FORMULATION DEVELOPMENT AND IN VITRO EVALUATION OF PULSATILE DRUG DELIVERY SYSTEM USING NOVEL NATURAL SUPERDISINTEGRANT
Full text
Figure
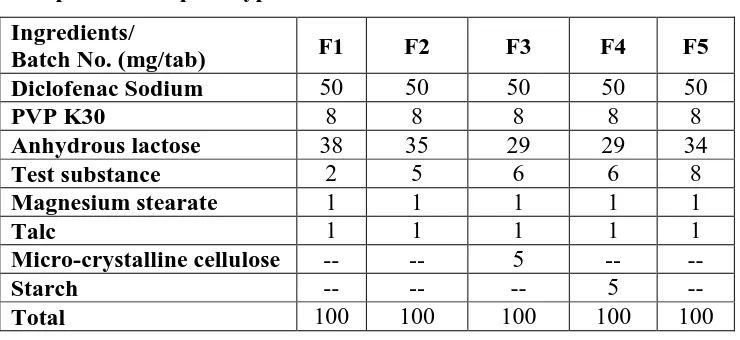
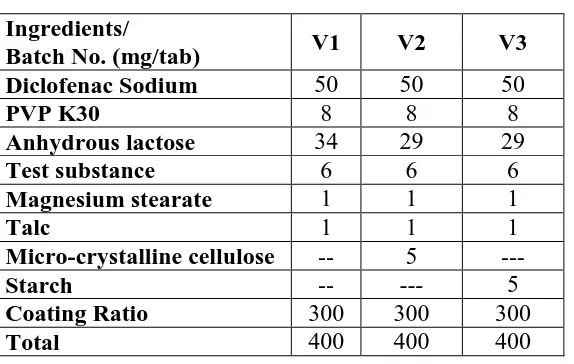
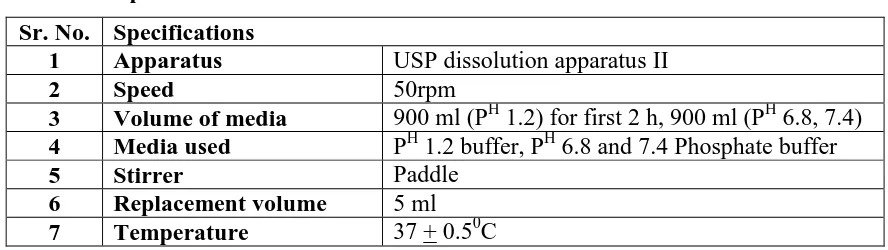
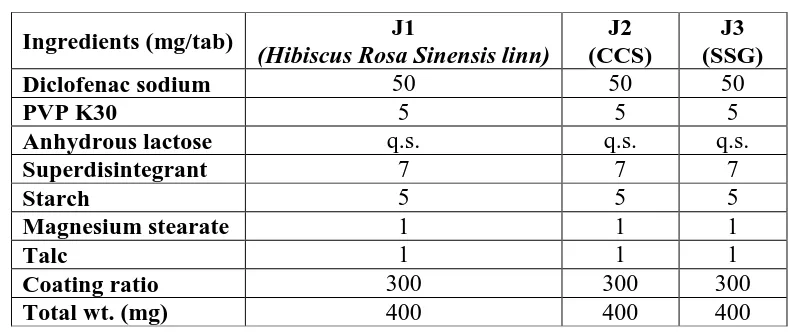
Related documents
The cross-border dimension of these M&A operations is a key indicator of the integration process of the euro-area banking sector, which is fundamental to the integration
Proposed study suggests that system quality, information quality, service quality, usefulness, citizens’ satisfaction, citizens’ trust and perceived e-government
Taken together, after PH CD1d-/- mice previously exposed to ConA, exhibited significantly lower IL-6 levels compare to wt mice, these results correlate with reduced liver regen-
Analysis of the partial E1 gene sequences of 19 represen- tative strains of each genotype of CHIKV published in GenBank, including sequences of three representative strains of
DAVID: the Database for Annotation, Visualization and Integrated Discovery; FDR: False discovery rate; GO: Gene Ontology; GS: Gene significance; HBV: Chronic hepatitis B virus;
In this present work, a one dimensional simulation program called a analysis of microelectronic and photonic structures (AMPS-1D) [3] is used to simulate the
Two methods are available for emergency contraception, namely emergency contraception pills (ECPs) and copper-bearing intrauterine devices (Cu-IUDs). There are two regimens of
Keywords: Body Sensor Network, Dynamic Transmission Threshold, Energy