PRIMARY RESEARCH
Circ-DENND4C up-regulates TCF4 expression
to modulate hepatocellular carcinoma cell
proliferation and apoptosis via activating Wnt/
β-catenin signal pathway
Xialei Liu
1†, Lewei Yang
2†, Dong Jiang
3, Wuzhu Lu
4and Yongyu Zhang
5*Abstract
Background: Hepatocellular carcinoma (HCC) is a common malignant tumor in China. Advanced treatment like transcatheter hepatic arterial chemoembolization (TACE) has prolonged the lives of many HCC patients. However, the prognosis of most HCC patients remains unsatisfactory. Recently, circular RNAs (circRNAs) have been gradually unveiled to exert considerable functions in cancer. Promising circRNAs in HCC remains to be further elucidated.
Methods: Gene expression was assessed by qRT-PCR and western blot. The function of circ-DENND4C in HCC was estimated by both in vitro and in vivo experiments. The location of circ-DENND4C in HCC cells was determined by subcellular fractionation and FISH assays. The association among molecules were analyzed through RNA pull down, RIP and luciferase reporter assays.
Results: circ-DENND4C (DENN domain containing 4C), an oncogene identified in breast cancer, was overexpressed in HCC cells. Also, circ-DENND4C exerted pro-tumor functions in HCC through activating Wnt/β-catenin pathway. Importantly, circ-DENND4C could augment transcription factor 4 (TCF4) expression to activate Wnt/β-catenin signal-ing via sequestersignal-ing miR-195-5p. Moreover, followsignal-ing rescue assays disclosed that circ-DENND4C mediated malignant phenotypes in HCC cells via up-regulating TCF4 through sponging miR-195-5p.
Conclusion: circ-DENND4C boosted TCF4 expression to modulate malignant behaviors of HCC cells via activating Wnt/β-catenin pathway, which might offer a promising target for HCC treatment.
Keywords: Circ-DENND4C, Hepatocellular carcinoma, ceRNA, Wnt/β-catenin pathway
© The Author(s) 2020. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creat iveco mmons .org/licen ses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creat iveco mmons .org/publi cdoma in/ zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Background
Hepatocellular carcinoma (HCC) is a common pri-mary cancer around the word, which ranks the 6th in
cancer morbidity and the 3rd in cancer mortality [1,
2]. In recent years, advanced traditional treatments
like transcatheter hepatic arterial chemoembolization (TACE) and the application of chemotherapy drug like paclitaxel [3] and doxorubicin [4], have extended HCC patients’ lives to a certain degree. However, the progno-sis of most HCC cases remains disappointing with the 5-year survival rate of approximately 7% [5]. With the development of science and technology, targeted ther-apy has been an option for HCC treatment. Hence, it is urgent to figure out possible targets for HCC.
It is discovered that many factors and mechanisms can affect the development of cancer. As reported, c-Fos
Open Access
*Correspondence: zyybo@126.com
†Xialei Liu and Lewei Yang are co-first authors
5 Department of Interventional Radiology, The Fifth Affiliated Hospital
of Sun Yat-sen University, No. 52, Meihuadong Road, Xiangzhou District, Zhuhai City, Guangdong Province 519000, China
promotes cell stemness in head and neck squamous cell carcinoma [6]. In recent years, circular RNAs (circR-NAs), a class of non-coding RNAs, are reported as new regulators that participate in diverse biological functions in eukaryotic organisms [7]. Recently, increasing studies manifested that circRNAs could regulate cancer develop-ment through a competing endogenous RNA (ceRNA) network via sponging certain microRNAs (miRNAs) [8,
9]. MiRNAs are another type of non-coding RNAs that also play important regulatory roles in cancer [10, 11]. For example, miR-24-3p restrains cell cycle and inva-sion in pancreatic ductal adenocarcinoma by targeting LAMB3 [12]. MiR-203 is upregulated in breast cancer and regulates cell growth and stemness via targeting SOCS3 [10]. Previously, a circRNA derived from DENN domain containing 4C (circ-DENND4C) has been newly recognized [13]. As a hypoxia-associated RNA, circ-DENND4C exhibited a high level and promoted cell pro-liferation in breast cancer [14]. It was also reported that circ-DENND4C was involved in the regulation of blood-tumor barrier in glioma [13]. However, the function and mechanism of circ-DENND4C in HCC remain largely unknown.
Our study investigated the role and molecular mecha-nism of DENND4C in HCC. It was found that circ-DENND4C was up-regulated in HCC cells and activated Wnt pathway to aggravate cell growth, stemness and invasion in HCC by acting as a ceRNA. These findings might be helpful to develop novel therapeutic strategies for HCC patients.
Materials
Cell culture
HCCLM3, Huh7, HepG2 and Hep3B were purchased as the human liver cancer cell lines, and THLE-3 served as a normal liver cell line. All cell lines were obtained from Cell Biology of the Chinese Academy of Sciences (Shang-hai, China). All above cells were cultured in DMEM medium (Invitrogen, Carlsbad, CA) containing 100 U/ml penicillin, 10% fetal bovine serum (FBS) and 100 mg/ml streptomycin (all, Invitrogen) at 37°C with 5% CO2.
