287
Copyright © 2016. Vandana Publications. All Rights Reserved.
Volume-6, Issue-4, July-August 2016
International Journal of Engineering and Management Research
Page Number: 287-291
Heavy Metals Removal by Nickel-Oxide Nanoparticles Synthesised by
Lemon Juice Extract
Ashish Panji1, L. Udaya Simha2, B.M. Nagabhushana3 1
Previously Postgraduate Student, Department of Civil Engineering, B.M.S. College of Engineering, Bangalore, INDIA; Presently with Honda Motors, Kolar, INDIA
2Department of Civil Engineering, B.M.S. College of Engineering, Bangalore – 560018, INDIA
3Department of Chemistry, M.S. Ramaiah Institute of Technology, Bangalore – 560054, INDIA
ABSTRACT
Applicability of nickel-oxide nanoparticles for the selective removal of heavy metals from synthetic metal solutions was investigated. An average size of 26 nm were synthesized using lemon juice extract as fuel by solution combustion method and characterized by X-Ray diffraction, SEM and FTIR. Batch experiments were conducted to determine adsorption kinetics and mechanisms of Cr (VI), Cu (II) and Ni (II). The adsorption process was found to be highly pH dependent. Data were well defined with Langmuir isotherms. Adsorption mechanisms were found to follow pseudo second order for Cr (VI), Cu (II) and Ni (II).
Keywords--- Nickel-oxide nanoparticles, solution combustion method, heavy metal removal, adsorption kinetics, adsorption isotherms
I.
INTRODUCTION
World’s population is increasing steadily. The issues concerned with it like food security, water shortage problem are also increasing alarmingly. Due to socio-economic conditions in the third world countries, wastewater is used for agricultural purposes. Application of heavy metal contaminated wastewater, such as cadmium, copper, lead, iron and manganese etc., would pollute soil and water bodies. The heavy metals taken up by plants in the vicinity, directly or indirectly affect human and animal health [1]. Heavy metal ions could be eliminated by several traditional techniques such as reverse osmosis, chemical precipitation, electrochemical treatment techniques, ion exchange, membrane filtration, coagulation, extraction, irradiation and adsorption. Adsorption technology would be most promising due to its low cost-effective, high efficiency, and simple to operate [2]. The materials like activated carbons, chelating materials, clay minerals, and chitosan/natural zeolites have been adopted to adsorb metal ions from aqueous solutions. Although these traditional materials
could remove heavy metal ions from effluent, the low sorption capacities limit their application [2]. To overcome these defects of traditional materials, nanomaterials could be the novel option to remove heavy metal ions in wastewater. Nanotechnology application to water treatment has been the current research field of interest. In particular, nanomaterials as adsorbent could remove contaminants from water during municipal treatment or in point of use applications in efficient and less costly fixed bed absorbers [3]. T. Mimani and K.C. Patil [4] described the solution combustion method for
synthesis of nanoscale CeO2-ZrO2, t-ZrO2
-Al2O3andY2O3-ZrO2 and their composites, which were
characterized by X-ray diffraction (XRD) and
transmission electron microscope (TEM).
The studies on adoptability of nano-oxides of nickel in treating lead, hexavalent chromium, arsenic, COD and colour revealed the reduction in lead, chromium and colour to the tune of more than 95%. The COD reduction was reported to be about 50% [3, 5- 9]. Synthesised metal oxides of iron were reported to reduce lead and Cu(II) [7,10, 11]. Synthesised composite nanoparticles of iron and nickel were found to reduce concentration of Pb and Cr (VI) [5, 10]. Jing Hu et al. [11] investigated the removal of Cr (VI), Cu (II), and Ni(II) by maghemite nanoparticles. They concluded that the adsorption of heavy metals attained equilibrium rapidly within 10 min and the adsorption data were found to follow the Langmuir isotherm. KirilHristovski et al. [3] investigated the arsenic removal by aggregated metal oxide nanoparticles, viz., TiO2, Fe2O3, ZrO2 and NiO, in packed bed columns. Valerie A. Grover et
al.,[12] adopted commercially prepared hematite
288
Copyright © 2016. Vandana Publications. All Rights Reserved.
The solution combustion method was adopted to develop nanoparticles in the present study. Solution combustion synthesis (SCS) is a simple, versatile and rapid method, which allows effective synthesis of a variety of nanosize materials. It involves a self-sustained reaction in homogeneous solution of different oxidizers and fuels (e.g., urea, glycine, hydrazides, hexamine, sucrose, citric acid) [13-16]. In present investigation,the ability of NiO nanoparticles synthesised using lemon juice extract as a fuel was investigated in removal of heavy metals like Cr (VI), Cu (II) and Ni (II). The nanoparticles were characterised by powder X-Ray diffraction (PXRD), Scanning Electron Microscopy (SEM) and Fourier Transform Infrared Spectroscopy (FTIR).
