BACTERIAL ENDOTOXIN TEST FOR CERTAIN
PHARMACEUTICAL COMPOUNDS
P.Jyothi Chaitanya, R.Chandrashekar, and N.Lakshmi Bhavani*
Department of Botany, University College of science, Saifabad, Osmania University, Hyderabad, Andhra Pradesh, India-5000004.
ABSTRACT
Endotoxins are Lipopopolysaccharide 1complexs associated with the cell wall of the gram negative bacteria such as E.coli, Salmonella, Pseudomonas, Heamophilus and other pathogens. Antibiotics with endotoxin contamination can cause pyrogenic effects in patients when treated with these antibiotics. In order to prevent the side effects of the endotoxin contamination antibiotics have to be thoroughly tested for endotoxins. LAL method is one of the endotoxin estimating method that is used in some pharmaceutical industries to test their end products for free of endotoxins. In bulk sterilization process of drugs in pharmaceutical industries, LAL method is the method for detection of endotoxins that is widely used to test antibiotics. In present investigation products like the pharmaceutical compounds cloxillin sodium sterile, sulbactum sodium sterile, ceftriaxone sodium sterile, piperacillin sodium sterile, amoxicillin sodium sterile are tested for the endotoxins,
KEY WORDS: Endotoxins, Gram-negative bacteria, Pharmaceutical compounds.
INTRODUCTION
Endo toxins are cell associated bacterial toxins. Which are on the outer membrane of the cell walls of the gram-ve bacteria such as E.coli, salmonella, pseudomonas etc. the biological activity of endotoxin is associated with the lipopolysaccharide in which toxicity is due to the lipid content (lipid A) and immunogenecity is associated with the polysaccharide
components. These endotoxins elicits a variety of inflammatory responses in man as well as human being as it activates the alternative pathways which is the part of the pathology of
Volume 2, Issue 4, 950-958. Research Article ISSN 2277 – 7105
Article Received on 15 April 2013,
Revised on 02 May 2013, Accepted on 20 June 2013
*Correspondence for Author:
N.Lakshmi Bhavani
Department of Botany,
University College of science,
Saifabad, Osmania University,
Hyderabad, Andhra Pradesh,
India
jyothichaitanya1983@gmai l.com
gram –ve bacterial infections. Endotoxins are heat stable but can be degraded by using certain powerful oxidizing agents such as superoxide, peroxide, and hypochlorite.
The chemical structure of endotoxin has been derived from the species salmonella and E.coli with the extraction from whole cells by treating with 45% phenol at 90ºc. Mild hydrolysis of LPS yields lipid A+ polysaccharides. LPS generally consists of three components or regions i, e. Region 1 Lipid A, Region 2 core antigen or R polysaccharide and Region 3 somatic antigen or O polysaccharide.
Endotoxins are toxic to most of the mammals and regardless of bacterial source all endotoxins produce the same range of biological effects in the animal host. The injection of living or killed gram –ve cells or purified LPS into experimental animals causes a wide spectrum of non specific pathophysiological reactions such as fever, changes in white blood cell counts, tumor necrosis, hypotension, shock lethality. The physiological activities of endotoxins are mediated mainly by the Lipid A component of LPs that can stimulate the mammalian immune system.
The pharmaceutical compounds tested are Cloxacillin sodium sterile, ceftriaxone sodium sterile, piperacillin sodium sterile, sulbactum sodium sterile, Amoxicillin sodium sterile. Cloxacillin sodium sterile is a semi synthetic broad spectrum penicillin and is a resistant and acid stable.
Ceftriaxone sodium sterile is semi synthetic broad spectrum cephalosporin antibiotic for intravenous intramascular administration is penetrated into the inflamed meninges of infants and pediatric patients. It has the high stability in presence of the β lactums. Bactericidal activity results from inhibition of cellwall synthesis.
Piperaxicillin sodium sterile is an antibiotic and a semi synthetic broad spectrum pencillin. It penetrates cerebral spinal fluid in the presence of inflamed meninges.