Cell transfection
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used to transfect cells with indicated plasmids. Cells were put in the six-well plate (2 × 105 cells/well) for 24-hour
cultivation. Lipofectamine RNAiMAX reagent (Invitro-gen) was utilized for shRNA transfection in line with the protocols of suppliers. HepG2 and Huh7 cells were sub-jected to transfection with 2 μg of shRNA for two days. qRT-PCR was used to estimate transfection efficiency.
RNA extraction and quantitative real‑time PCR (qRT‑PCR)
Based on protocols of suppliers, TRIzol method (Invit-rogen) was utilized to separate the total RNA. Briefly, cells were supplemented with 1 ml of TRIzol for dis-solution and cultivation for five minutes. Then, cells were subjected to centrifugation for separating after they were mixed forcefully with chloroform. After that, isopropanol (1:1) was used to precipitate the superna-tant and 75% ethanol was used to wash them. In the end, RNase-free H2O was applied to dissolve the RNA
pellet. The RT-PCR kit (Promega, Madison, WI) was adopted to produce complementary DNA from RNA. In accordance with the standard procedures, real-time PCR reaction was carried out.
Colony‑formation assay
Six well plates (800 cells/well) were used to cultivate cells in the drippy incubator at 37 °C. The process lasted for 14 days. After fixing cells with 4% formalde-hyde, 0.5% Crystal Violet (Sigma-Aldrich, Miamisburg, OH) was adopted to stain the colonies. Finally, colonies with over 50 cells were calculated manually.
Transwell invasion assay
For transwell invasion, the top chamber coated with Matrigel (BD Biosciences, Franklin Lakes, NJ) was adopted to cultivate the cells (3 × 105 cells/well) in
serum-free medium. The medium which included 10% FBS was put into the lower chamber. After 24 h, a cot-ton swab was used to eliminate the cells which did not invade into the lower chamber. Thereafter, cells crossed the membrane were fixated by 4% formaldehyde and dyed with crystal violet. Finally, Nikon EclipseTi micro-scope (Olympus) was applied to obtain the images.
Cell cycle assay
After transfection, cells were cultured in 6-well plates (3 × 105 cells/well) and collected after 10 min of
cen-trifugation. Afterwards, cells were rinsed in PBS and then subjected to propidium iodide (PI) dying in the dark. Then, cell proportions in G0/G1, S, and G2/M phases were examined by flow cytometry (BD Bio-sciences) as guided.
TUNEL assay
One Step TUNEL Apoptosis Assay Kit (Beyotime, Jiangsu, China) was used to estimate cell apoptosis. In short, cells in 6-well plates (1 × 105 cells/well) were
(Beyotime) staining. By fluorescent microscope (Olym-pus), apoptotic cells was imaged.
Fluorescence In Situ Hybridization (FISH)
In order to test the localization of circ-DENND4C, RNAscope Multiplex Fluorescent Reagent Kit v2 (Advanced Cell Diagnostics, Inc., Newark, NJ, USA) was utilized to conduct FISH assay. Briefly, 4% paraformalde-hyde was adopted to fix cells (1 × 104) for half an hour.
After digested by protease III prior, cells were subjected to take hybridization by the specific probes of target RNA at 40 °C for two hours. TSA plus Cyanine3, HRP-labeled oligos, amplifier and preamplifier were subjected to the hybridization by turn at 40 °C. Hoechst was adopted to stain the nuclei. Images were obtained by Zeiss LSM 710 confocal microscope (Jena, Germany).
Immunofluorescence assay
The pre-cooled PBS was used to rinse the cells in 6-well plates (2 × 104 cells/well) for 3 times and 4%
paraformal-dehyde was employed to fix the cells deposited in plates of 48-well. After that, cells were subjected to permeabi-lization by 0.5% Triton X-100 at 37°C for ten minutes. Then, 3% BSA was added for blockading the nonspecific binding. With Cy3-conjugated goat anti-mouse IgG (Mil-lipore) as the control, cells were stained with β-catenin antibody to obtain the location of β-catenin. For observ-ing Ki67 expression, Ki67 cell proliferation Detection Kit (Beyotime) was adopted to culture cells at least one hour. Afterwards, PBS was adopted to rinse the cells for 3 times and DAPI was taken to stain them at 37 °C for 15 min. With the help of a Nikon Eclipse Ti Fluorescence Microscopy (Olympus), the images were obtained.
Subcellular fractionation assay
PARIS™ Kit (Ambion, Austin, TX) was used to conduct
subcellular fractionation assay in 1 × 104 cells. Firstly, cell
fractionation buffer was used to resuspend the collected cells. Then, cells were placed on ice for ten minutes. After centrifugation, the cell disruption buffer was utilized to conserve the nuclear pellet and supernatant for the extraction of RNA.
