organic papers
o1518
Shashidharet al. C15H16N2O2S doi:10.1107/S1600536806009433 Acta Cryst.(2006). E62, o1518–o1519
Acta Crystallographica Section E
Structure Reports Online
ISSN 1600-5368
1,3-Bis(4-methoxyphenyl)thiourea
Shashidhar,aVijay
Thiruvenkatam,b
S. A. Shivashankar,aM. B. Hallic
and T. N. Guru Rowb*
aMaterials Research Centre, Indian Institute of
Science, Bangalore 560 012, Karnataka, India,
bSolid State and Structural Chemistry Unit,
Indian Institute of Science, Bangalore 560 012, Karnataka, India, andcDepartment of
Chemistry, Gulbarga University, Gulbarga 585106, Karnataka, India
Correspondence e-mail: ssctng@sscu.iisc.ernet.in
Key indicators Single-crystal X-ray study
T= 290 K
Mean(C–C) = 0.003 A˚
Rfactor = 0.054
wRfactor = 0.127
Data-to-parameter ratio = 12.5
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 23 February 2006 Accepted 14 March 2006
#2006 International Union of Crystallography All rights reserved
In the crystal structure of the title compound, C15H16N2O2S,
the The C S group lies on a mirror plane. The molecules are packed in a centrosymmetric manner through intermolecular N—H S hydrogen bonds.
Comment
Compounds containing the thiourea unit show strong anti-fungal and antibacterial activity and find applications in medicine and agriculture (Lin et al., 2004). The title compound, (I), crystallized in the space groupPnmawithZ= 4. The C S group lies on a mirror plane and thus there is one half-molecule in the asymmetric unit.
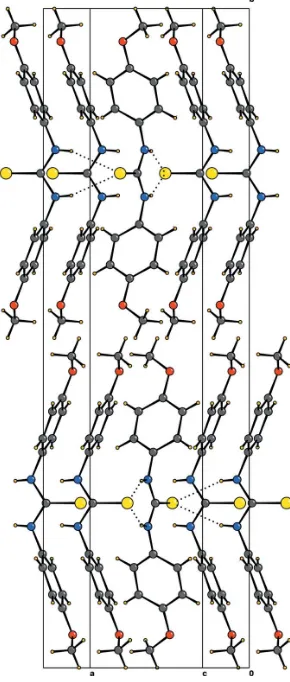
The molecule of (I) is shown in Fig. 1 and a packing diagram is shown in Fig. 2. The dihedral angle between the planes formed by N1/C1/S1 and C2–C7 is 90.26 (1), resulting in a
bent ‘butterfly-like’ molecular shape. The molecules are arranged parallel to each other and extend along the longb axisviapurely due to purely van der Waals interactions. Two well defined N—H S hydrogen bonds involving the S atom lying on the mirror plane generate the packing motif in theac plane [N—H S: N S = 3.551 (3) A˚ ]. As a result, alternate molecules generated by the glide planes are held perpendi-cular to each other, as shown in Fig. 2.
Experimental
To a solution of p-anisidine (37 g), carbon disulfide (24 ml) and rectified spirit (40 ml) kept at 283–287 K, a small amount of aqueous ammonia (41 ml) was added, accompained by constant shaking. The intermediate thiocarbamate was recovered and washed with small amounts of diethyl ether. It was subjected to steam distillation after adding lead nitrate and water. The compound which separated was further distilled and crystallized. The NMR data for the compound have been reported previously (Natarajanet al., 2005).