II.
MATERIALS AND METHODS
A. Preparation and Characterization of Adsorbent:
Nickel-oxide nanoparticles were prepared by solution combustion method using Ni(NO3)2 as an oxidizer and lemon extract served as a bio-fuel which is the alternate form for the commonly used organic fuels. Stoichiometric quantity of Ni(NO3)2 is dissolved in distilled water to make homogenous solution. It was mixed with lemon extract and stirred well to obtain uniform solution. The solution was transferred to the ceramic crucible and heated at 400°C for five to ten minutes in a muffle furnace until complete combustion.
The crystalline phase analyses were investigated by Powder X-Ray Diffraction (PXRD). PXRD of the as-synthesized powders was performed using CuKα radiation (λ=1.54 Å). The morphological properties of the synthesized powder were determined by scanning electron microscopy (SEM). The phase purity was further confirmed by Fourier Transform Infrared Spectroscopy (FTIR). The metal ion concentration was detected by Atomic absorption spectrophotometer (AAS).
B. Batch Adsorption:
The batch experiments on pH, adsorbent dosage, and contact time were conducted for the investigation of adsorption of metals ions by the synthesized metal oxide nanoparticles. The synthetic solution of metal salt with concentration 10 ppm was prepared. A known volume (50 mL) was pipette into the series of glass vial to which varying amounts of adsorbent was added. After attaining the contact time (in minutes) between adsorbent and adsorbate, the solution was centrifuged and the supernatant was filtered. The filtrate was analysed by Atomic absorption spectrophotometer for determination of metal ion concentration. Each experiment was repeated with varying pH and contact time. The pH was adjusted by 0.1M HCl and 0.1M NaOH solution. All adsorption experiments were carried out at room temperature (29±2°C). The removal efficiency of metal ions in percentile was calculated using the following equation:
E = [(C0−Ct)/C0] × 100 …… (1)
Where C0 and Ct
III.
RESULTS AND DISCUSSION:
Characterization of Adsorbent:
X-ray diffraction analysis is a widely used method to identify the mean crystallite size, purity and structure of the nanoparticles. CuKα radiation of wavelength 1.54 Å was used for characterization of nickel oxide nanoparticles. 100% intensity was observed at the angle 43.27° (Fig. 1) and the peaks of the graph fits with the JCPDS card no. 22‐1189. XRD data were used to determine the mean size of nickel oxide nanoparticles by using Debye-Scherrer equation. The size of nickel oxide nanoparticles corresponding to 100% intensity peak was found to be 26nm. The SEM analysis revealed the fact that the particles were agglomerated, random shaped and multi dispersed (Fig. 2). FTIR spectrum shown in Fig.3 is typical for metal oxide nanomaterials. FTIR spectrum of NiO presents an absorption band at 411cm
are the concentration of metal ions in the reaction solution before and after treatment, respectively.
-1 representing presence of
Ni-O bond and the absorption bands in the region of 1000– 1500 cm−1 are symmetric and asymmetric stretching vibrations which are assigned to the O-C=O and the C–O stretching vibration.The broad absorption band centred at 3440 cm−1 is attributable to the band O–H stretching vibrations. The weak band near 1635cm−1
0 10 20 30 40 50 60 70 80 90
0 1000 2000 3000 4000 5000 6000 7000
Int
ens
ity
(C
PS
)
2 theta (deg)
is assigned to H–O–H bending vibrations mode.
Figure1: XRD graph of Nickel-oxide nanoparticles
289
Copyright © 2016. Vandana Publications. All Rights Reserved.