RNA immunoprecipitation
Magna RIP™ RNA-Binding Protein Immunoprecipitation
Kit (Millipore, Billerica, MA) was adopted for conduct-ing the RIP assays in 1 × 107 cells. Ago2 antibody was
utilized to conduct the RIP assay and qRT-PCR was uti-lized to detect the co-precipitated RNA. IgG was seen as a negative control.
RNA pull down assay
For pull down assays, Biotin RNA Labeling Mix (Roche, Basel, Switzerland) and T7 RNA polymerase were utilized to take the in vitro transcription for getting circ-DENND4C sequence to obtain biotin-labelled circ-DENND4C, and sequence without biotin labelling acted as the negative control. Then, cell lysates from 1
× 107 cells were subjected to circ-DENND4C sequences
labelled with or without biotin for four hours at room temperature (RT). After that, streptavidin-conjugated agarose beads were added and the mixtures were fur-ther incubated for 4 h. Then, the pulled down RNAs were detected by qRT-PCR.
Western blotting
Cells were crushed by radioimmune precipitation assay buffer (Beyotime) and then taken to SDS-PAGE. Then, cells were transferred by Nitrocellulose membrane (Bey-otime Biotechnology) and cultivated by the primary anti-bodies against Cyclin D1, CDK4, β-catenin, Bcl-2, Bax and loading control GAPDH. After that, the secondary antibodies conjugated with horseradish peroxidase were adopted for visualizing. All antibodies were procured from Abcam (Cambridge, MA).
Luciferase reporter assay
Cells (1.5 × 105) were seeded in 96-well plates and the
consistence of each well was 5000 cells. At 24-hour culti-vation, the mixture was formed firefly luciferase reporter (Promega, Madison, WI), pRL-CMV Renilla luciferase reporter (Promega) and small RNA was adopted to transfect the cells transiently. 48 h later, dual-luciferase reporter assay system (Promega) was employed to meas-ure the luciferase activity.
In vivo tumor formation assay
The male nude mice, aged about 6-week old, were pro-cured from the National Laboratory Animal Center (Bei-jing, China) and housed under SPF-condition, with the approval from the Animal Research Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University. 5
× 106 transfected cells were subcutaneously injected into
mice for 28-day of tumor formation purposes. Tumor volume was monitored every 4 days. The mice were killed prior to excising tumors, and then tumor samples were used for weight assessment and further analysis.
Immunohistochemistry (IHC)
consecutive sections of 4 μm thick were used for IHC assay using the specific primary antibodies and second-ary antibodies (Abcam).
Statistical analysis
Mean ± standard deviation (SD) was used for data pres-entation. Each experiment was repeated at least three times. The results were estimated by Student’s t-test and one-way ANOVA using GraphPad PRISM 6 (GraphPad, San Diego, CA). P < 0.05 was supposed to be significant.
Results
Circ‑DENND4C regulated HCC cell proliferation, apoptosis, invasion and stemness
We initially detected circ-DENND4C expression in HCC cell lines (HCCLM3, HepG2, Huh7 and Hep3B) and nor-mal human liver epithelial cell line (THLE-3). As mani-fested in Fig. 1a, the expression of circ-DENND4C was notably higher in HCC cells than that in THLE-3 cells. HepG2 and Huh7 cells were used to conduct following assays for that they exhibited highest expression of circ-DENND4C. Then, we started to verify the circular char-acteristic of circ-DENND4C. Convergent primers were designed to amplify DENND4C while divergent primers were designed to amplify circ-DENND4C. cDNA and gDNA were applied as the templates. Gel electrophoresis revealed that circ-DENND4C was amplified by divergent primers in only cDNA while DENND4C was amplified by convergent primers in both cDNA and gDNA (Fig. 1b). Next, we treated HepG2 and Huh7 cells with actinomy-cin D (ActD). The results disclosed that circ-DENND4C was not impacted while the level of linear DENND4C was obviously decreased under ActD treatment (Fig. 1c). Above results verified the abnormal expression and cir-cular characteristic of circ-DENND4C in HCC. Thus, we continued to explore the function of circ-DENND4C in HCC. Before functional assays, knockdown efficiency of circ-DENND4C was verified (Fig. 1d). Then, we disclosed that circ-DENND4C knockdown decreased the prolifera-tion of HCC cells (Fig. 1e, f, Additional file 1: Figure S1A). Further, flow cytometry analysis showed that silenced circ-DENND4C induced cell cycle arrest (Fig. 1g). In TUNEL assay, circ-DENND4C depletion elevated the
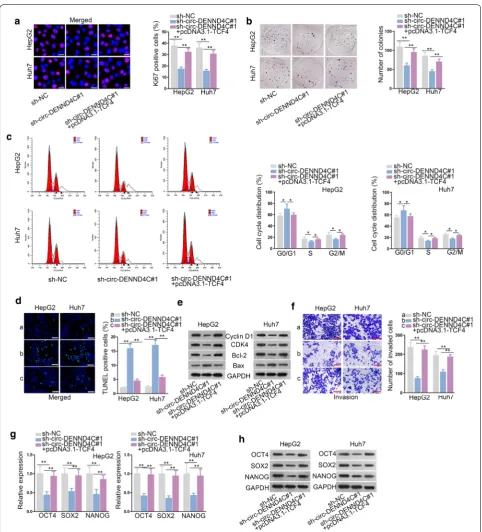
apoptosis rates of HCC cells (Fig. 1h, Additional file 1: Figure S1B). Later, the levels of cell cycle-related teins (Cyclin D1, CDK4) and apoptosis-associated pro-teins (Bcl-2, Bax) were tested in sh-circ-DENND4C#1/2 transfected cells. The results demonstrated that circ-DENND4C deficiency decreased the levels of Cyclin D1, CDK4 and Bcl-2 while increased that of Bax (Fig. 1i). Besides, transwell assay indicated the weakened HCC cell invasive ability in response to circ-DENND4C deple-tion (Fig. 1j). It was well known that tumor formation was associated with high differentiation of stem cells. Thus, the mRNA and protein levels of OCT4, SOX2 and NANOG (biomarkers of stemness) were examined by qRT-PCR and western blot. Results implied that OCT4, SOX2 and NANOG levels were all decreased in HCC cells transfected with sh-circ-DENND4C#1/2 (Fig. 1k, l). All data suggested that circ-DENND4C facilitated HCC cell proliferation, cell cycle, stemness, invasion and repressed HCC cell apoptosis.
