Original Article
Transferrin receptor monoclonal antibody exacerbates
curcumin-mediated apoptotic effect in castration
resistant prostate cancer cells
Chunguang Yang1*, Zhiquan Hu1*, Zhihua Wang1, Shuanglin Liu2, Xing Zeng1, Ye Wang1, Zhangqun Ye1,
Guanxin Shen3
1Department of Urology, Tongji Hospital Affiliated Tongji Medical College of Huazhong University of Science and
Technology (HUST), Wuhan, P. R. China; 2Department of Urology, No. 1 Hospital of Wuhan City, Wuhan, P. R. China; 3Department of Immunology, Tongji Medical College of Huazhong University of Science and Technology (HUST),
Wuhan, P. R. China. *Equal contributors.
Received December 25, 2015; Accepted March 12, 2016; Epub May 1, 2016; Published May 15, 2016
Abstract: Curcumin is a biologically active iron chelator and induces cytotoxicity in a series of cell lines. Whereas anti-human transferrin receptor monoclonal antibody (TfR-mAb), which can specifically recognize the outer segment of TfR and result in iron deprivation of tumor cells, has been proved to synergize the antitumor effect of many che-motherapeutic drugs. Thus the aim of this study was to investigate the apoptosis inducing activity of curcumin alone or in combination with TfR-mAb in castration resistant prostate cancer (CRPC) PC3 cells. Here we demonstrated that curcumin induces apoptosis in PC3 cells, as assessed by flow cytometry, TUNEL assay and caspase activation. Combining curcumin with TfR-mAb, synergizes the apoptotic effect of curcumin. At apoptotic inducing concentration, curcumin evaluates the expression of TfR1 and IRP1, but inhibits AKT phosphorylation in PC3 cells. Furthermore, iron deprivation and AKT phosphorylation inhibition effect of curcumin was enhanced by TfR-mAb. All together, our results suggest that TfR-mAb enhances the apoptosis inducing activity of curcumin in CRPC cells, iron deprivation and AKT phosphorylation inhibition are potential mechanisms.
Keywords: Curcumin, transferring receptor monoclonal antibody, castration resistant prostate cancer, apoptosis, iron deprivation
Introduction
Prostate carcinoma is still the most common cancer and the second leading cause of can-cer-related death in American males [1]. Ad- vanced prostate cancer responses to castra-tion therapy on average 14-20 months with eventual progression to hormone-refractory disease named as castration resistant pros-tate cancer (CRPC) [2]. CRPC carries a worse prognosis and has a mean survival time of 16-18 months from the beginning of progres-sion [3]. Systemic therapies have been an option in CPRC treatment, but chemotherapy is not well tolerated in the management to these patients [4], and therefore, new and effective therapies against CRPC are urgently needed. Derived from the roots of Curcuma longa, Curcumin possesses anticancer,
anti-inflam-matory, antioxidative and antimicrobial proper-ties [5]. Studies have shown that curcumin induces caspase-related apoptosis in both androgen-dependent and castration refractory prostate cancer cells [6, 7]. Moreover, chemical and biological experiments have shown that curcumin is a biologically active iron chelator and induces cytotoxicity through iron chelation [8, 9]. These findings indicate that curcumin may induce apoptosis in CRPC cells through its iron chelating properties, and if so, combina-tion therapy with iron deprivacombina-tion agents may enhance its efficacy while minimizing its toxi- city.
fer-TfR-mAb exacerbates curcumin-induced apoptosis
ritin and TfR1 mRNA. The IRE-IRP interactions inhibit the translation of ferritin mRNA but sta-bilize TfR1 mRNA, thereby inhibiting ferritin syn-thesis, but enhancing the expression of TfR1. Therefore, the expression levels of TfR1, ferri-tin, and IRP1 can reflect the intracellular status of iron. Of note, TfR has been used as a target for antibody-based cancer therapy [12], in which anti-TfR antibodies (TfR-mAb) can spe-cifically recognize the outer segment of TfR leading to iron deprivation of tumor cells [13]. Importantly, TfR-mAb has been recognized to synergize with antitumor drugs deferoxamine, nimustine and sinomenine hydrochloride in studies of cancer cell lines [14-16]. Therefore, combination therapy of TfR-mAb with antitumor drugs could be an effective strategy in clinical settings. We, thus, in the present report, inves-tigated whether TfR-mAb could enhance cur-cumin-mediated apoptotic effect and its relat-ed underlying mechanisms. We found that TfR-mAb enhances curcumin induced apoptosis in CRPC cells, which involves iron deprivation along with attenuated AKT activity.