4000 3000 2000 1000 0
0 20 40 60 80 100 120 140 160 180
Tr
ans
m
itt
anc
e (
%
)
Wavenumbers (per cm)
Figure 3: FTIR graph of Nickel-oxide nanoparticles
A. Batch Studies Effect of pH:
The effect of solution pH on the removal of chromium, copper, and nickel during adsorption process is shown in Fig. 4. The results are very much in support with the findings of Jing, et al., [11]for maghemite nanoparticle. The removal efficiency was found to be highly pH dependent. The percentage of removal of Cu(II) and Ni(II) is found to be increased with an increase in pH, whereas uptake percentage of Cr(VI) gradually decreased with an increase in pH. The maximum removal of Cr(VI) was found to occurred at about pH 3. With an increase in pH to 7, the removal efficiency of Ni reached 95.8%; and 93.9% for Cu. When the pH was further increased to 11, 98.3% of the Ni and 99.1% of Cu was removed. Thus, controlling of pH of the solution has the effect on removal of these three selective metals. The metal removal efficiency with respect to pH can be explained from the perspective of surface chemistry in an aqueous phase; the hydroxyl group, which, generally, covers the surfaces of metal oxides, would vary in form at different pH levels[11]. At zero point of charge (pHpzc), the surface charge is neutral. Below zero point of charge, the surface of adsorbent is positively charged. The surface of adsorbent is negatively charged for the values above pHpzc. The decrease in uptake of Cr(VI) with increase in pH, could be due the presence of concentration OH− ions in the solution which compete with Cr(VI) species CrO42− for adsorption sites. On the other hand, if the adsorption surface is negatively charged, would result in a release of the adsorbed HCrO4 and CrO42− due to increase in electrostatic repulsion between negatively charged nanoparticles and negatively charged Cr(VI) species. The increase in removal of Cu and Ni are concerned, a lower columbic repulsion of the adsorbed metal would be resulting in due to a decline in competition between metal species and proton under increase pH of solution [11]. The adsorption capacity of Cu and Ni should be reduced when pH of solution is below zero point of charge. But in the present investigation though pH of solution is lower than pHpzc,there was still adsorption of Cu and Ni on to the nickel oxide, which could be due to ion exchange between Cu2+/Ni2+ and H+ may play a role in this pH range [11]. The behaviour of adsorption of Cu(II) and Ni(II) on to nickel oxide nano particles could
be due the reason based on electronegativity values. Electro negativity values for Cu (II) and Ni (II) are 2.00 and 1.91, respectively; hence Cu exhibited a stronger attraction than Ni to nickel oxide [5]. In the present case, Cu exhibited a stronger attraction than Ni to nickel oxide nanoparticles upto pH 7.0.
Figure 4: Effect of solution pH on adsorption of Cu, Cr and Ni
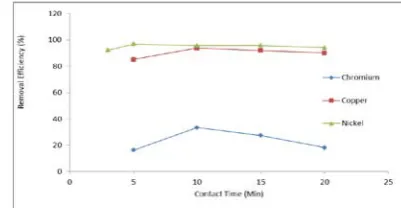
Effect of contact time:
The effect of contact time on the removal of chromium, copper and nickel is shown in Fig. 5. The removal efficiency was dependent on contact time. As the contact time was increased upto 5 to10 minutes, there was increase in removal efficiency. Further increase in contact time reduced the removal efficiency for chromium, copper, and nickel. At 10 minutes of contact time, copper removal efficiency was about 94% and that of hexavalent chromium about 33.3%.The efficiency of removal of nickel was about 97% at 5 min. It is observed that uptake rate of metals by nickel oxides initially was quite high, followed by a much slower removal rate which has led gradually to equilibrium. The rapid adsorption of metals by nickel-oxide nanoparticles is attributed to the external surface adsorption, where all the adsorption sites exist in the exterior of the adsorbent. Hence the adsorbate can access these active sites, resulting in rapid approach of equilibrium.
Figure 5: Effect of contact time on adsorption of Cu, Cr and Ni
Effect of Adsorbent dosage
290
Copyright © 2016. Vandana Publications. All Rights Reserved.
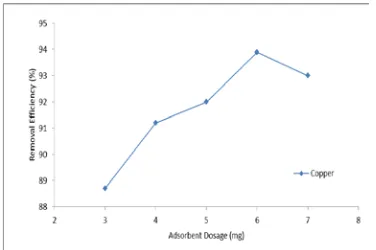
respectively. The increase in the efficiency of removal may be attributed to the reason that with an increase in the adsorbent dose, more adsorbent surface is available for the solute to be adsorbed. This is true for a particular value of dosage. After this, the increase of dosage reduces the removal efficiency could due to reduction in the effective surface area.
Figure 6: Effect of adsorbent dosage on adsorption of Cu
Figure 7: Effect of adsorbent dosage on adsorption of Cr
and Ni
Kinetic Studies:
To determine the mechanism involved in adsorption process, various kinetic models like first order, pseudo first order, pseudo second order and intra particle diffusion equations were adoted in the study. Table 1 shows the kinetic parameters for the removal of heavy metals by nickel-oxide
The Figures 8 and 9 show graphical representation. The kinetics follows the pseudo second order for copper, nickel and chromium.
Figure 8: Pseudo second order kinetics for Cu and Ni
Figure 9: Pseudo second order for Cr
Adsorption Isotherms:
The Figures 10 and 11 show the isothermal behaviour of the adsorption of considered heavy metals. The constants of the model for heavy metals are shown in Table 3. The correlation coefficients reported in the study showed that Cr (VI) adsorption was defined by Langmuir isotherm model and Cu (II) and Ni (II) were found to follow Freundlich isotherm model.