Activation of Wnt/β‑catenin signaling pathway rescued circ‑DENND4C depletion‑mediated effects on HCC cells
Then, we sought the potential downstream pathways of circ-DENND4C in HCC. Activators of Hedgehog path-way, NOTCH pathpath-way, Wnt/β-catenin pathway and PI3K/AKT pathway were used. The result revealed that only LiCl (activator of Wnt/β-catenin pathway) could rescue the suppressive effect of circ-DENND4C knock-down on HCC cell proliferation (Fig. 2a). Then, we detected levels of Wnt/β-catenin pathway associated pro-teins upon circ-DENND4C knockdown. It was disclosed in western blot analysis that protein levels of p-GSK3β, c-Myc, β-catenin and MMP7 were all reduced in cells transfected with sh-circ-DENND4C#1/2 (Fig. 2b). Later, western blot analysis and Immunofluorescence assay confirmed that circ-DENND4C knockdown could hin-der the translocation of β-catenin into nucleus (Fig. 2c, d). Next, rescue assays were conducted to explore the influence of Wnt/β-catenin pathway on HCC cells with circ-DENND4C depletion. Colony formation assay dis-closed that the treatment of LiCl could counteract the suppressive effect of silenced circ-DENND4C on HCC cell proliferation (Fig. 2e). Further, the inhibitory role of
Fig. 1 Circ-DENND4C regulated HCC cell growth, invasion and stemness. a qRT-PCR examined circ-DENND4C expression in HCC cells and normal liver epithelial cells. b, c Gel electrophoresis and ActD treatment verified circular characteristic of circ-DENND4C. d qRT-PCR verified knockdown efficiency of sh-circ-DENND4C in HCC cells. e, f. Immunofluorescence staining assay (scale bar = 30 μm) and colony formation assay detected the proliferation of HCC cells transfected with sh-circ-DENND4C#1/2. g Cell cycle was analyzed by flow cytometry upon circ-DENND4C knockdown.
h TUNEL assay (scale bar = 100 μm) revealed HCC cell apoptosis with transfection of sh-circ-DENND4C#1/2. i The protein levels of Cyclin D1, CDK4, Bcl-2 and Bax were determined through western blot analysis. j Transwell assay (scale bar = 100 μm) detected cell invasion in HCC cells transfected with sh-circ-DENND4C#1/2. k, l qRT-PCR and western blot detected mRNA and protein levels of stemness biomarkers in HCC cells upon circ-DENND4C depletion. *P < 0.05, **P < 0.01
circ-DENND4C knockdown in cell cycle was also neu-tralized by treating LiCl (Fig. 2f). TUNEL assay displayed that LiCl abolished the facilitative effect on HCC cell apoptosis caused by circ-DENND4C depletion (Fig. 2g, Additional file 1: Figure S1C). It was also observed that the effect of circ-DENND4C deficiency on proteins related to cell cycle and apoptosis was abrogated after the treatment of LiCl (Fig. 2h). Besides, LiCl treatment recovered the weakened HCC cell invasion caused by circ-DENND4C knockdown (Fig. 2i). Further, treatment of LiCl countervailed the expression changes of OCT4, SOX2, NANOG at both mRNA and protein levels in
circ-DENND4C-inhibited cells (Fig. 2j, k). Thus, we sum-marized that circ-DENND4C regulated HCC cell growth, invasion and stemness via activating Wnt/β-catenin signaling.