Sulbactum sodium sterile is an antibiotic and a semi synthetic broad spectrum pencillin is an inhibitor of β lactamase enzyme in microorganisms for intravenous and intra mascular administration.
MATERIALS AND METHODS
Lal method is applied for estimating concentration of bacterial endotoxins that may be present or not. Determination of end point is made with dilutions from the material under test in direct comparison with parallel dilutions of reference endotoxins and qualities of endotoxins are expressed in defined endotoxin units.
SPECIAL REAGENTS
The reference standard endotoxins (RSE) are the freeze dried endotoxins from E.coli which are maintained by the central drug laboratory, Calcutta or any other suitable position. These are the reference standards that have the potency of 1000 USP endotoxin units (EU) per vial. The entire contents of one vial of the RSE and 5 ml of LAL reagent water are mixed thoroughly for 30 minutes using vortex mixer. This concentrate is refrigerated for making subsequent dilutions. Before every use the solution is mixed with vortex mixer for about 3 minutes before use. Do not store dilutions not more than 14 days because of loss of activity, by absorption in absence of supporting data.
The control standard endotoxins (CSE) are endotoxins preparation other than RSE. It has been standardized each new lot of CSE prior to use in the test. Calibration of a CSE in terms of the RSE must be specific.
LAL (limulus amoebocyte lysate) is developed with the innovative work of john Hopkins university investigators. Frederick bang observed that bacteria caused intravascular coagulation in the American horse shoe crab, limulus poly phenus. Lal has been prepared and characterized for use as a reagent for gel clot formation.
pippetes and disposable tips,incubate device(heating block set to 37˚C+/-1˚C, Timer, racks, Parafilm “M” or aluminium foil, Endotoxin free buffer, PH meter, weighing balance.
To the 50µl of the test sample (already prepared as 2λ dilution) add 50µl of PFW and to that
add 100µl of LAL is added to respective reaction tubes. Apart from these CSE also prepared
as a control with to the 100µl of CSE add 100µl of reconstituted LAL reagent.CSE tube acts
as a control to the test. Using calibrated mechanical pipettor fitted with a disposable tip all the prepared tubes are shaken to assure mixing of sample and lysate. Then the tubes inserted into the heating block where they will incubate temperature is set to 37˚C for 60 minutes. The temperature and the incubation period are recorded.
RESULTS AND DISSCUSSION
After incubation period tubes are removed from the heating block one at a time smoothly rotating at 180˚ direction. A positive test defined as a clot that maintain its integrity with inversion of the test tube and also as a visible increase in turbidity or viscosity of the contents in the tube.
Antibiotics with endotoxin contaminations can cause pyrogenic effects in patients treated with these antibiotics. In order to prevent the side effects the antibiotics have to be thoroughly tested for endotoxin contamination. Previously pyrogen test in rabbits was used to estimate endotoxin contaminations which is a time taking one. Now a days Limulus amoebocyte lysate is used as a test for endotoxin detection in pharmaceutical industries. From the present investigation it is found that the limulus amoebocyte lysate test is very accurate and sensitive in bulk drug sterilization of antibiotics. The result is known within two hours. The negative result indicates that there is no endotoxin contamination in the antibiotics tested. Filter sterilization through 0.2µ filters is very effective to control endotoxin contamination to certain extent as an aseptic measure in the industry.