Materials and methods
Cell culture and reagents
The castration resistant prostate cancer cell line (PC3) was obtained from the American Type Culture Collection (ATCC, Rockville, MD). Cells were cultured in DMEM medium (GIBCO, USA) supplemented with 10% fetal calf serum (GIBCO, USA) at 37°C in a 5% CO2 humidified atmosphere. Curcumin (Sigma-Aldrich, USA) was dissolved in DMSO (Sigma-Aldrich, USA) at 50 mM. Antibodies against caspase 9, AKT, phosphor-AKT (Ser 473), β-actin (Santa Cruz, USA), and HRP-conjugated goat anti-rabbit IgG were purchased from Santa Cruz (Santa Cruz, CA, USA), while transferring receptor monoclo-nal antibody (TfR1) was generated and purified as previously described [17]). Antibodies for iron regulatory protein 1 (IRP1) and caspase 9 were obtained from Proteintech Group (Rose- mont, USA).
Assay for cell viability
Cell viability was assessed by MTT assay (Sigma-Aldrich, USA) according to the instruc-tion. PC3 cells were cultured in 96-well plates and incubated with different concentrations of
curcumin for 24 h, followed by analysis of the absorbance of each well at 570 nm wavelength. Cell viability was expressed as percentage of the control cells, and the experiments were conducted in triplicate.
Apoptosis assay
PC3 cells were placed in 12-well plates and treated with curcumin (50 μmol/L) alone or in combination with TfR-mAb (100 mg/L) for 24 h. After washes with ice-cold PBS, the cells were subjected to flow cytometry analysis (Becton-Dickinson LSR II) using an Annexin V-FITC apo- ptosis detection kit (Roche Diagnostics, Mann- heim, Germany).
TUNEL assay
The above prepared PC3 cells were also ana-lyzed for apoptosis by TUNEL assay (Roche Diagnostics, Germany). Briefly, the treated cells were fixed with 4% paraformaldehyde for 40 min at room temperature and permeabilized in 0.1% Triton X-100 in 0.1% sodium citrate for 2 min, followed by incubating with a mixture of TdT solution and fluorescein isothiocyanate dUTP solution at 37°C. The cells were finally were examined microscopically at 400× mag- nification.
Western blotting
The expression levels for TfR1, IRP1, caspase 3, caspase 9, AKT and pAKT 473 expression in PC3 cells were analyzed by Western blot analy-sis as previously described [18]. The density of a target band was quantified using the ImageJ software (Version 1.38×), and β-actin was served as a loading control. The relative expression of a target protein was expressed as a ratio with β-actin.
Statistical analysis
Results
Curcumin induces cytotoxicity and caspase-related apoptosis in PC3 cells
MTT assay revealed that curcumin induced cytotoxicity in PC3 cells (Figure 1A and 1C). Flow cytometry analysis indicated that curcum-in dose-dependently curcum-induced PC3 cells under-going apoptosis (Figure 1B). In line with this observation, the cleavage of caspase 3 and 9 was significantly increased in curcumin treated cells as determined by Western blot analysis (Figure 3C). TUNEL assay of above curcumin-treated cultures further confirmed that curcum-in curcum-induced apoptosis curcum-in PC3 cells (Figure 3D).
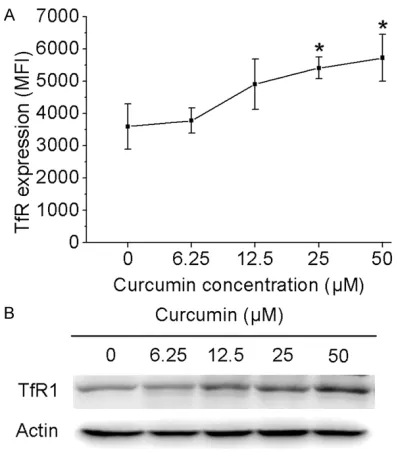
Curcumin up-regulates TfR1 expression in PC3 cells
[image:3.612.93.522.71.331.2]Next, we sought to address whether curcumin affects TfR1 expression in PC3 cells. For this purpose, we first examined the expression lev-els of TfR1 in curcumin treated tumor cells by flow cytometry as manifested by the mean fluo-rescence intensity (MFI). As shown in Figure
Figure 1.Curcumin induces cytotoxicity and apoptosis in PC3 cells. A, C. PC3 cells were incubated for 24 hours with various concentrations of curcumin. Survival rate of PC3 cells was determined by MTT method. Samples were ex-amined microscopically at 200× magnification. B. PC3 cells were treated with curcumin at different concentrations for 24 h, and the percentage of apoptotic cells was analyzed by flow cytometric analysis of Annexin V and PI double staining. Results are expressed as means ± S.E.M.