Crystal data
C15H16N2O2S
Mr= 288.37 Orthorhombic,Pnma a= 8.427 (5) A˚
b= 31.628 (19) A˚
c= 5.292 (3) A˚
V= 1410.3 (14) A˚3
Z= 4
Dx= 1.358 Mg m 3
MoKradiation Cell parameters from 732
reflections
= 2.6–27.4
= 0.23 mm1
Data collection
Bruker SMART CCD area-detector diffractometer
’and!scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996)
Tmin= 0.903,Tmax= 0.923
11344 measured reflections
1572 independent reflections 1433 reflections withI> 2(I)
Rint= 0.022
max= 27.4
h=10!10
k=40!39
l=6!6 Refinement
Refinement onF2
R[F2> 2(F2)] = 0.054
wR(F2) = 0.127
S= 1.21 1572 reflections 126 parameters
All H-atom parameters refined
w= 1/[2(F
o2) + (0.0502P)2
+ 0.7475P]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001
max= 0.33 e A˚
3
min=0.19 e A˚
[image:2.610.43.299.70.144.2] [image:2.610.366.511.76.417.2]3
Table 1
Selected geometric parameters (A˚ ,).
S1—C1 1.693 (3) O1—C5 1.375 (3) O1—C8 1.417 (4)
N1—C1 1.339 (2) N1—C2 1.431 (3)
C5—O1—C8 117.5 (2) C1—N1—C2 124.68 (18) N1—C1—N1i 114.7 (2) S1—C1—N1 122.66 (12)
N1—C2—C3 119.41 (19) N1—C2—C7 120.56 (18) O1—C5—C6 124.4 (2) O1—C5—C4 115.5 (2)
Symmetry code: (i)x;yþ1 2;z.
Table 2
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
N1—H1 S1ii
0.80 (3) 2.79 (3) 3.551 (3) 160 (2)
Symmetry code: (ii)xþ1 2;yþ
1 2;zþ
1 2.
All the H atoms were located and refined isotropically. The C—H bond lengths are 0.89 (3)–1.05 (3) A˚ and the N—H bond length is 0.80 (3) A˚ .
Data collection:SMART(Bruker, 1998); cell refinement:SMART; data reduction: SAINT (Bruker, 1998); program(s) used to solve structure: SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
ORTEP-3 for Windows(Farrugia, 1997) andCAMERON(Watkinet al., 1993); software used to prepare material for publication:
PLATON(Spek, 2003).
We thank the Department of Science and Technology, India, for use of the CCD facility set up under the IRHPA– DST programme at IISc.
References
Bruker (1998).SMART(Version 5.628) andSAINT(Version 6.02). Bruker AXS Inc., Madison, Wisconsin, USA.
Farrugia, L. J. (1997).J. Appl. Cryst.30, 565.
Lin, Q., Zhang, Y.-M., Wei, T.-B. & Wang, H. (2004).Acta Cryst.E60, o580– o582.
Natarajan, A., Guo, Y.-H., Arthanari, H., Wagner, G., Halperin, J. A. & Chorev, M. (2005).J. Org. Chem.70, 6362–6368.
Sheldrick, G. M. (1996).SADABS. University of Gottingen, Germany. Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of
Gottingen, Germany.
Spek, A. L. (2003).J. Appl. Cryst.36, 7–13.
Watkin, D. M., Pearce, L. & Prout, C. K. (1993). Chemical Crystallography Laboratory, University of Oxford, England.
Figure 1
View of the molecular structure of (I), showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 50% probability level. H atoms are shown as small spheres of arbitrary radii. [Symmetry code: (a)x,1
2y,z.]
