Two types of graviresponse of swimming protists have been experimentally verified, gravitaxis and gravikinesis. The more recent literature primarily reflects the search for physiological pathways of either gravitaxis or gravikinesis (Machemer and Bräucker, 1992; Häder and Hemmersbach, 1997; Machemer, 1998; Hemmersbach et al., 1999). This is due, in part, to the limited and still developing body of methods and tools for the investigation of graviresponses in cells, requiring that an experimenter concentrates on a few, well-established procedures. A further reason for the current emphasis on physiology is that gravitational signalling promises to be among the most sensitive mechanisms of mechanotransduction known among eukaryotes.
The existing separation of research into gravitaxis or gravikinesis, physiological or physical mechanisms, is artificial because protist cells are likely to use gravitaxis in conjunction with gravikinesis, and an ecologically useful graviresponse may be generated by mechanisms of physical torque or via sensory transduction. We have previously developed and refined methods documenting the orientation- and gravity-dependent speed of locomotion, the effects of sedimentation and gravikinesis in various ciliates, and most recently in a flagellate (Machemer-Röhnisch et al., 1999). Attempts were made to improve the assessment of cellular gravitaxis
(Machemer and Bräucker, 1992), and we have proposed a method of screening gravitaxis for a possible physical mechanism (Machemer and Bräucker, 1996). Such screening suggested that the search for mechanisms of gravitaxis should not be limited to mutual exclusion of the physical or physiological signalling pathways. The responses of a single species and between species differed in directions taken, degree and time course. Observations of the precision of gravitaxis indicate that mechanisms exist that can antagonize a taxis so that at no time do all the cells swim precisely in one direction. It was suggested that brief kinks (‘reversals’) in swimming direction, which are ubiquitous in protists, randomize orientation to antagonize perfect gravitaxis.
Here, we present results of experiments aiming at the analysis of both physical and physiological mechanisms of graviresponses in Paramecium tetraurelia giving special attention to the role of swimming reversals in modulating gravitaxis. We use, apart from the wild type, a mutant known for local and global defects of cortex morphology and thought to have an abnormal distribution of mechanoreceptor channels along the antero-posterior cell axis (Jerka-Dziadosz et al., 1992). The data form part of a dissertation by U.N. at the Faculty of Biology, Ruhr-Universität Bochum, Germany (Nagel, 1998).
Printed in Great Britain © The Company of Biologists Limited 2000 JEB2469
Wild-type and the morphological mutant kin 241 of Paramecium tetraurelia showed improved orientation away from the centre of gravity (negative gravitaxis) when accelerations were increased from 1 to 7 g. Gravitaxis was more pronounced in the mutant. A correlation between the efficiency of orientation and the applied g value suggests a physical basis for gravitaxis. Transiently enhanced rates of reversal of the swimming direction coincided with transiently enhanced gravitaxis because reversals occurred more often in downward swimmers than in upward swimmers. The results provide evidence of a physiological modulation of gravitaxis by means of the randomizing effect of depolarization-dependent swimming reversals. Gravity bimodally
altered propulsion rates of wild-type P. tetraurelia so that sedimentation was partly antagonized in upward and downward swimmers (negative gravikinesis). In the mutant, only increases in propulsion were observed, although the orientation-dependent sensitivity of the gravikinetic response was the same as in the wild-type population. Observed swimming speed and sedimentation rates in the wild-type and mutant cells were linearly related to acceleration, allowing the determination of gravikinesis as a linear (and so far non-saturating) function of gravity.
Key words: gravikinesis, gravitaxis, mechanotransduction, physical mechanisms, mutant, Paramecium tetraurelia.
Summary
Introduction
PHYSICAL AND PHYSIOLOGICAL COMPONENTS OF THE GRAVIRESPONSES OF
WILD-TYPE AND MUTANT PARAMECIUM TETRAURELIA
UTE NAGEL* ANDHANS MACHEMER
Arbeitsgruppe Zelluläre Erregungsphysiologie, Fakultät für Biologie, Ruhr-Universität, D-44780 Bochum, Germany
*e-mail: ute.nagel@ruhr-uni-bochum.de
Materials and methods
Cells and solutions
Paramecium tetraurelia wild type (Sonneborn; stock d4-2)
and the mutant kin 241 (line 12A 2a) were kindly provided by Dr J. Beisson (Centre de Génétique Moléculaire, Gif-Sur-Yvette, France). The mutation is monogenic and recessive. The mutant kin 241 is homozygous and thermosensitive above 35 °C (Jerka-Dziadosz et al., 1992). Culture medium contained 0.8 % wheat straw in double-distilled water (w/v). It was autoclaved and buffered at 22 °C at pH 7.0 using Sörensen buffer (1.8 mmol l−1 Na
2HPO4 plus 0.2 mmol l−1 NaH2PO4).
The medium was bacterized with Enterobacter aerogenes 24 h before inoculation with Paramecium. Cells were cultured at 22 °C in a 14 h:10 h L:D photoperiod and harvested after 3 days in the early stationary phase. Cells were washed in the experimental solution (1 mmol l−1 CaCl
2, 1 mmol l−1
KCl/KOH, 1 mmol l−1 Mops buffered at pH 7.0; 22 °C) and
collected using gravitactic accumulation. Experimental protocol
Cells were equilibrated for 4 h in an experimental chamber made from Plexiglas with a glass cover for viewing; the chamber had a swimming volume of 35 mm×35 mm×2 mm and a field of view of 13.5 mm×17.5 mm (Nagel et al., 1997). Chambers were slowly rotated, at 1.5 ° s−1(or at 3 ° s−1in the
centrifuge), from the horizontal to the vertical position. After completion of turning and the passage of a predetermined time interval (60 s; or 0 s in the centrifuge), cells were monitored using a video camera under dark-field illumination (48 green light-emitting diodes, 565 nm, 700 lx corresponding to 4 W m−2). Abrupt changes in swimming direction (‘reversals’
or ‘turns’) were documented if the swimming track preceding a reorientation was pursued for a sufficient time (1 s) to allow determination of orientation. Only those reorientations that were more than 30 ° off the axis of the previous path were recorded. With these restrictions, results were used in only 50–65 % of reorientation events.
We used a computer-controlled low-velocity centrifuge microscope (NIZEMI; Dornier, Germany; Jena Optronics, Germany) at accelerations between 1 and 7 g (for experimental arrangement, see Bräucker et al., 1994). The sequence of levels of acceleration was randomized. The centre of the experimental chamber was positioned in the radial plane at a distance of 20.1 cm from the centre of the horizontal turntable. The cells were monitored as described above. A motor-driven swing device mounted on a turntable turned the major plane of the fluid volume from a perpendicular to a parallel position with respect to the resulting g vector. While the acceleration of the centrifuge increased or decreased (at 0.05 g s−1), the
chamber was held in the perpendicular position to reduce an undesirable loss of cells from the viewing field. Prior to recording, the chamber was turned to the parallel position (at 3 ° s−1; Nagel et al., 1997; Nagel, 1998). The experimental
chamber swang out as a result of eccentric mass with rising acceleration. All experiments were carried out at room temperature (21–23 °C).
