther 2005;6:1931-42.
10. Lewis EJ, Hunsicker LG, Clarke WR, Bert T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin receptor blocker irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345: 851-860.
11. Brenner BM,S Cooper ME, de Zeuw D, Keane WF, Mitch WE, Parvings HH, et al. Effects of Losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy . N Engl J Med 2001; 345:861-869.
12. Shastri NJ, Shendrmkar N, Nayak U, Kotecha PV. Quantification of protienuria by urine protein/creatinine ratio. Indian Pediatr 1994;31:334-337.
13. Gaede P, Vedel P, Parving HH and Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbumiuria. Lancet 1999; 353:617-622.
14. Heart outcomes prevention evaluation (HOPE) study investigation. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus. Lancet 2000;355:253-9. 15. Lacourciere Y, Nadeau A, Poirier L, Tancrede G. Captopril conventional therapy in hypertensive type 2 diabetes. Hypertension 1993;21:786-94.
16. Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000; 23(Suppl 2) :B54-B64.
17. National institutes of health: Excerpts from United States renal data system's 2000 annual data report: atlas of end stage renal disease in the United States: economic costs of ESRD. Am J Kidney Dis 2000;36:163-76.
18. Rossing P. Diabetic nephropathy: worldwide epidemic and effects of current treatment on natural history. Curr Diabetes Rep 2006;6:479-83.
19. Basi S, Lewis JB. Microalbuminuria as a target to improve cardiovascular and renal outcomes. Am J Kidney Dis 2006;47:927-46.
20. Jensen JS. Microalbuminuria is a strong determinant for ischemic heart disease also among non diabetics. Ugeskr Laegr 2007;169:485-7.
21. Dahlof B, Devereux RB, Kjcldsen SE, Julius S, Beevers G, DeFaire U, et al. Cardiovascular morbidity and mortality in the losartan interventions for end point reduction in hypertension (LIFE) study. Lancet 2002; 359: 995-1003.
22. Pitt B, Segal R, Martinez FA. Randomized trial of losartan versus captopril in patients over 65 with heart failure. Lancet 1997; 349:747-752.
23. Dobovisek J, Kanic V, Niegowska J, Rekic S, Soucek M, Spinar J. Efficacy and tolerability of losartan in patients with essential hypertension. J Clin Bas Cardi2005;8 (1-4): 43-46.
24. Osei SY, Price DA, Laffel LMB, Lansemg MC, Hollenberg NK. Effect of angiotensin II antagonist eprosartan on hyperglycemia induced activation of the internal renin angiotensin system in healthy humans. Hypertension 2000;36:122-126.
25. American Diabetes Association (ADA). Standards of medical care for patients with diabetes mellitus. Diabetes Care, 2002; 25: 213-229.
26. Brenner BM,S Cooper ME, De Zeuw D, Keane WF, Mitch WE, Parvings HH, et al. Effects of Losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy . N Engl J Med 2001; 345:861-869.
27. Lacourcier Y, Belanger A, Godin C, Halle J, Ross S, Wright N, et al. Long term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney Inter 2000;58(2):762-769.
28. Shahanz Sh, Tania Z, Tultul A, Zhonxin Z, Denise R, Ronald D, et al. Losartan in patients with type 2 diabetes and proteinuria. Kidney Inter 2002;62:64-67.
29. Leehey DJ, Singh AK, Alvin N, Singh R. Role of angiotensin II in diabetic nephropathy. Kidney Inter 2000;77: 93-98.
30. Esmatjes E, Flores L, Inigo P, Lario S, Ruilope LM, Campistol JM. Effect of losartan on TGF-alpha 1 and urinary albumin excretion in patients with type 2 diabetes mellius and microalbuminuria. Nephrol Dial Transplant 2001;16 (1): 90-93.
31. Rodrigo E, Maeso R, Munoz-Garcia R, Navarro-cid J, Ruilope L, Cachofeiro V. Endothelial dysfunction in spontaneously hypertensive rats. Hypertention 1997;15: 613-618.
32. Dilek K, Usta M, Ersoy A, Ozdemir B, Yavuz M, Gullulu M, et al. Long term effects of losartan on proteinuria and renal function in patients with renal amyloidosis. Scand J Urol and Nephrol 2002; 36 (6): 443-446.
33. Steen G Terra. Angiotensin receptor blockers. American heart association . Circulation 2003;107: e215-e216.