Figure 2
[image:2.610.42.298.436.508.2]supporting information
sup-1 Acta Cryst. (2006). E62, o1518–o1519
supporting information
Acta Cryst. (2006). E62, o1518–o1519 [https://doi.org/10.1107/S1600536806009433]
1,3-Bis(4-methoxyphenyl)thiourea
Shashidhar, Vijay Thiruvenkatam, S. A. Shivashankar, M. B. Halli and T. N. Guru Row
1,3-Bis(4-methoxyphenyl)thiourea
Crystal data
C15H16N2O2S Mr = 288.37
Orthorhombic, Pnma
Hall symbol: -P 2ac 2n
a = 8.427 (5) Å
b = 31.628 (19) Å
c = 5.292 (3) Å
V = 1410.3 (14) Å3 Z = 4
F(000) = 608
Dx = 1.358 Mg m−3 Melting point: 202 K
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 732 reflections
θ = 2.6–27.4°
µ = 0.23 mm−1 T = 290 K Prism, colourless 0.45 × 0.40 × 0.35 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996)
Tmin = 0.903, Tmax = 0.923
11344 measured reflections 1572 independent reflections 1433 reflections with I > 2σ(I)
Rint = 0.022
θmax = 27.4°, θmin = 2.6° h = −10→10
k = −40→39
l = −6→6
Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.054 wR(F2) = 0.127 S = 1.21 1572 reflections 126 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
All H-atom parameters refined
w = 1/[σ2(F
o2) + (0.0502P)2 + 0.7475P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001 Δρmax = 0.33 e Å−3 Δρmin = −0.19 e Å−3
Special details
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
S1 0.30058 (9) 0.25000 0.61989 (16) 0.0457 (3) O1 0.3347 (2) 0.05014 (5) 0.5625 (4) 0.0607 (6) N1 0.5326 (2) 0.21436 (5) 0.3520 (4) 0.0451 (6) C1 0.4633 (3) 0.25000 0.4324 (5) 0.0369 (8) C2 0.4809 (2) 0.17261 (6) 0.4159 (4) 0.0388 (6) C3 0.3743 (3) 0.15207 (7) 0.2602 (5) 0.0452 (7) C4 0.3264 (3) 0.11132 (7) 0.3169 (5) 0.0487 (7) C5 0.3881 (3) 0.09076 (6) 0.5254 (4) 0.0427 (6) C6 0.4958 (3) 0.11110 (7) 0.6804 (4) 0.0458 (7) C7 0.5404 (3) 0.15240 (7) 0.6258 (4) 0.0449 (7) C8 0.4002 (4) 0.02715 (9) 0.7674 (7) 0.0700 (10) H1 0.603 (3) 0.2163 (8) 0.251 (5) 0.054 (7)* H3 0.336 (3) 0.1655 (8) 0.120 (5) 0.053 (7)* H4 0.257 (3) 0.0981 (8) 0.219 (5) 0.061 (7)* H6 0.536 (3) 0.0990 (8) 0.825 (5) 0.053 (7)* H7 0.614 (3) 0.1661 (8) 0.730 (5) 0.050 (6)* H8A 0.377 (4) 0.0421 (10) 0.940 (6) 0.084 (10)* H8B 0.518 (4) 0.0246 (10) 0.754 (6) 0.086 (10)* H8C 0.354 (4) 0.0004 (10) 0.765 (6) 0.077 (9)*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