Approximations of the gravity-free propulsion rate of Paramecium tetraurelia were obtained during free-fall experiments in a drop tower (ZARM, Bremen, Germany). An experimental arrangement including devices for recording was maintained at normal atmosphere inside a drop capsule, which was released to fall for 4.6 s over a vertical distance of 110 m in an evacuated steel tube (10 Pa, effective gravity, 10−4g;
Machemer et al., 1993a; Nagel, 1998). Data evaluation
Video images were digitized and superimposed to provide tracks from which swimming rates and track orientations were measured. Cell orientations were determined from track orientations by accounting for the sedimentation rate (Machemer et al., 1997). The numbers of reversals in upward and downward orientation were counted for all measured cells and were normalized to a time interval of 1 min.
The precision of orientation was determined using circular statistics. The coefficient of orientation (ro; Machemer and
Bräucker, 1992) integrates the coefficient of directivity (R value; Batschelet, 1981) and the error angle (the angle between the cell and stimulus vector); it corresponds to the mean cosine of all individual direction vectors of unity scalar value and varying error angle (Machemer and Bräucker, 1996). The orientation coefficient differentiates between the upward orientation (positive sign) and downward orientation (negative sign) of cells. We calculated orientation coefficients of a population (roC) after transforming observed individual track
orientations to cell orientations by accounting for errors in reading orientations introduced by sedimentation (Machemer et al., 1997).
Possible physical mechanisms of gravitaxis in P. tetraurelia were screened by plotting orientation coefficients from experimental data as a function of applied acceleration and testing for fit with a torque-dependent sigmoidal relationship between roC and g (Machemer and Bräucker,
1 shows that roincreases with increasing eccentricity of mass and shape (k) as well as with gravity (g):
Gravikinesis, ∆, of single cells at the orientation, o, was determined according to the equation:
using swimming rates (V), track orientations (θ; inclination with respect to 0 °, where 0 ° is upwards), gravity-independent propulsion rates (P) and sedimentation rates (S) (Machemer and Bräucker, 1992). V and θwere determined from individual cells. S and P were calculated as medians for immobilized cells (Nagel et al., 1997) and from populations of swimming cells under weightless conditions, respectively (Nagel, 1998). Orientational data are represented, unless specified otherwise, using sectors of 10 ° for ∆oand of ±45 ° for gravikinesis of upward swimmers, ∆U, and of downward swimmers, ∆D:
∆U= P−VU−S , (3)
∆D =VD−P−S , (4)
where VUand VDare upward and downward swimming rates, respectively. For an extensive description of methods, see Nagel (1998).
Statistical analyses
We employ nonparametric statistics (medians with 95 % confidence intervals) because Gaussian distributions were not assured in all cases. In the case of normal distributions, the medians coincide with the means. Tests of statistical significance of speed differences are based on the Wilcoxon–Mann–Whitney U-test (level of significance 5 %).
Orientational distributions were determined using the Rayleigh test and were statistically compared using the Kuiper test (Batschelet, 1981). For regression analysis, we applied the method of least squares. Significance of the linear relationship was tested (level of significance 5 %). The correlation coefficient (−1⭐R⭐+1) is a relative measure of the strength and direction of a linear relationship. The square of the correlation coefficient, R2, gives the coefficient of
determination. The value of R2(for instance, 0.95) indicates
how well a regression curve is in agreement with given data (for instance, at 95 %).
Results Graviorientation
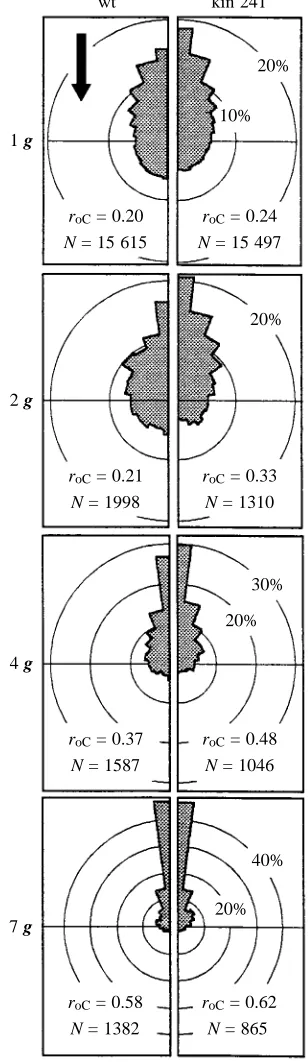
Wild-type and mutant cells tended to be oriented in the upward direction (negative gravitaxis). Negative gravitaxis was significantly more pronounced in the mutant (roC=0.24)
than in the wild type (roC=0.20; Fig. 1), and this applied
to accelerations of up to 7 g (wild-type roC=0.58; mutant
roC=0.62).
(2) ∆o=
冪
V2+ S2+ 2Vcosθ −P(1)
ro= .
kg
k2g2+ 1
冪
1 g
roC= 0.20
wt
20%
roC= 0.24
N = 15 615 N = 15 497 10%
2 g
roC= 0.21
20%
roC= 0.33
N = 1998 N = 1310
4 g
roC= 0.37
30%
roC= 0.48
N = 1587 N = 1046 20%
7 g
roC= 0.58
40%
roC= 0.62
[image:3.609.357.511.77.608.2]N = 1382 N = 865 20% kin 241
Fig. 1. Comparison of polar histograms of orientation of Paramecium tetraurelia wild type (wt) and mutant (kin 241) at different accelerations recorded at least 50 s after turning the chamber to the vertical position (the arrow indicates the direction of gravity vector). For clarity, frequencies of orientation in the left and right hemispheres are superimposed and averaged. N, number of tracks; roC, cell orientation coefficient of the total population. In
The more pronounced gravitaxis of the mutant compared with the wild type might be due, in part, to differences in eccentricity of the centre of shape from the geometric centre of the cell (Table 1). Asymmetries in density distribution of the cell body were not tested by shape relationships, but are possible.
Cell orientation coefficients, plotted as functions of acceleration, are in satisfactory agreement with the roC–g transfer function predicted from a mechanism of orientation by mechanical torque (Machemer and Bräucker, 1996; Fig. 2A). At all accelerations tested, cellular gravitaxis was most pronounced immediately after completion of turning the chamber from the horizontal to the vertical position (Fig. 2B). Mutants were more oriented than wild-type cells (Fig. 2B). More than 50 s after completion of the turn to the vertical position, orientation coefficients were reduced at all g values (Fig. 2C). The observed time-dependent depression of orientation suggests that an unknown mechanism interfered with orientation to reduce gravitaxis. According to the coefficients of determination of the regression analysis (R2; see Materials and methods), the early (Fig. 2B) and late (Fig. 2C) orientation data are in agreement (⭓94 %) with the hypothesis
that gravitaxis is effected by a physical mechanism of mechanical torque.