Table 1: Bacterial endotoxin test report of cloxicillin sodium sterile
Endotoxin limit: 0.04EU/mg 2×MVC= 0.190 mg/ml
S.NO Product and PPC
where
λ=0.03EU/ml
results Acceptance
criteria
Test Duplicate Test Duplicate
1 Product - - - -
3 PPC + + + +
CONTROL STANDARD ENDOTOXIN DILUTIONS
“+” gel present, “-“ no gel, PPC: product positive control MVC: Minimum Valid Concentration EU: Endotoxin Units PFW: Pyrogen Free Water λ: Sensitivity of LAL LAL: Limulus Amoebocyte Lysate
Table 2: Bacterial endotoxin test report of Ceftriaxone sodium sterile
Endotoxin limit: 0.2EU/mg 2×MVC= 0.35 mg/ml
S.NO Product and PPC
where
λ=0.03EU/ml
Results Acceptance
criteria
Test Duplicate Test Duplicate
1 Product - - - -
2 Negative(PFW) - - - -
3 PPC + + + +
CONTROL STANDARD ENDOTOXIN DILUTIONS
4 0.06EU/ml - 2λ + + + +
5 0.03EU/ml – λ + + + +
6 0.015EU/ml – λ/2 _ _ +/- +/-
7 0.0075EU/ml – λ/4 _ _ _ _
“+” gel present, “-“no gel, PPC: product positive control MVC: Minimum Valid Concentration EU: Endotoxin Units PFW: Pyrogen Free Water λ: Sensitivity of LAL LAL: Limulus Amoebocyte Lysate
4 0.06EU/ml - 2λ + + + +
5 0.03EU/ml – λ + + + +
6 0.015EU/ml – λ/2 _ _ +/- +/-
Table 3: Bacterial endotoxin test report of Piperacillin sodium sterile
Endotoxin limit: 0.07 EU/mg 2×MVC= 0.96 mg/ml
S.NO Product and PPC
where
λ=0.03EU/ml
results Acceptance
criteria
Test Duplicate Test Duplicate
1 Product - - - -
2 Negative(PFW) - - - -
3 PPC + + + +
CONTROL STANDARD ENDOTOXIN DILUTIONS
4 0.06EU/ml - 2λ + + + +
5 0.03EU/ml – λ + + + +
6 0.015EU/ml – λ/2 _ _ +/- +/-
7 0.0075EU/ml – λ/4 _ _ _ _
“+” gel present, “-“no gel, PPC: product positive control MVC: Minimum Valid Concentration EU: Endotoxin Units PFW: Pyrogen Free Water λ : Sensitivity of LAL
LAL: Limulus Amoebocyte Lysate
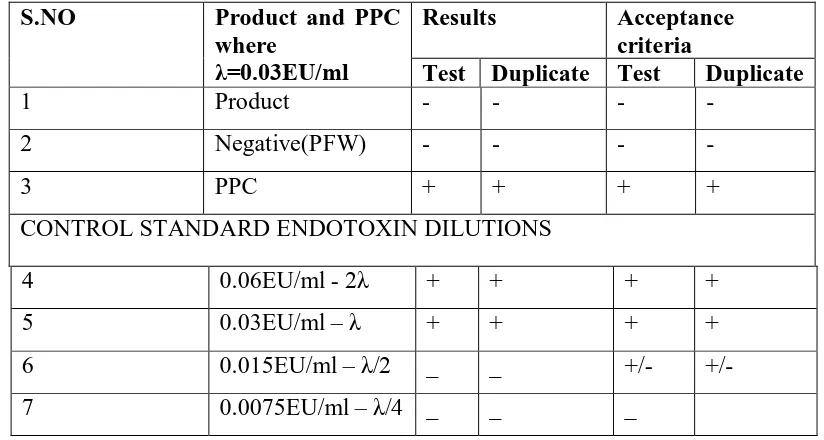
Table 4 : Bacterial endotoxin test report of Sulbactum sodium sterile
Endotoxin limit: 0.171 EU/mg 2×MVC= 0.4 mg/ml
S.NO Product and PPC
where
λ=0.03EU/ml
Results Acceptance
criteria
Test Duplicate Test Duplicate
1 Product - - - -
2 Negative(PFW) - - - -
3 PPC + + + +
CONTROL STANDARD ENDOTOXIN DILUTIONS
4 0.06EU/ml - 2λ + + + +
5 0.03EU/ml – λ + + + +
6 0.015EU/ml – λ/2 _ _ +/- +/-
[image:6.595.107.519.539.759.2]“+” gel present, “-“no gel, PPC: product positive control MVC: Minimum Valid Concentration EU: Endotoxin Units
PFW : Pyrogen Free Water λ : Sensitivity of LAL
LAL : Limulus Amoebocyte Lysate
Table 5: Bacterial endotoxin test report of Amoxycillin sodium sterile
Endotoxin limit: 0.25EU/mg 2×MVC= 0.31 mg/ml
S.NO Product and PPC
where
λ=0.03EU/ml
results Acceptance
criteria
Test Duplicate Test Duplicate
1 Product - - - -
2 Negative(PFW) - - - -
3 PPC + + + +
CONTROL STANDARD ENDOTOXIN DILUTIONS
4 0.06EU/ml - 2λ + + + +
5 0.03EU/ml – λ + + + +
6 0.015EU/ml – λ/2 _ _ +/- +/-
7 0.0075EU/ml – λ/4 _ _ _
