FORMULATION AND EVALUATION OF MICROSPHERES CONTATING LAMAVUDINE
Full text
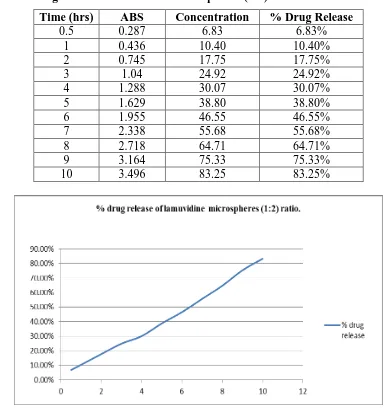
Figure



Related documents
In the great majority of individuals with classical form and preserved speech variant there was a mutation in the MECP2 gene with a correlation between clinical evaluation and
German MNCs global CPS adoption, globalization induced internal shifts in competence allocation and new lower wage capacity subcontractors inside Germany all pose interesting
The finding that the 19- to 24-year-old group was divergent from the general population was reexamined by rerunning the analysis with the inclusion of a second cohort of 212
The use of this approach in vaccinology, further enhanced by advances in immune ontogeny and human in vitro culture systems as well as in adjuvantation and formulation science,
The performance and robustness of the 13-plex dLIA were monitored over time beyond validation through the quality control samples (QCS), which are included on each assay plate
The following research paper is based on gaining an in-depth understanding of Cross-cultural mistakes by renowned brands for evaluating the success and failures of brands in
D-Dimer plasma levels parallel the clinical response to omalizumab in patients with severe chronic spontaneous urticaria.. Int Arch