Changes in swimming direction
Behavioural tests of Paramecium tetraurelia showed that the frequency of reversal responses varied. The frequency of reversals declined during the first 5 min following infusion of cells into the chamber (Table 2). During the first 3 min, reversals occurred significantly more often in downward swimmers than in upward swimmers (Table 2).
[image:4.609.41.291.108.325.2]At 1 g and after 4 h of equilibration, the frequency of reversals was no longer orientation-dependent (Fig. 3). In hypergravity, however, downward swimmers were more likely to change swimming direction than upward swimmers (Fig. 3). The frequency of reversals of upward-swimming wild-type cells showed no significant relationship with increasing g, but the number of reversals rose significantly in downward swimmers (Fig. 3A). The same pattern was observed in the mutants. Increases in g caused a significant increase in reversal frequency in upward and, to a lesser degree, in downward swimmers (Fig. 3B).
Table 1. Comparison of the dimensions and physical
properties of Paramecium tetraurelia wild type and mutant kin 241
Wild type Mutant
Length (µm) 114.86 120.89
(−1.28/+1.14) (−1.14/+0.98)
Width (µm) 33.94 36.78
(−0.79/+0.66) (−0.71/+0.83)
Centre of shape 0.5014 0.5031
(fraction of cell length) (−0.0044/+0.0036) (−0.0024/+0.0032) Number of cilia per cell 3501 5314 Geometric surface area (cm2) 8.91×10−5 10.21×10−5
(rotational ellipsoid)
Surface area of soma (cm2) 13.36×10−5 15.32×10−5
(excluding cilia)
Membrane surface area (cm2) 40.86×10−5 57.06×10−5
(including cilia)
Volume (cm3) 6.93×10−8 8.56×10−8
Apparent mass (g) 2.77×10−9 3.43×10−9
Force from 1 g (N) 2.72×10−11 3.36×10−11
Values in parentheses are 95 % confidence intervals of the median. Length, width and the centre of shape are medians (wild type, N=308, mutant, N=488). For the centre of shape the anterior end=0, the posterior end=1.
The number of cilia has been approximated according to Jerka-Dziadosz et al. (1992).
Note that the geometric surface area of a rotational ellipsoid that approximates the dimensions of P. tetraurelia is only a fraction of the area of the total surface membrane.
[image:4.609.309.561.123.197.2]The apparent (or differential) mass of a submerged cell is its true mass minus the mass of the displaced water.
Table 2. Frequencies of reversal events in Paramecium tetraurelia after 2 h of equilibration to the experimental
solution and subsequent incubation in the vertical chamber at 1 g
Time after incubation Downward swimmers Upward swimmers (min) (reversals min−1) (reversals min−1)
2 3.7 (414) 3.0 (782)
3 3.1 (385) 2.1 (646)
4 2.4 (333) 1.9 (592)
5 1.9 (327) 1.5 (639)
Downward and upward swimmers were identified within ±45 ° of vertical orientation.
Values in parentheses are the total number of tracks tested for reversals.
Table 3. Membrane properties of Paramecium tetraurelia wild
type and mutant kin 241
Wild-type, Mutant kin 241,
N=13 N=11
Resting potential (mV) −32 (−10/+4) −32 (−17/+4) Input resistance (MΩ) 46.0 (−18/+7) 29.7 (−4/+4)
Input conductance (nS) 21.7 33.7
Soma membrane specific 6.15×103 4.55×103
resistance (Ωcm2)
[image:4.609.309.561.576.667.2]Electric membrane properties
The properties of the membrane in the wild-type and mutant cells are shown in Table 3. The resting membrane potentials of the wild-type and mutant cells were identical (−32 mV), whereas the input resistance in the mutant (29.7 MΩ) was significantly smaller than that of the wild type (46 MΩ). Part of this difference is due to the larger soma membrane surface area of the mutant (115 %) compared with the wild type (Table 1). The variable that most affects input resistance, however, is the specific resistance of the soma membrane, which in the mutant is reduced to 74 % compared with that of the wild type (Table 3). Input conductances of the wild type (21.7 nS) and the mutant (33.7 nS) were measured in horizontally oriented cells under 1 g conditions. These conductances, the membrane potential and the equilibrium
potentials of Ca2+and K+ in the experimental solution (1 mmol l−1 Ca2+, 1 mmol l−1 K+) were used to calculate conductance ratios (gCa/gK; Table 4).
Gravikinesis
Modulation of swimming speed in the wild type and mutant by gravity was investigated by determining the speed V and angle of inclination θ of swimming tracks, the median sedimentation rate (S) and the gravity-independent propulsion rate (P) (equation 2). In wild-type P. tetraurelia, the value of
P was taken as the speed of horizontally swimming cells (VH) at 1 g because the swimming speed during the weightless condition in drop-tower experiments did not differ significantly from VH(Nagel, 1998). In the mutant, P was found to be 89 % of VH. Moreover, VHof the mutant was less (73 %) than VHof
1
0.8
0.6
0.4
Orientation coefficient,
roC
0.01 0.1 1 10
0.2
0
100
A
k = 6.4 1.6 0.4 0.1
1
0.8
0.6
0.4
0.01 0.1 1 10
Acceleration (g) 0.2
0
100
B
k = 0.34 k = 0.22 t = 0 s
1
0.8
0.6
0.4
0.01 0.1 1 10
0.2
0
100
C
[image:5.609.65.560.72.228.2]k = 0.13 k = 0.10 t⭓50 s
Fig. 2. Precision of orientation (roC) of Paramecium tetraurelia wild type (䊉) and mutant (䊊) plotted as a function of acceleration (g). Curves
of best fit from particular coefficients to acceleration (k values; equation 1) are superimposed on the data points to test the applicability of the physical mechanisms of gravitaxis. A k value is an empirical constant of the physical eccentricity of a particular cell species. (A) Curves to illustrate how arbitrary k values (6.4, 1.6, 0.4, 0.1) affect cell orientation for a given acceleration (dotted line). With decreasing k, cell orientation will be less dependent on cell density or shape. An increase in k shifts the sigmoidal acceleration–orientation transfer function to the left on the x-axis, suggesting an increasing contribution of mechanical torque to orientation. (B) Orientation coefficients recorded from cells immediately after turning the experimental chamber from the horizontal to the vertical position (t=0 s). (C) Orientation coefficients recorded from cells at least 50 s after completion of turning the experimental chamber. The dotted lines in C are the dashed lines from B and show the time-dependent shift of roC/g curve (arrows).