“+” gel present, “-“no gel, PPC: product positive control MVC: Minimum Valid Concentration EU: Endotoxin Units
PFW : Pyrogen Free Water λ : Sensitivity of LAL
LAL: Limulus Amoebocyte Lysate
CONCLUSION
tested. Previously pyrogen test in rabbits was used to estimate endotoxin contaminations which is a time taking one. In recent days Limulus amoebocyte lysate (LAL) is used as an effective test for endotoxin detection in pharmaceutical industries inorder to prevent the side effects of the endotoxins contaminated. We conclude in the present study that antibiotics like cloxillin sodium sterile, sulbactum sodium sterile, ceftriaxone sodium sterile, piperacillin sodium sterile, amoxicillin sodium sterile are tested for thier endotoxins contamination. Among all the products tested the tubes of PPC product positive control and CSE control standard endotoxin dilutions showed the positive result.
ACKNOWLDEMENTS
The author expresses sincere thanks to Department of Microbiology, KAVITHA MEMORIAL DEGREE AND PG COLLEGE , Khammam.
We acknowledge Dr.D.Vijaya Lakshmi HOD, Department of microbiology KAVITHA MEMORIAL DEGREE AND PG COLLEGE Khammam for her valuable guidance.
REFERENCES
1. Twohy, C. W., A. P. Duran, and T. E. Munson. 1984. Endotoxin contamination of parenteral drugs and radiopharmaceuticals as determined by the Limulus amebocyte lysate method. J.Parenter.Sci.Technol. 38:190 201.
2. M. E. Dawson. 1995. Maximum Valid Dilution and Minimum Valid Concentration. LAL Update, 13 (4), 1-3.
3. Bacterial Endotoxins Test. Chapter 85. USP 28, 2264-2267, United States Pharmacopeial Convention, Rockville, MD.
4. Endotoxins Test, European Pharmacopoeia 5, chapter 2.6.14, 161-168, 5. Endotoxins Test, Japanese Pharmacopoeia XIV, chapter 6, 20-23.
6. "Guideline on Validation of the Limulus Amebocyte Lysate test as an End-Product Endotoxin Test for Human and Animal Parenteral Drugs, Biological Products, and Medical Devices" US, Food and Drug Administration, 1987.
7. M. E. Dawson. 1996. Preliminary Testing. LAL Update, 14 (1), 1-5.
8. Roth, R.I., F.C. Levin, and J. Levin. 1990. Optimization of the detection of bacterial endotoxin in plasma with the Limulus test.J. Lab. Clin. Med. 116:153-161.
10. Roslansky, P. F., Dawson, M. E., and Novitsky, T. J. (1991) Plastics, Endotoxins, and the Limulus Amebocyte Lysate Test. J.Parenter. Sci. Technol. 45(2), 83- 87.
11. Piluso, L. G., and M. Y. Martinez. 1999. Resolving liposomal inhibition of Quantitative LAL Methods. J. Pharm. Sci. Technol.53 (5):260-263.
12. Yu S; Nakashima N; Xu BH; Matsuda T; Izumihara A; Sunahara N; Nakamura T; Tsukano M, and Matsuyama T. Pathological significance of elevated soluble CD14 production in rheumatoid arthritis: in the presence of soluble CD14, lipopolysaccharides at low concentrations activate RA synovial fibroblasts. Rheumat Internat. 1998; 17(6):237-43.