S1 0.0436 (4) 0.0384 (4) 0.0551 (5) 0.0000 0.0144 (3) 0.0000 O1 0.0656 (11) 0.0357 (8) 0.0807 (12) −0.0093 (7) −0.0080 (9) 0.0056 (8) N1 0.0434 (10) 0.0353 (9) 0.0567 (11) −0.0005 (7) 0.0165 (9) −0.0012 (8) C1 0.0370 (14) 0.0353 (14) 0.0384 (14) 0.0000 −0.0014 (12) 0.0000 C2 0.0382 (10) 0.0297 (9) 0.0485 (11) 0.0016 (8) 0.0092 (9) −0.0025 (8) C3 0.0455 (12) 0.0395 (11) 0.0507 (12) 0.0061 (9) −0.0036 (10) 0.0018 (10) C4 0.0466 (12) 0.0406 (11) 0.0589 (14) −0.0044 (9) −0.0093 (11) −0.0064 (10) C5 0.0414 (11) 0.0318 (10) 0.0548 (12) −0.0003 (8) 0.0045 (10) −0.0024 (9) C6 0.0492 (12) 0.0440 (12) 0.0441 (12) −0.0026 (10) −0.0010 (10) 0.0051 (9) C7 0.0463 (12) 0.0434 (11) 0.0451 (12) −0.0081 (9) −0.0001 (10) −0.0043 (9) C8 0.084 (2) 0.0420 (13) 0.084 (2) −0.0078 (14) 0.0002 (17) 0.0162 (14)
Geometric parameters (Å, º)
supporting information
sup-3 Acta Cryst. (2006). E62, o1518–o1519
N1—C1 1.339 (2) C4—H4 0.89 (3) N1—C2 1.431 (3) C6—H6 0.92 (3) N1—H1 0.80 (3) C7—H7 0.93 (3) C2—C7 1.376 (3) C8—H8A 1.05 (3) C2—C3 1.381 (3) C8—H8B 1.00 (3) C3—C4 1.384 (3) C8—H8C 0.93 (3) C4—C5 1.382 (3)
S1···C3 3.688 (3) C7···H3vii 2.84 (3) S1···C7 3.690 (3) C8···H6 2.56 (3) S1···C3i 3.688 (3) C8···H8Bx 3.09 (3) S1···C7i 3.690 (3) H1···S1xi 2.79 (3) S1···H1ii 2.79 (3) H1···S1vii 2.79 (3) S1···H7iii 3.19 (3) H1···C3vii 3.06 (3) S1···H7iv 3.19 (3) H1···H1i 2.13 (4) S1···H1v 2.79 (3) H3···C2v 3.01 (3) O1···H8Cvi 2.75 (3) H3···C7v 2.84 (3) C2···C3vii 3.504 (4) H4···H8Aviii 2.52 (4) C3···C2v 3.504 (4) H4···C6v 3.08 (3) C3···S1 3.688 (3) H6···C8 2.56 (3) C3···C6viii 3.485 (4) H6···H8A 2.32 (4) C3···C7v 3.477 (4) H6···H8B 2.39 (4) C6···C3ix 3.485 (4) H6···C5xii 3.08 (3) C7···C3vii 3.477 (4) H7···S1xiii 3.19 (3) C7···S1 3.690 (3) H7···S1xii 3.19 (3) C8···C8x 3.441 (5) H8A···C4ix 2.99 (3) C2···H3vii 3.01 (3) H8A···C6 2.77 (3) C3···H1v 3.06 (3) H8A···H4ix 2.52 (4) C4···H8Aviii 2.99 (3) H8A···H6 2.32 (4) C5···H6iii 3.08 (3) H8B···C6 2.77 (3) C6···H4vii 3.08 (3) H8B···H6 2.39 (4) C6···H8B 2.77 (3) H8B···C8x 3.09 (3) C6···H8A 2.77 (3) H8C···O1xiv 2.75 (3)
C4—C5—C6 120.12 (19) H8B—C8—H8C 110 (3) C5—C6—C7 119.5 (2)
C8—O1—C5—C4 177.2 (2) C7—C2—C3—C4 0.6 (3) C8—O1—C5—C6 −2.9 (4) C3—C2—C7—C6 1.0 (3) C1—N1—C2—C3 91.8 (3) C2—C3—C4—C5 −1.6 (4) C1—N1—C2—C7 −90.3 (3) C3—C4—C5—O1 −179.0 (2) C2—N1—C1—S1 −1.0 (3) C3—C4—C5—C6 1.1 (4) C2—N1—C1—N1i 179.2 (2) O1—C5—C6—C7 −179.5 (2) N1—C2—C7—C6 −176.8 (2) C4—C5—C6—C7 0.5 (4) N1—C2—C3—C4 178.5 (2) C5—C6—C7—C2 −1.5 (4)
Symmetry codes: (i) x, −y+1/2, z; (ii) x−1/2, −y+1/2, −z+1/2; (iii) x−1/2, y, −z+3/2; (iv) x−1/2, −y+1/2, −z+3/2; (v) x−1/2, y, −z+1/2; (vi) −x+1/2, −y,
z−1/2; (vii) x+1/2, y, −z+1/2; (viii) x, y, z−1; (ix) x, y, z+1; (x) −x+1, −y, −z+2; (xi) x+1/2, −y+1/2, −z+1/2; (xii) x+1/2, y, −z+3/2; (xiii) x+1/2, −y+1/2, −z+3/2; (xiv) −x+1/2, −y, z+1/2.
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
N1—H1···S1xi 0.80 (3) 2.79 (3) 3.551 (3) 160 (2) N1—H1···S1vii 0.80 (3) 2.79 (3) 3.551 (3) 160 (2)