1.8
1.5
1.2
0.9
Reversal frequency (min
-1)
0 1 2 3
0.6
0.3
4
A
wt
Acceleration (g) 0
5 6 7
1.8
1.5
1.2
0.9
0 1 2 3
0.6
0.3
4
B
kin 241
Acceleration (g) 0
[image:5.609.199.463.566.739.2]5 6 7
the wild type (Nagel, 1998). Active propulsion is composed of
P and the gravikinetic change (±∆) from the level of P at the
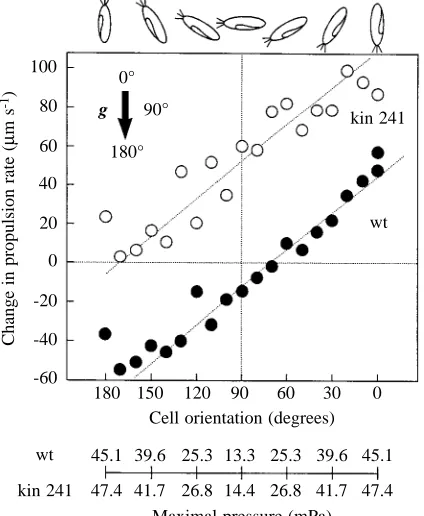
orientation (o) taken by the cell (P±∆o). The change in active propulsion rate was found to be a continuous function of cell orientation for both wild-type and mutant cells (Fig. 4). The sensitivity of the gravikinetic responses in the wild type and kin 241 was the same (0.64µm s−1degree−1). However, the gravikinetic response of the mutant was shifted in the positive direction by approximately 65µm s−1.
Despite identical sensitivity, only wild-type cells were able to generate a largely symmetrical gravikinetic response during downward and upward swimming (maxima near 50µm s−1, see Fig. 4). The mutant kin 241 showed no gravikinesis at 180 ° (oriented vertical downwards) and gave an extreme response at 0 ° (oriented vertically upwards; kinesis approximately 90µm s−1, see Fig. 4). The unilateral shift in gravikinesis of the mutant compared with the wild-type is equivalent to a
hyperpolarization-dependent augmentation of the ciliary motor response.
Assessment of gravitational pressure
[image:6.609.41.560.109.248.2]Approximating the shape of the cell by a rotational ellipsoid, Table 4. Membrane properties of the wild type (wt) and mutant kin 241 (kin) under normal gravity (1 g) in
downward-(Down-1g) and upward- (Up-1g) oriented cells and under microgravity derived from measured electrical variables for horizontally oriented cells at 1 g (Horiz.-1g, values in bold; see Table 3)
Microgravity Horiz.-1g Down-1g Up-1g
wt kin wt kin wt kin wt kin
Vm(mV) −32.0 −30.4 −−32.0 −−32.0 −30.4 −30.4 −33.6 −33.6
∆Vm(mV) 0 −1.6 +1.6 0 −1.6 −3.2
gCa/gK(nS) 0.400 0.414 0.400 0.400 0.414 0.414 0.384 0.384
Rinp(MΩ) 46.5 30.8 46.0 29.7 45.7 30.4 45.2 29.2
Ginp(nS) 21.5 32.5 21.7 33.7 21.9 32.9 22.1 34.3
gCa(nS) 6.1* 9.5* 6.2 9.6 6.4 9.6 6.1 9.5
gK(nS) 15.4* 23.0 15.5 24.1 15.5 23.3 16.0 24.8
∆gCa(nS) 0.062 0.096 0.277 0.125 0.0* 0.0*
∆gK(nS) 0.155 1.119 0.1* 0.3* 0.598 1.808
Conductance ratios (gCa/gK) were calculated from the equilibrium potentials of Ca2+ (+116 mV) and K+(−91 mV) and the membrane
potentials (Vm) at 1 g and microgravity.
Total conductances (Ginp) and partial ionic conductances (gCa, gK) were calculated from the input resistance (Rinp).
A difference in the gravity-induced change in swimming speed between wild-type and mutant (65µm s−1, see Fig. 4) cells corresponding to a
1.6 mV shift (∆Vm) from the resting potential (Vm at microgravity) was approximated from the properties of electromotor coupling in Paramecium caudatum (Gebauer et al., 1999).
Arbitrary assumptions (*) are (i) that 1 % of the total conductance in a horizontally swimming wild-type cell is due to open mechanoreceptor channels at 1 g; (ii) that no Ca2+mechano-sensitive channels are open in upward-oriented cells; (iii) that gravity-induced mechanoconductance
in downward-oriented mutants is three times that in the wild-type cells.
[image:6.609.330.542.479.737.2]Inferred values: values in roman type are inferred from values in bold types; values in italic type are inferred from values in bold type and from values marked with an asterisk. Minor discrepancies are due to rounding.
Fig. 4. Orientation-dependent up and down modulation of actively driven swimming speed of Paramecium tetraurelia wild type (wt) (䊉) and mutant kin 241 (䊊). 15 000 tracks were used for data from wild-type and mutant cells. Data from sectors of 10 ° of the left orientational hemisphere (180–360 °) are combined with data from corresponding sectors of the right orientational hemisphere (0–180 °) because helically swimming cells do not identify laterality. The arrow shows the direction of gravitational acceleration; the diagrams above the figure represent the cell oriented at the angles shown. See Fig. 5 and the text for the association between pressure and orientation.
100
80
60
40
Change in propulsion rate (
µ
m s
-1)
180 150 120 20
0
90 0°
Cell orientation (degrees) -60
60 30 0
-20
-40
180° 90°
g kin 241
wt
wt
kin 241
45.1 39.6 25.3 13.3 25.3 39.6 45.1
the dimensions of Paramecium tetraurelia give a volume of 6.9×10−8cm3for the wild type and 8.6×10−8cm3for the mutant kin 241 (Table 1). With a cell density of 1.04 g cm−3 (Machemer and Bräucker, 1992), the apparent masses (cell mass minus mass of displaced body of water) are 2.8×10−9g and 3.4×10−9g, respectively. At normal acceleration (9.81 m s−2), the gross gravitational force can be calculated (wild type, 2.7×10−11N; mutant, 3.4×10−11N; Table 1). This force may be linked to gravisensitive receptors in the soma membrane as follows.