Circ‑DENND4C sponged miR‑195‑5p
To further explore the molecular mechanism of circ-DENND4C in HCC, we first detected the subcellular localization of circ-DENND4C. It was disclosed that circ-DENND4C was mainly located in cytoplasm of HCC cells (Fig. 3a, b). Cytoplasmic distribution of circ-DENND4C indicated the post-transcriptional regulation
Fig. 2 Activated Wnt/β-catenin pathway rescued the effects of circ-DENND4C suppression on HCC cells. a Activators of different signaling pathways were used in immunofluorescence staining assay. b Western blot detected Wnt pathway-associated proteins upon circ-DENND4C depletion. c, d The distribution of β-catenin in cells transfected with sh-NC or sh-circ-DENND4C#1 was determined by western blot analysis and immunofluorescence assay (scale bar = 30 μm). e Colony formation assay detected proliferation of HCC cells transfected with sh-NC, sh-circ-DENND4C#1 or sh-circ-DENND4C#1+LiCl. f Flow cytometry assessed cell cycle in sh-NC, sh-circ-DENND4C#1 or sh-circ-DENND4C#1+LiCl group. g TUNEL assay (scale bar = 100 μm) revealed HCC cell apoptosis with transfection of sh-NC, sh-circ-DENND4C#1 or sh-circ-DENND4C#1+LiCl.
of circ-DENND4C in HCC cells. Since ceRNA mecha-nism is a typical post-transcriptional network, we sup-posed that circ-DENND4C might serve as a ceRNA in HCC. By using “starBase” [15], 9 miRNAs were identi-fied under the screening condition that strict stringency of CLIP data and at least 5 supported AGO CLIP-seq experiments. Next, it was displayed that only miR-195-5p and miR-6838-5p were significantly pulled down by circ-DENND4C biotin probe, while other not (Fig. 3c). The following RIP assay indicated that circ-DENND4C co-existed with miR-195-5p rather than miR-6838-5p in anti-Ago2 group (Fig. 3d). Meanwhile, we discovered that the expression of miR-195-5p was down-regulated in HCC cells while that of miR-6838-5p didn’t exhibit difference (Fig. 3e). In this regard, miR-195-5p was then proposed as the downstream of circ-DENND4C in HCC. Further, we found the binding sequences of miR-195-5p to circ-DENND4C and mutated these sequences as well (Fig. 3f). Besides, the overexpression efficiency of miR-195-5p was verified in qRT-PCR analysis (Fig. 3g). As revealed in luciferase reporter assay, miR-195-5p overex-pression notably decreased the luciferase activity of circ-DENND4C-wt vectors while that of circ-DENND4C-mut vectors was not affected (Fig. 3h). Similarly, RNA pull down assay further verified the interaction of circ-DENND4C with miR-195-5p at predicted binding sequences (Fig. 3i). In conclusion, circ-DENND4C func-tioned as a sponge of miR-195-5p in HCC.
MiR‑195‑5p targeted TCF4 and negatively modulated TCF4
To support ceRNA hypothesis, we explored the target genes of miR-195-5p. According to the result of “star-Base”, TCF4 was predicted as target gene of miR-195-5p. More importantly, TCF4 has been widely reported to activate Wnt/β-catenin pathway in many cancers, includ-ing HCC [16–18]. We first observed that TCF4 was nota-bly overexpressed in HCC cells compared with normal THLE-3 cells (Fig. 4a). Then, RIP assay was conducted to verify the interaction between miR-195-5p and TCF4 mRNA. The result disclosed that both miR-195-5p and TCF4 were significantly enriched in RNA-induced silenced complexes (RISCs) (Fig. 4b). Next, the binding sites between TCF4 3′UTR and miR-195-5p were dis-closed and we mutated the binding sequences in TCF4
(Fig. 4c). RNA pull down assay revealed that TCF4 was notably pulled down by biotinylated miR-195-5p-wt while had no response to biotinylated miR-195-5p-mut (Fig. 4d). Further, we down-regulated the expression of miR-195-5p in HCC cells as verified by qRT-PCR analy-sis (Fig. 4e). Subsequently, luciferase reporter assay was carried out and results revealed that luciferase activity of wild type TCF4 reporter was significantly decreased by 195-5p overexpression and enhanced by miR-195-5p knockdown. However, the luciferase activity of mutant type TCF4 showed no change in cells transfected with miR-195-5p-mimics/inhibitor (Fig. 4f). Further, we examined the effect of miR-195-5p overexpression or circ-DENND4C knockdown on TCF4 expression in HCC. As expected, TCF4 mRNA and protein expres-sions were both down-regulated by miR-195-5p overex-pression or by circ-DENND4C knockdown in HCC cells (Fig. 4g, h). Thus, we concluded that TCF4 was down-stream of circ-DENND4C/miR-195-5p axis in HCC.