[image:3.612.90.289.414.641.2]TfR-mAb exacerbates curcumin-induced apoptosis
2A, TfR1 MFI in PC3 cells after 24 h of curcum-in treatment was significantly higher than that of control cells (P<0.05). To confirm this obser-vation, we conducted Western blotting for anal-ysis of TfR1 protein levels. Indeed, curcumin can significantly enhanced TfR1 expression in PC3 cells.
TfR-mAb exacerbates curcumin-mediated apoptosis in PC3 cells
Based on the above observation, a TfR1 block-ing monoclonal antibody (TfR-mAb, 100 mg/L) was next added into the cultures along with cur-cumin. Remarkably, addition of TfR-mAb signifi-cantly exacerbated curcumin-induced apopto-sis, and similarly, the activation of caspase 3 and 9 was significantly increased (Figure 3A,
3B and 3C). Indeed, fluorescence microscopy analysis revealed that the characteristic chang-es of apoptosis induced by curcumin were sig-nificantly exacerbated by the addition of TfR-mAb (Figure 3D).
TfR-mAb enhances curcumin-induced iron de-privation along with attenuated AKT signaling
[image:4.612.92.522.74.414.2]ated curcumin-induced TfR1 expression. In line with this observation, TfR-mAb was noted to synergize with curcumin to promote IRP1 acti-vation, indicating that TfR-mAb exacerbates curcumin-induced iron deprivation in PC3 cells. Recent studies have elucidated that iron chela-tors attenuate oncogenic p-AKT activity in pros-tate cancer as part of their anti-tumor activity [20]. We next examined AKT signaling and noted that addition of TfR-mAb significantly exacerbated curcumin-induced attenuation of AKT phosphorylation.
Discussion
Over the past several years, a number of nutri-tional supplements, such as soybean, garlic, green tea and vitamin D3, have been found to be beneficial for the prevention and treatment of prostate cancer [21]. In fact, some medical centers are now combining their conventional therapies with some form of alternative and complementary medicine, the most popular of which are nutritional modification and the use of herbal or other micronutrients [22].
Derived from the roots of Curcuma longa, Curcumin possesses anticancer, anti-inflam-matory, anti-oxidative and antimicrobial activi-ties [5]. Moreover, curcumin has been reported to induce apoptosis through intrinsic and extrin-sic pathways in several cancer cell lines, includ-ing prostate cancer cells [6, 7]. Similarly in our experimental approach, we found that curcum-in could curcum-induce caspase-related apoptosis curcum-in
PC3 cells, as assessed by flow cytometry, cas-pase activation and TUNEL assay (Figures 1B,
3C and 3D, respectively). Furthermore, we fo- und that at the concentration for induction of apoptosis, curcumin elevated the expression of TfR1 and IRP1 in PC3 cells, indicating that its impact on apoptosis in PC3 cells is associated with its iron chelating properties.
[image:5.612.94.522.71.231.2]TfR-mAb exacerbates curcumin-induced apoptosis
development of chemo-immunotherapy in pros-tate cancer.
In conclusion, we demonstrated that TfR-mAb synergizes with curcumin to promote PC3 cells undergoing apoptosis, which involves the de- privation of iron and the inhibition of AKT phosphorylation.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81101944 & No. 81173608). The authors thank Prof. Guan- xin Shen for the critical reading and editing of the manuscript. This work was also supported by Hubei Provincial Natural Science Foundation of China (No. 2015CFB239).
Disclosure of conflict of interest
None.
Address correspondence to: Dr. Zhihua Wang, Department of Urology, Tongji Hospital Affiliated Tongji Medical College of Huazhong University of Science and Technology (HUST), Wuhan, P. R. China. Tel: +86-13995574598; Fax: +86-27-83665368; E-mail: zhwang_hust@hotmail.com
References
[1] Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87.
[2] Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, Blumenstein BA, Davis MA, Goodman PJ. A controlled trial of leuprolide with and without flutamide in pros-tatic carcinoma. N Engl J Med 1989; 321: 419. [3] Harris WP, Mostaghel EA, Nelson PS,
Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of re-sistance and optimizing androgen depletion. Nat Clin Pract Urol 2009; 6: 76.
[4] Schweizer MT, Antonarakis ES. Chemotherapy and its evolving role in the management of ad-vanced prostate cancer. Asian J Androl 2014; 16: 334.
[5] Shanmugam MK, Rane G, Kanchi MM, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP, Sethi G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015; 20: 2728.
[6] Dorai T, Gehani N, Katz A. Therapeutic poten-tial of curcumin in human prostate cancer-I. curcumin induces apoptosis in both androgen-dependent and androgen-inandrogen-dependent
pros-tate cancer cells. Prospros-tate Cancer Prostatic Dis 2000; 3: 84.