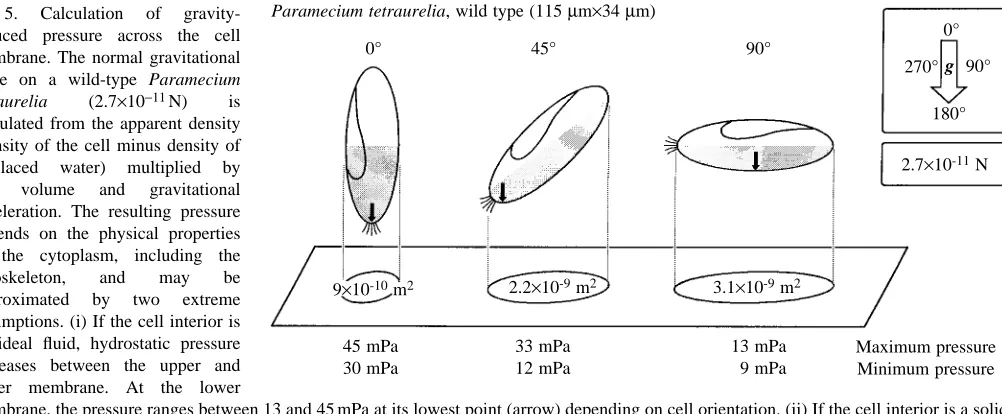
Two extreme assumptions regarding the physical properties of the cytoplasm approximate the local pressure: (i) if the body is an ideal fluid, the hydrostatic pressure on the surrounding membrane results from the height of the column of cytoplasm per unit area; (ii) if the cytoplasm including the cytoskeleton is a rigid body, static pressure on the lowest point of the membrane results from the total gravitational force acting on the horizontal cross section (shadow) of the cell (Fig. 5). The cytoplasm has viscoelastic properties (Todd, 1994; Ehrenstein and Iwasa, 1996). Assumptions i and ii above, therefore, mark the upper and lower extremes of a description of gravitational pressure at the membrane excluding additional cellular mechanisms of gravitational focusing. Fig. 5 illustrates, for three orientations, the lowest points on the membrane (arrow), the maximal pressure, as derived from the hydrostatic approach, and the minimal pressure, as derived from the static approach. The range of pressures applicable in Paramecium
tetraurelia is therefore between 9 and 45 mPa (0.009–0.045 N m−2).
Inclusion of the values of maximal pressures for wild type and kin 241 in Fig. 4 shows that pressure alone does not explain the divergent motor responses of downward and upward swimmers, nor do differences in pressure between wild type and kin 241 explain the constant offset in the motor responses of these cells.
Effects of hypergravity
The speed of wild-type upward swimmers (VU) decreased, and the speed of downward swimmers (VD) increased with increasing gravitational acceleration (Fig. 6B). Linear regressions of these relationships intersected at 0 g at a common speed, 829µm s−1 (Fig. 6B). As for the swimming rate, the median sedimentation rates (S) of immobilized cells (wild-type and mutant) were a linear function of acceleration intersecting at the origin (Fig. 6A; wild type 67.4µm s−1; mutant 73.5µm s−1). To account for sedimentation during swimming in wild-type cells, the slope of S was added to the slope of VU, and was subtracted from the slope of VD(Fig. 6C). The resulting rates of upward propulsion increased and rates of downward propulsion decreased with increasing acceleration. The slopes of these functions give values of orientation-dependent gravikinesis, and the intersection of the slopes gives the rate of gravity-free propulsion (P). Referring gravikinesis to sedimentation as a vector (positive sign), negative gravikinesis of downward swimmers (−54µm s−1g−1) significantly exceeded negative gravikinesis of upward swimmers (−25µm s−1g−1; Fig. 6D). The viability of the mutant kin 241 suffered during the phasic run-up and run-down accelerations of the centrifuge so that, for these cells, data were obtained from sedimentation experiments only.
Discussion
Gravitaxis, adaptation and reversal rates
Our investigation of gravitaxis in Paramecium tetraurelia confirmed previous findings suggesting that hypergravity potentiates gravitaxis in ciliates (Bräucker et al., 1994) and
Euglena gracilis (Häder et al., 1995) (Fig. 1). Moreover,
[image:7.609.63.564.71.278.2]gravitaxis to some degree relaxed over time (Fig. 2), a phenomenon commonly called adaptation. Adaptation of gravitaxis in protists is controversial in the literature. There are Fig. 5. Calculation of
gravity-induced pressure across the cell membrane. The normal gravitational force on a wild-type Paramecium tetraurelia (2.7×10−11N) is
calculated from the apparent density (density of the cell minus density of displaced water) multiplied by cell volume and gravitational acceleration. The resulting pressure depends on the physical properties of the cytoplasm, including the cytoskeleton, and may be approximated by two extreme assumptions. (i) If the cell interior is an ideal fluid, hydrostatic pressure increases between the upper and lower membrane. At the lower
membrane, the pressure ranges between 13 and 45 mPa at its lowest point (arrow) depending on cell orientation. (ii) If the cell interior is a solid body, pressure increases within the shaded half of the cell and is maximal (between 9 and 30 mPa) depending on orientation. The real values of pressure are likely to range between these extremes.
0°
Paramecium tetraurelia, wild type (115 µm×34 µm)
45° 90°
9×10-10m2 2.2×10-9m2 3.1×10-9m2
45 mPa 30 mPa
33 mPa 12 mPa
13 mPa 9 mPa
Maximum pressure Minimum pressure
0°
90°
180° 270° g
both reports of adaptation in P. caudatum and P. biaurelia (Bräucker et al., 1998; Hemmersbach-Krause and Häder, 1990; Machemer et al., 1993b) and claims that adaptation to gravity does not exist, at least under hypergravity (Häder et al., 1995; Hemmersbach et al., 1996; Machemer-Röhnisch et al., 1993). A stable acceleration/gravitaxis relationship (Figs 1, 2) implies a baseline reversal rate. In the absence of reversals, even a weak acceleration would perfectly orient all cells; this was not observed in the present study. The observed transition from an initially high degree of orientation (following completion of turning of the experimental chamber from horizontal to vertical at 1 g; Fig. 2B) to a reduced degree of orientation (Fig. 2C) confirms findings in P. caudatum (Bräucker et al., 1998). This phenomenon might be based on a time-dependent change in
rates of reversals in Paramecium spp. as previously documented, for example, for adaptation following a K+ -induced shift in the potential of the cell membrane (Machemer, 1989).
We believe that an interaction between gravitactic orientation and orientational randomization by reversals (Machemer and Bräucker, 1996) can explain the time-dependence of gravitaxis in the follwing ways.