Circ‑DENND4C mediated HCC cell growth, invasion and stemness via up‑regulating TCF4
Finally, rescue assays were conducted to examine the effects of TCF4 on circ-DENND4C knockdown-medi-ated HCC cell development. Immunofluorescence stain-ing and colony formation assay disclosed that TCF4 overexpression restored the suppressive effect of circ-DENND4C depletion on HCC cell proliferation (Fig. 5a, b). Furthermore, circ-DENND4C silencing-mediated inhibition on cell cycle was also recovered by TCF4 over-expression (Fig. 5c). TUNEL assay disclosed that TCF4 up-regulation restored the facilitating effect of circ-DENND4C depletion on HCC cell apoptosis (Fig. 5d). Through western blot analysis, overexpressed TCF4 res-cued the effect of silenced circ-DENND4C on the levels of proteins involved in cell cycle and apoptosis (Fig. 5e). Moreover, TCF4 overexpression reversed the weakened HCC cell invasion caused by circ-DENND4C depletion (Fig. 5f). Additionally, the expression levels of OCT4, SOX2 and NANOG decreased by knockdown of circ-DENND4C were further normalized by TCF4 up-regula-tion at both mRNA and protein levels (Fig. 5g, h). In sum, circ-DENND4C mediated HCC cell growth, invasion and stemness via up-regulating TCF4.
Fig. 3 Circ-DENND4C sponged miR-195-5p. a, b Subcellular fractionation assay and FISH assay (scale bar = 10 μm) were carried out to detect the subcellular location of circ-DENND4C in HCC cells. c RNA pull down assay detected enrichments of 9 candidate miRNAs in no-biotin/biotin-labeled circ-DENND4C probe. d RIP assay detected miR-195-5p, circ-DENND4C and miR-6838-5p enrichments in Ago2 and IgG group. e qRT-PCR examined miR-195-5p and miR-6838-5p expressions in HCC cells and normal THLE-3 cells. f Binding sequences between circ-DENND4C and miR-195-5p was predicted from starBase. g qRT-PCR verified the overexpression efficiency of miR-195-5p. h Luciferase activity of circ-DENND4C-wt/mut reporters was examined after miR-195-5p overexpression. i RNA pull down assay examined enrichment of circ-DENND4C pulled down by biotinylated miR-195-5p-wt/mut. **P < 0.01, n.s. meat no significance
Circ‑DENND4C up‑regulates TCF4 to facilitate HCC tumor growth in vivo
Subsequently, we investigated whether circ-DENND4C regulate tumor growth by increasing TCF4 expres-sion. At first, we subcutaneously injected HepG2 cells transfected with sh-NC, sh-circ-DENND4C#1 or
sh-circ-DENND4C#1+pcDNA3.1-TCF4 into nude
mice. As a result, we observed that the size of subcu-taneous tumors was remarkably decreased by circ-DENND4C knockdown but was further normalized in sh-circ-DENND4C#1+pcDNA3.1-TCF4 group
(Fig. 6a). Accordingly, we recorded that the tumor
growth was obstructed in circ-DENND4C-depleted group but recovered after TCF4 upregulation (Fig. 6b). Also, the weight of tumors was evidently reduced in sh-circ-DENND4C#1 group but was further restored in the group of sh-circ-DENND4C#1+pcDNA3.1-TCF4
(Fig. 6c). IHC assay unveiled that TCF4 overexpression could offset the inhibitory effect of circ-DENND4C depletion on the positivity of Ki67 and PCNA (two well-known proliferation markers) in above tumors (Fig. 6d). All experimental data suggested that circ-DENND4C accelerated HCC progression by targeting miR-195-5p/TCF4 axis and activating Wnt/β-catenin pathway (Fig. 6e).
Discussion
The present study first found the high expression of circ-DENND4C in HCC cells, which established the research value of circ-DENND4C in HCC. Then, cell proliferation, apoptosis and invasion assays were con-ducted to examine the function of circ-DENND4C in HCC cells. All the results disclosed that circ-DENND4C facilitated HCC cell proliferation, invasion and repressed HCC cell apoptosis. Also, expression levels of stemness biomarkers were examined, and it was disclosed that silencing circ-DENND4C notably down-regulated the expression of stemness biomarkers at both mRNA and protein levels. Next, we sought for the downstream pathway of circ-DENND4C in HCC.
Interestingly, LiCl, the activator of Wnt/β-catenin path-way, was found to rescue circ-DENND4C knockdown-mediated effects on HCC cells. The oncogenic role of Wnt pathway was initially recognized by that ectopic expression of Wnt-1 could facilitate tumor formation in mammary tissues of mouse [19]. Our study found that circ-DENND4C could activate Wnt/β-catenin pathway to exacerbate cell growth, invasion and stemness in HCC.