[7] Yallapu MM, Khan S, Maher DM, Ebeling MC, Sundram V, Chauhan N, Ganju A, Balakrishna S, Gupta BK, Zafar N, Jaggi M, Chauhan SC. Anti-cancer activity of curcumin loaded na- noparticles in prostate cancer. Biomaterials 2014; 35: 8635.
[8] Bernabe-Pineda M, Ramirez-Silva MT, Ro- mero-Romo MA, Gonzalez-Vergara E, Rojas-Hernandez A. Spectrophotometric and electro-chemical determination of the formation con-stants of the complexes Curcumin-Fe(III)-water and Curcumin-Fe(II)-water. Spectrochim Acta A Mol Biomol Spectrosc 2004; 60: 1105-13. [9] Jiao Y, Wilkinson JT, Di X, Wang W, Hatcher H,
Kock ND, D’Agostino RJ, Knovich MA, Torti FM, Torti SV. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 2009; 113: 462. [10] Parenti R, Salvatorelli L, Magro G. Anaplastic
thyroid carcinoma: current treatments and po-tential new therapeutic options with emphasis on TfR1/CD71. Int J Endocrinol 2014; 2014: 685396.
[11] Kuhn LC. Iron regulatory proteins and their role in controlling iron metabolism. Metallomics 2015; 7: 232.
[12] Shen X, Zhu HF, He FR, Xing W, Li L, Liu J, Yang J, Pan XF, Lei P, Wang ZH, Shen GX. An anti-transferrin receptor antibody enhanced the growth inhibitory effects of chemotherapeutic drugs on human non-hematopoietic tumor cells. Int Immunopharmacol 2008; 8: 1813. [13] Rodriguez JA, Luria-Perez R, Lopez-Valdes HE,
Casero D, Daniels TR, Patel S, Avila D, Leuchter R, So S, Ortiz-Sanchez E, Bonavida B, Martinez-Maza O, Charles AC, Pellegrini M, Helguera G, Penichet ML. Lethal iron deprivation induced by non-neutralizing antibodies targeting trans-ferrin receptor 1 in malignant B cells. Leuk Lymphoma 2011; 52: 2169.
[14] Kemp JD, Smith KM, Kanner LJ, Gomez F, Thorson JA, Naumann PW. Synergistic inhibi-tion of lymphoid tumor growth in vitro by com-bined treatment with the iron chelator defer- oxamine and an immunoglobulin G monoclo-nal antibody against the transferrin receptor. Blood 1990; 76: 991.
[15] Xu G, Wen X, Hong Y, Du H, Zhang X, Song J, Yin Y, Huang H, Shen G. An anti-transferrin recep-tor antibody enhanced the growth inhibirecep-tory effects of chemotherapeutic drugs on human glioma cells. Int Immunopharmacol 2011; 11: 1844.
recep-tor antibody-dependent on the COX-2 pathway in human hepatoma cells. Cancer Immunol Immunother 2013; 62: 447.
[17] Qing Y, Shuo W, Zhihua W, Huifen Z, Ping L, Lijiang L, Xiaorong Z, Liming C, Daiwen X, Yu H, Wei X, Min F, Zuohua F, Guanxin S. The in vitro antitumor effect and in vivo tumor-specificity distribution of human-mouse chimeric anti-body against transferrin receptor. Cancer Immunol Immunother 2006; 55: 1111.
[18] Zhang M, Guo Y, Fu H, Hu S, Pan J, Wang Y, Cheng J, Song J, Yu Q, Zhang S, Xu J F, Pei G, Xiang X, Yang P, Wang CY. Chop deficiency pre-vents UUO-induced renal fibrosis by attenuat-ing fibrotic signals originated from Hmgb1/ TLR4/NFκB/IL-1β signaling. Cell Death Dis 2015; 6: e1847.
[19] Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J 2011; 434: 365. [20] Dixon KM, Lui GY, Kovacevic Z, Zhang D, Yao M,
Chen Z, Dong Q, Assinder SJ, Richardson DR. Dp44mT targets the AKT, TGF-beta and ERK pathways via the metastasis suppressor NDRG1 in normal prostate epi thelial cells and prostate cancer cells. Br J Cancer 2013; 108: 409-419.
[21] Stacewicz-Sapuntzakis M, Borthakur G, Burns JL, Bowen PE. Correlations of dietary patterns with prostate health. Mol Nutr Food Res 2008; 52: 114.
[22] Braun DP, Gupta D, Birdsall TC, Sumner M, Staren ED. Effect of naturopathic and nutri-tional supplement treatment on tumor re-sponse, control, and recurrence in patients with prostate cancer treated with radiation therapy. J Altern Complement Med 2013; 19: 198.