(1) Reversal frequency depends on orientation
We have documented that reversals in downward swimmers occurred significantly more often than reversals in upward swimmers during the first few minutes after incubation (Table 2). This non-stochastic distribution of reversals will 500
400
300
200
Sedimentation rate (
µ
m s
-1)
0 1 2 3
100
4
A
0
5 6 7
0 1 2 3 4
Acceleration (g)
5 6 7
y=73.5x
y=67.4x
1200
1000
800
Active swimming rate (
µ
m s
-1)
600
C
4001200
1000
800
Swimming rate (
µ
m s
-1)
0 1 2 3
600
4
B
400
5 6 7
VD
VU
VU+ S
VD– S
P
0 1 2 3 4
Acceleration (g)
5 6 7
0
-100
-200
Gravikinesis (
µ
m s
-1)
-300
D
-400∆U
∆
[image:8.609.106.496.74.418.2]∆D
Fig. 6. Changes in vertical locomotion under hypergravity conditions. (A) Linear regressions of medians of sedimentation rates (S) of immobilized Paramecium tetraurelia wild type (filled symbols; y=67.37x; R>0.998) and mutant (open symbols; y=73.46x; R>0.999). 95 % confidence intervals are smaller than the symbols. (B) Observed median swimming rates of wild-type populations (VU upwards, 䉱; VD
downwards, 䉲; orientations ±45 ° from vertical included). Linear regressions: VU, y=828.29−42.09x (R=−0.98); VD, y=830.33+12.94x
(R=0.82). (C) Swimming rates after accounting for sedimentation rate (S=67.37µm s−1g−1). The intersection of regressions at 0 g (mean
829.31µm s−1; also seen in B) gives the value of the gravity-free propulsion rate (P). Slopes give values of gravikinesis. Linear regressions:
VU+S, y=828.29+25.27x (R=0.94); VD−S, y=830.33−54.43x (R=−0.99). (D) Gravikinesis of upward swimmers (∆U, 䉱) and downward
swimmers (∆D, 䉲) isolated from the active swimming rates (C) after accounting for gravity-free propulsion rate (P). The negative sign
indicates that this kinesis acts to neutralize the effects of sedimentation (S is positive). The arithmetic mean of ∆Uand ∆Dgives the generalized
value of gravikinesis, ∆. Linear regressions: ∆U, y=1.01−25.27x (R=−0.94); ∆D, y=1.01−54.43x (R=−0.99); ∆, y=1.01−39.85x (R=−0.99).
Corrected regressions passing through the intersection of the axes are: ∆U, y=−25.07x (R=−0.94); ∆D, y=−54.22x (R=−0.99); ∆, y=−39.64x
necessarily favour an improvement in upward orientation. The differences in the reversal frequency between upward and downward swimmers declined with time (Table 2). This decline logically explains the observed time-dependent relaxation of gravitaxis (the shift from initially large to lower
k values; Fig. 2C). The observed orientational preference of
reversals generates a bias favouring negative gravitaxis. Jennings (1906) was the first to note that reversals were rare in upward-swimming Paramecium spp. and more frequent during downward swimming.
(2) Reversal frequency links gravitaxis to membrane potential The orientation and gravity-dependence of reversals (Fig. 3) suggests a coupling between cellular reversal rates and the membrane potential. A pronounced sensitivity of downward swimmers for reversals was postulated by Naitoh and Eckert (1974) because depolarizing mechanoreceptor channels accumulate in the anterior membrane of Paramecium
caudatum (Naitoh and Eckert, 1969). In agreement with this,
increasing acceleration augmented reversal rates in downward swimmers but not in upward swimmers (Fig. 3A). Moreover, the downward swimming speed was reduced compared with the gravity-free propulsion P (Fig. 4), in agreement with depolarization-dependent gravikinesis (Gebauer et al., 1999). The mutant kin 241 were more precisely oriented than the wild-type cells (Fig. 2), mutant reversals were more rare (74 % of the value for wild-type cells, data not shown), and mutant cells were more hyperpolarized under gravity (Table 4; see change in Vmfrom microgravity to 1 g). These observations agree with the view that the membrane potential can modulate gravitaxis by means of reversal frequency.
(3) Adapting reversal frequency modulates non-adapting
gravitaxis
The established electrophysiological basis for reversal responses in Paramecium spp. necessarily links the observed time-dependence of gravitaxis (Fig. 2) with mechanisms of physiological adaptation in P. caudatum (Oka et al., 1986). The apparent contradiction between the documented adaptation of gravitaxis and the postulated non-adapting graviresponses is thereby resolved.
Hypergravity experiments
The observed rates of swimming and sedimentation were linearly related to the acceleration applied. These relationships allowed the gravikinetic components during upward and downward swimming to be determined using linear regressions (Fig. 6). The resulting values of gravikinesis (equations 3, 4; medians from ±45 ° sectors; ∆U=−25µm s−1; ∆D=−54µm s−1) largely agree with values obtained in noncentrifuged cells (equation 2; Fig. 4, medians from 10 ° sectors: ∆U=−48µm s−1, ∆D=−36µm s−1). Calculating the data of Fig. 4 for ±45 ° sectors, gives ∆U=−34µm s−1, ∆D=−44µm s−1. A 2 % error (i.e. of 9.5µm s−1) in assessment of the value of P under microgravity conditions would explain the remaining discrepancy between the hypergravity results (Fig. 6) and the
1 g results (Fig. 4). The results obtained using several levels of acceleration are likely to be superior to those derived from exclusively 1 g conditions because regression analysis reduces the effects of uncertainties regarding single data points.
Wild-type and mutant gravikinesis
The gravikinesis of wild-type P. tetraurelia neutralizes, in part, the effects of sedimentation by increasing the upward and reducing the downward speed of swimming (Fig. 4). This type of gravikinesis is common in ciliates and has been demonstrated previously in P. caudatum (Bräucker et al., 1998; Machemer-Röhnisch et al., 1996; Watzke et al., 1998), P.
biaurelia (Hemmersbach-Krause et al., 1993), Tetrahymena pyriformis (Kowalewski et al., 1999), Loxodes striatus
(Machemer-Röhnisch et al., 1993) and Bursaria truncatella (Krause, 1999). The mutant kin 241 of P. tetraurelia had the same orientational sensitivity as the wild-type. However, gravikinesis of upward swimmers was augmented, and gravikinesis of downward swimmers acted in the ‘wrong’ direction (Fig. 4). Thus, the well-balanced and quite efficient gravikinetic response of the wild type has apparently turned, in part, to a nonsense response in the mutant.
Organisation of gravikinesis in protists
Among wild-type protists so far investigated, two species show an interesting correspondence with the monopolar gravikinetic response of the P. tetraurelia mutant. (i) Didinium
nasutum can actively reduce forward swimming speed under
gravitational stimulation (Bräucker et al., 1994) by using depolarizing mechanoreceptor channels in more anterior parts of the cell membrane. D. nasutum cannot augment ciliary propulsion (Pernberg and Machemer, 1995). Gravikinesis in D.
nasutum thereby neutralizes sedimentation only during
downward orientations, whereas P. tetraurelia kin 241 neutralizes sedimentation only during upward orientations. (ii)
Euglena gracilis shows an apparently even more primitive
gravikinetic response, augmenting swimming rates at all orientations in the gravity field, albeit more effectively while swimming upwards (Machemer-Röhnisch et al., 1999). A comparison of various types of gravikinesis suggests that
Paramecium spp., by fine-tuning opposing gradients of
mechanoreceptor channels, generates a useful kinetic response to the gravity vector. This balance appears to be lost in the mutant kin 241 of P. tetraurelia.