After that, we examined the subcellular location of circ-DENND4C, and data revealed that circ-DENND4C was principally distributed in the cytoplasm of HCC cells. Thus, we wondered if circ-DENND4C func-tioned as a ceRNA to up-regulate certain mRNA so as to activate Wnt/β-catenin pathway. CeRNA network is a typical post-transcriptional regulatory mechanism which has been reported in various cancers, includ-ing HCC. For example, lncRNA MEG3 represses HCC cell growth via up-regulating SOX11 through spong-ing miR-9-5p [20]. LncRNA miat serves as a ceRNA to modulate sirt1 by acting as a miR-22-3p sponge in HCC cellular senescence [21]. LncRNA MALAT1 inhibits miR-124-3p expression and promotes slug expression to induce tumor metastasis in HCC [22]. In our study, we identified that circ-DENND4C acted as a sponge of miR-195-5p to up-regulate TCF4 expression in HCC cells. TCF4 is a famous transcription factor which has been widely reported to activate Wnt/β-catenin path-way. Previous study indicated that miR-155 targets TCF4 to exacerbate acute kidney injury via regulat-ing Wnt/β-catenin signalregulat-ing pathway [23]. LINC01197 inhibits the activity of Wnt/β-catenin pathway via dis-turbing the binding of TCF4 to β-catenin in pancreatic adenocarcinoma [24]. The final rescue assays indicated that circ-DENND4C mediated HCC cellular progres-sion in vitro and in vivo via up-regulating TCF4.
Conclusion
In conclusion, our research unveiled that circ-DENND4C functioned as a ceRNA to facilitate HCC cell proliferation, cell cycle, invasion, stemness via up-regulation of TCF4 and activation of Wnt/β-catenin pathway. This research might give innovative directions to explore new potential therapies for HCC patients.
Supplementary information
Supplementary information accompanies this paper at https ://doi. org/10.1186/s1293 5-020-01346 -0.
Additional file 1: Figure S1. A. The detailed images of the merged data in Fig. 1e. B. The detailed images of the merged data in Fig. 1h. C. The detailed images of the merged data in Fig. 2g.
Abbreviations
HCC: Hepatocellular carcinoma; circRNA: Circular RNA; DENND4C: DENN domain containing 4C; TCF4: Transcription factor 4; ceRNA: Competing endogenous RNA; miRNA: MicroRNA; qRT-PCR: Quantitative Real-time PCR; FISH: Fluorescence in Situ Hybridization; RIP: RNA Immunoprecipitation; ChIP: Chromatin immunoprecipitation; ActD: Actinomycin D.
Acknowledgements
We sincerely appreciate all lab members.
Authors’ contributions
Xialei Liu and Lewei Yang designed this study and analyzed data. Dong Jiang contributed to experiments. Wuzhu Lu devoted to investigation and methods, Yongyu Zhang wrote this paper. All authors read and approved final manuscript.
Funding
This work was supported by grants from Medical Scientific Research Founda-tion of Guangdong Province of China (No.A2017421); Science and Technology Planning Project of Zhuhai City of China (No. 20171009E030080).
Availability of data and materials Research data and material are not shared.
Ethics approval and consent to participate
This study was approved from the Animal Research Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University.
Consent for publication
All authors have read and approved the final manuscript for publication.
Competing interests
The authors declare that they have no competing interests.
Author details
1 Department of Hepatobiliary Surgery, The Fifth Affiliated Hospital of Sun
Yat-sen University, Zhuhai City, Guangdong Province 519000, China. 2
Depart-ment of Radiotherapy for Abdominal Neoplasms, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai City, Guangdong Province 519000, China.
3 Department of Urology, The Fifth Affiliated Hospital of Sun Yat-sen University,
Zhuhai City, Guangdong Province 519000, China. 4 Department of Ultrasound,
The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai City, Guangdong Province 519000, China. 5 Department of Interventional Radiology, The Fifth
Affiliated Hospital of Sun Yat-sen University, No. 52, Meihuadong Road, Xiang-zhou District, Zhuhai City, Guangdong Province 519000, China.
Received: 20 January 2020 Accepted: 13 June 2020
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mor-tality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
2. Gong D, Feng PC, Ke XF, Kuang HL, Pan LL, Ye Q, Wu JB. Silencing Long Non-coding RNA LINC01224 Inhibits Hepatocellular Carcinoma Progres-sion via MicroRNA-330-5p-Induced Inhibition of CHEK1. Molecular therapy Nucleic acids. 2019;19:482–97.
3. Meena AS, Sharma A, Kumari R, Mohammad N, Singh SV, Bhat MK. Inher-ent and acquired resistance to paclitaxel in hepatocellular carcinoma: molecular events involved. PLoS ONE. 2013;8(4):e61524.
4. Mohammad N, Singh SV, Malvi P, Chaube B, Athavale D, Vanuopadath M, Nair SS, Nair B, Bhat MK. Strategy to enhance efficacy of doxorubicin in solid tumor cells by methyl-beta-cyclodextrin: Involvement of p53 and Fas receptor ligand complex. Scientific reports. 2015;5:11853. 5. Carr BI. Hepatocellular carcinoma: current management and future
trends. Gastroenterology. 2004;127(5 Suppl 1):S218–24.
•fast, convenient online submission •
thorough peer review by experienced researchers in your field • rapid publication on acceptance
• support for research data, including large and complex data types •
gold Open Access which fosters wider collaboration and increased citations maximum visibility for your research: over 100M website views per year •
At BMC, research is always in progress.