Figure 10: Langmuir adsorption isotherm for chromium
Figure 11: Freundlich adsorption isotherm for copper and nickel
IV.
CONCLUSION
291
Copyright © 2016. Vandana Publications. All Rights Reserved.
The adsorption was dependent on adsorbent dosage and contact time. The optimum dosage and contact time for copper, nickel, and chromium were 6 mg/50 ml and 10 min, 10 mg/50 mL and 5 min, 25mg/50 mL and 10 min respectively. The removal efficiency followed the following trend, Ni >Cu >Cr. The adsorption mechanism was found to follow Pseudo second order. The Cr adsorption was defined by Langmuir isotherm model and Cu (II) and Ni (II) were found to follow Freundlich isotherm model.
REFERENCES
[1] Maria Mussarat, AmanUllahBhatti and Farman Ullah Khan, Concentration of metals in sewage and canal water used for irrigation in Peshawar, J. Agric. Vol. 23, No. 2, 335-338, 2007.
[2] Xiangtao Wang, YifeiGuo, Li Yang, Meihua Han, Jing Zhao1 and Xiaoliang Cheng., Nanomaterials as sorbents to Remove heavy metal ions in waste water treatment, J. Environ Anal Toxicol., Volume 2, Issue 7, 2-7, 2012.
[3] Hristovski. K, Andrew Baumgardner, Paul Westerhoff, Selecting metal oxide nanomaterials for arsenic removal in fixed bed columns: From nanoparticles to aggregated media, 17;147(1-2), 265-74, 2007.
[11] Jing Hu; Guohua Chen; and Irene M. C. Lo, M., Selective Removal of Heavy Metals from Industrial
Wastewater Using Maghemite Nanoparticle: Performance and Mechanisms, Journal of Environmental Engineering, ASCE, Vol. 132, No. 7, 709-715, 2006.
[4] T. Mimani and K.C. Patil, Solution Combustion synthesis of nanoscale oxides and their composites, J. Mater.Phys.Mech. Vol. 4, 134-137, 200, 2001.
[5] Linsen Wei, Gang Yang, Ren Wang, Wei Ma, Selective adsorption and separation of chromium (VI) on the magnetic iron-nickel oxide from waste nickel liquid, J. Journal of Hazardous Materials. Vol. 164, 1159-1163, 2009.
[6] A. Naeem, M.T. Saddique, S. Mustafa, Y. Kim, B. Dilara, Cation exchange removal of Pb from aqueous solution by sorption onto NiO, Journal of Hazardous Materials. Vol. 168, 364-368, 2009.
[7] Xue Song Wang, Lei Zhu, HaiJie Lu, Surface chemical properties and adsorption of Cu (II) on nanoscale magnetite in aqueous solutions, J. Desalination 276, 154–160, 2011.
[8] N.K. Srivastava, M.K. Jha and T.R. Sreekrishnan, Removal of Cr (VI) from waste water using NiO nanoparticles, J. International Journal of Science, Environment and Technology. Vol. 3, No. 2, 395-402, 2014.
[9] R.D. Kale, Prerana Kane, NamrataPhulaware, Decolourization of C.I. Reactive Black 5 by PVP stabilized Nickel nanoparticles, J. International Journal of Engineering Science and Innovative Technology, Vol. 3, Issue 2, 109-117, 2014.
[10] A. Imtiaz and U.Rafique, Synthesis of Metal Oxides and its Application as Adsorbent for the Treatment of Wastewater Effluents, J. International Journal of Chemical and Environmental Engineering. Vol. 2, No. 6, 309-405, 2011.
[12] Valerie A. Grover, Jinxuan Hu, Karen E. Engates, Heather J. Shipley; Adsorption and desorption of bivalent metals to hematite nanoparticles, 2012
[13] Singanahally T. Aruna, Alexander S. Mukasyan, Combustion synthesis and nanomaterials, Current Opinion in Solid State and Materials Science, 44–50, 2008.
[14] Poonam Sharma, Gurmeet Singh Lotey, Sukhpreet Singh, N. K. Verma, Solution-combustion: the versatile route to synthesize silver nanoparticles, J Nanopart Res (2011) 13:2553–256m, 2011
[15] S. Balamurugan,1 L. Ashna,2 and P. Parthiban1, Synthesis of Nanocrystalline MgO Particles by Combustion Followed by Annealing Method Using Hexamine as a Fuel, Journal of Nanotechnology, Volume 2014, Article ID 841803, 2014.