The linear gravikinetic curve of P. tetraurelia is at variance with the orientation-dependent gravikinetic curve of P.
caudatum, which follows a sigmoidal course, where
horizontally swimming cells occur in the centre of maximal orientational sensitivity (Gebauer et al., 1999). By comparison with P. caudatum, the curve for wild-type P. tetraurelia (Fig. 4) does not include orientations of pronounced sensitivity.
Role of gravitational pressure in gravikinesis
assuming ideal fluidity of the cell interior (Fig. 5), ignores the comparatively high viscosity of the cytoplasm and excludes a role of the cytoskeleton, via hydrodynamic coupling, as a potential agent of gravitational focusing. The minimal estimate of pressure, 9 mPa for a horizontal cell, is equally unrealistic because the cytoplasm is not a solid body, and gravitational focusing by such a body is also excluded. These hypothetical extreme values therefore simply delimit a range within which the value of gravitational pressure must lie.
We have included in Fig. 4 values of maximal pressure for the wild type and for the larger mutant (see Table 1) as functions of orientation. From Fig. 4, pressure is identical for cells at 0 ° and 180 ° orientation, and for cells at 45 ° and 135 ° orientation, etc. Gravikinetic responses, however, do not distribute symmetrically in this way but follow antero-posterior gradients. The comparison suggests that gravikinesis is determined by gravitational pressure in conjunction with cell orientation. A maximal transmembrane pressure difference of 13.3 mPa for a horizontal cell was sufficient to elicit a gravikinetic response (Fig. 4). Similar results have been obtained from smaller cells such as Tetrahymena pyriformis (9 mPa; Kowalewski et al., 1999) and Euglena gracilis (5 mPa; Machemer-Röhnisch et al., 1999). With channel gating distance in most sensitive mechanoreceptors presumably being restricted to no more than 3.5 nm (Howard et al., 1988), the primary energy derived from normal acceleration is very small; for a horizontal P. tetraurelia, it is 9×10−20N m (only a maximum of 45 times above the thermal noise level at room temperature).
Local or global gravisensation?
Deformation of the tension-dependent architecture of the cell by gravity has been suggested as a primary step in gravisensation (Ingber, 1999). According to this author, transmembrane mechanoreceptors could be activated by gravity via the cytoskeleton. In agreement with this general view, an observed potentiation of gravikinesis in Paramecium
caudatum exposed to a weak direct current field was explained
by postulating Ca2+-sensitive filaments linked to the gates of mechanoreceptor channels (Machemer-Röhnisch et al., 1996). Ingber’s (1999) suggested gravisensation relies on the presence of a three-dimensional link between the skeletal elements of the cytoplasm and the membrane, which can store mechanical tension and thereby spread a local cellular deformation (Wang et al., 1993). Direct application of this view of ‘tensegrity’ to gravitransduction in free-swimming protists is a problem because global deformations of the cell (elongations or rounding up) would necessarily cause events of global gravitransduction. Our data suggest, however, that gravity first activates channels of the lower membrane. These channels are part of antero-posterior gradients of mechanosensitivity that are an indispensable basis for active compensation of sedimentation of the cell irrespective of its orientation.
Experimental step transitions from normal acceleration to the weightless condition induced vertically oriented
Paramecium caudatum and Didinium nasutum transiently to
maintain gravikinesis which, however, changed sign to act co-parallel with the gravity vector (Bräucker et al., 1998; Machemer-Röhnisch et al., 1998). This ‘gravikinetic paradox’ was interpreted as a viscoelastic transfer of gravity-dependent deformation of the lower membrane to deformation of the upper membrane, implicating a mechanically charged cytoskeleton. Such transfer of local deformation representing an early step in the relaxation from a gravity-induced deformation of the cell is, to our understanding, difficult to reconcile with the idea of the global transfer of cytoskeletal tension.
Sensory organization
[image:10.609.311.558.399.629.2]Inspection of basic electrophysiological data obtained under horizontal 1 g conditions (Table 3) revealed the same resting potential in the wild-type and mutant (−32 mV) cells, but a significantly lower specific resistance of the soma membrane in the mutant (4.55 kΩcm2) than in the wild-type (6.15 kΩcm2) cells. This change in resistance suggests a major increase in resting conductance, in particular of the K+ channels (Table 4). Fig. 7 illustrates how the resting and gravity-induced conductances of Ca2+and K+channels might be altered in the mutant compared with the wild-type cells. Using orthodox electrophysiological calculations, we suggest that, in the mutant, the mechanosensitive system of two
Fig. 7. Overview of how the altered membrane conductances (gCa,
gK) of the mutant kin 241 could modify gravikinetic behaviour (see
Fig. 4) and membrane properties (Tables 3, 4) compared with those of wild-type Paramecium tetraurelia. Conductances (y-axis) along the antero-posterior axis of the cell (x-axis) are illustrated. Resting conductances, white fields; gravity-induced conductances, shaded fields (shown 10 times amplified for clarity). The mutation affects both resting and gravity-induced conductances; the latter are shifted towards an augmentation of K+-dependent sensitivity.
gCa
Wild type
gK
gCa
gK
Anterior end down
kin 241
Horizontal Posterior end down 6.2 nS
15.5 nS
9.6 nS
24.6 nS
1.1 nS 0.3 nS
0.1 nS 0.1 nS 0.3 nS
0.6 nS
opposing and overlapping gradients of Ca2+and K+channels present in the wild type is shifted in favour of hyperpolarizing K+ channels (Table 4). An overall increase in the number of gravisensitive open K+ channels and/or reduction in the number of gravisensitive open Ca2+channels could explain the shift in the gravikinetic curve (Fig. 4).
Concluding remarks
Gravisensory transduction in Paramecium tetraurelia profoundly interferes with the regulation of ciliary propulsion and with normal swimming behaviour; forward propulsion at any orientation of the cell is modulated in such a way as to counteract the passive settling effect of gravity. We show here for the first time that gravitransduction interferes indirectly with gravitaxis at 1 g and hypergravity in that reversals of the swimming direction occur more often in downward swimmers than in upward swimmers. Since reversals randomize orientation, upward orientation is favoured over downward orientation. Negative gravitaxis as a phenomenon of gradual upward orientation is shown to be compatible with static or hydrodynamic mechanisms of torque. The mutant kin 241 of
Paramecium tetraurelia has abandoned, in part, a behaviour
that neutralizes the settling effect of gravity. This is presumably due to disturbance of the fine-tuned topological balance of depolarizing and hyperpolarizing mechanoreceptors of the wild type, which is believed to be the basis of orientation-dependent up- and down-regulation of swimming speed in ciliates.
Financial support for this study was provided by the Deutsches Zentrum für Luft- und Raumfahrt (DLR), grant 50WB93193, and the Minister für Wissenschaft und Forschung of the state of Nordrhein-Westfalen, grant IV A1-21600588. We thank Professor H. J. Rath and his collaborators at ZARM, Bremen, for a most fruitful cooperation during the drop-tower experiments.