Learn more biomedcentral.com/submissions
Ready to submit your research? Choose BMC and benefit from: Neck Squamous Cell Carcinoma. Clinical cancer research : an official
jour-nal of the American Association for Cancer Research. 2017;23(12):3120–8. 7. Barrett SP, Salzman J. Circular RNAs: analysis, expression and potential
functions. Development (Cambridge, England). 2016;143(11):1838–47. 8. Jin C, Shi L, Li Z, Liu W, Zhao B, Qiu Y, Zhao Y, Li K, Li Y, Zhu Q. Circ_0039569
promotes renal cell carcinoma growth and metastasis by regulat-ing miR-34a-5p/CCL22. American journal of translational research. 2019;11(8):4935–45.
9. Rong X, Gao W, Yang X, Guo J. Downregulation of hsa_circ_0007534 restricts the proliferation and invasion of cervical cancer through regulat-ing miR-498/BMI-1 signalregulat-ing. Life Sci. 2019;235:116785.
10. Muhammad N, Bhattacharya S, Steele R, Ray RB. Anti-miR-203 suppresses ER-positive breast cancer growth and stemness by targeting SOCS3. Oncotarget. 2016;7(36):58595–605.
11. Wang Z, Liu W, Wang C, Ai Z. miR-873-5p Inhibits Cell Migration and Inva-sion of Papillary Thyroid Cancer via Regulation of CXCL16. OncoTargets and therapy. 2020;13:1037–46.
12. Huang W, Gu J, Tao T, Zhang J, Wang H, Fan Y. MiR-24-3p Inhibits the Progression of Pancreatic Ductal Adenocarcinoma Through LAMB3 Downregulation. Frontiers in oncology. 2019;9:1499.
13. Wu P, Gao Y, Shen S, Xue Y, Liu X, Ruan X, Shao L, Liu Y, Wang P. KHDRBS3 regulates the permeability of blood-tumor barrier via cDENND4C/miR-577 axis. Cell death & disease. 2019;10(7):536.
14. Liang G, Liu Z, Tan L, Su AN, Jiang WG, Gong C. HIF1alpha-associated circDENND4C Promotes Proliferation of Breast Cancer Cells in Hypoxic Environment. Anticancer Res. 2017;37(8):4337–43.
15. Li J-H, Liu S, Zhou H, Qu LH, Yang J-H. starBase v20: decoding miRNA-ceRNA, miRNA-ncRNA and protein–RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2013;42(D1):D92–7.
16. Liu Z, Zhong Y, Chen YJ, Chen H. SOX11 regulates apoptosis and cell cycle in hepatocellular carcinoma via Wnt/beta-catenin signaling pathway. Biotechnol Appl Biochem. 2019;66(2):240–6.
17. Zhang T, Ma Z, Liu L, Sun J, Tang H, Zhang B, Zou Y, Li H. DDX39 promotes hepatocellular carcinoma growth and metastasis through activating Wnt/beta-catenin pathway. Cell death & disease. 2018;9(6):675.
18. Cao MQ, You AB, Zhu XD, Zhang W, Zhang YY, Zhang SZ, Zhang KW, Cai H, Shi WK, Li XL, et al. miR-182-5p promotes hepatocellular carcinoma progression by repressing FOXO3a. Journal of hematology & oncology. 2018;11(1):12.
19. Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109.
20. Liu Z, Chen JY, Zhong Y, Xie L, Li JS. lncRNA MEG3 inhibits the growth of hepatocellular carcinoma cells by sponging miR-9-5p to upregulate SOX11. Brazilian journal of medical and biological research Revista = brasileira de pesquisas medicas e biologicas. 2019;52(10):8631. 21. Zhao L, Hu K, Cao J, Wang P, Li J, Zeng K, He X, Tu PF, Tong T, Han L.
lncRNA miat functions as a ceRNA to upregulate sirt1 by sponging miR-22-3p in HCC cellular senescence. Aging. 2019;11(17):7098–122. 22. Cui RJ, Fan JL, Lin YC, Pan YJ, Liu C, Wan JH, Wang W, Jiang ZY, Zheng XL,
Tang JB, et al. miR-124-3p availability is antagonized by LncRNA-MALAT1 for Slug-induced tumor metastasis in hepatocellular carcinoma. Cancer Med. 2019;8(14):6358–69.
23. Zhang XB, Chen X, Li DJ, Qi GN, Dai YQ, Gu J, Chen MQ, Hu S, Liu ZY, Yang ZM. Inhibition of miR-155 Ameliorates Acute Kidney Injury by Apoptosis Involving the Regulation on TCF4/Wnt/beta-Catenin Pathway. Nephron. 2019;143(2):135–47.
24. Ling J, Wang F, Liu C, Dong X, Xue Y, Jia X, Song W, Li Q. FOXO1-regulated lncRNA LINC01197 inhibits pancreatic adenocarcinoma cell proliferation by restraining Wnt/beta-catenin signaling. Journal of experimental & clinical cancer research : CR. 2019;38(1):179.
Publisher’s Note