References
Batschelet, E. (1981). Circular statistics in Biology. In Mathematics
in Biology (ed. R. Sibson and J. E. Cohen), pp. 3–353. London, New York: Academic Press.
Bräucker, R., Machemer-Röhnisch, S. and Machemer, H. (1994).
Graviresponses in Paramecium and Didinium examined under varied hypergravity conditions. J. Exp. Biol. 197, 271–294.
Bräucker, R., Murakami, A., Ikegaya, K., Yoshimura, K., Takahashi, K., Machemer-Röhnisch, S. and Machemer, H.
(1998). Relaxation and activation of graviresponses in Paramecium. J. Exp. Biol. 201, 2103–2113.
Ehrenstein, D. and Iwasa, K. H. (1996). Viscoelastic relaxation in
the membrane of the auditory outer hair cell. Biophys. J. 71, 1087–1094.
Gebauer, M., Watzke, D. and Machemer, H. (1999). The
gravikinetic response of Paramecium is based on orientation-dependent mechanotransduction. Naturwissenschaften 86,
352–356.
Häder, D. P. and Hemmersbach, R. (1997). Graviperception and
graviorientation in flagellates. Planta 203, 7–10.
Häder, D. P., Rosum, A., Schäfer, J. and Hemmersbach, R. (1995).
Gravitaxis in the flagellate Euglena gracilis is controlled by an active gravireceptor. J. Plant Physiol. 146, 474–480.
Hemmersbach-Krause, R., Briegleb, W., Vogel, K. and Häder, D. P. (1993). Swimming velocity of Paramecium under the conditions
of weightlessness. Acta Protozool. 32, 229–236.
Hemmersbach-Krause, R. and Häder, D. P. (1990). Negative
gravitaxis (geotaxis) of Paramecium – demonstrated by image analysis. Appl. Microgravity Techn. 2, 221–223.
Hemmersbach, R., Volkmann, D. and Häder, D. P. (1999).
Graviorientation in protists and plants. J. Plant Physiol. 154, 1–15.
Hemmersbach, R., Voormanns, R. and Häder, D. P. (1996).
Graviresponses in Paramecium biaurelia under different accelerations: studies on the ground and in space. J. Exp. Biol. 199, 2199–2205.
Howard, J., Roberts, W. M. and Hudspeth, A. J. (1988).
Mechanoelectrical transduction by hair cells. Annu. Rev. Biophys. Biophys. Chem. 17, 99–124.
Ingber, D. (1999). How cells (might) sense microgravity. FASEB J. 13 (Suppl.), S3–S15.
Jennings, H. S. (1906). Behavior of the Lower Organisms. New
York: Columbia University Press.
Jerka-Dziadosz, M., Garreau de Loubresse, N. and Beisson, J.
(1992). Development of surface pattern during division in Paramecium. II. Defective spatial control on the mutant kin241. Development 115, 319–335.
Kowalewski, U., Bräucker, R. and Machemer, H. (1999).
Responses of Tetrahymena pyriformis to the natural gravity vector. Microgravity Sci. Techn. 11, 167–172.
Krause, M. (1999). Elektrophysiologie, Mechanosensitivität und
Schwerkraftbeantwortung von Bursaria truncatella. Diplomarbeit, Fakultät für Biologie, Ruhr-Universität Bochum, Germany.
Machemer, H. (1989). Cellular behaviour modulated by ions:
electrophysiological implications. J. Protozool. 36, 463–487.
Machemer, H. (1998). Unicellular responses to gravity transitions.
Space Forum 3, 3–44.
Machemer, H. and Bräucker, R. (1992). Gravireception and
graviresponses in ciliates. Acta Protozool. 31, 185–214.
Machemer, H. and Bräucker, R. (1996). Gravitaxis screened for
physical mechanism using g-modulated orientational cellular behaviour. Microgravity Sci. Techn. 9, 2–9.
Machemer, H., Bräucker, R., Murakami, A. and Yoshimura, K.
(1993a). Graviperception in unicellular organisms: a comparative behavioural study under short-term microgravity. Microgravity Sci. Techn. 5, 221–231.
Machemer, H., Machemer-Röhnisch, S. and Bräucker, R.
(1993b). Velocity and graviresponses in Paramecium during adaptation and varied oxygen concentration. Arch. Protistenkd.
143, 285–296.
Machemer, H., Nagel, U. and Bräucker, R. (1997). Assessment of
g-dependent cellular gravitaxis: determination of cell orientation from locomotion track. J. Theor. Biol. 185, 201–211.
Machemer, H. and Ogura, A. (1979). Ionic conductances of
membranes in ciliated and deciliated Paramecium. J. Physiol., Lond. 296, 49–60.
Machemer-Röhnisch, S., Bräucker, R. and Machemer, H. (1993).
Neutral gravitaxis of gliding Loxodes exposed to normal and raised gravity. J. Comp. Physiol. A 171, 779–790.
Machemer-Röhnisch, S., Bräucker, R. and Machemer, H. (1998).
transition to the weightless condition. Microgravity Sci. Techn. 11, 35–43.
Machemer-Röhnisch, S., Machemer, H. and Bräucker, R. (1996).
Electric-field effects on gravikinesis in Paramecium. J. Comp. Physiol. A 179, 213–226.
Machemer-Röhnisch, S., Nagel, U. and Machemer, H. (1999). A
gravity-induced speed regulation in Euglena gracilis. J. Comp. Physiol. A (in press).
Nagel, U. (1998). Mechanismen physiologischer
Schwerkraftbeantwortung beim Wildtyp und einer Mutante von Paramecium tetraurelia. Dissertation, Fakultät für Biologie, Ruhr-Universität Bochum, Germany.
Nagel, U., Watzke, D., Neugebauer, D. C., Machemer-Röhnisch, S., Bräucker, R. and Machemer, H. (1997). Analysis of
sedimentation of immobilized cells under normal and hyper-gravity. Microgravity Sci. Techn. 10, 41–52.
Naitoh, Y. and Eckert, R. (1969). Ionic mechanisms controlling
behavioral responses in Paramecium to mechanical stimulation. Science 164, 963–965.
Naitoh, Y. and Eckert, R. (1974). The control of ciliary activity in
protozoa. In Cilia and Flagella (ed. M. A. Sleigh), pp. 305–352. London, New York: Academic Press.
Oka, T., Nakaoka, Y. and Oosawa, F. (1986). Changes in membrane
potential during adaptation to external potassium ions in Paramecium caudatum. J. Exp. Biol. 126, 111–117.
Pernberg, J. and Machemer, H. (1995). Voltage dependence of
ciliary activity in the ciliate Didinium nasutum. J. Exp. Biol. 198, 2537–2545.
Roberts, A. M. (1970). Geotaxis in motile micro-organisms. J. Exp.
Biol. 53, 687–699.
Todd, P. (1994). Mechanical analysis of statolith action in roots and
rhizoids. Adv. Space Res. 14, 121–124.
Wang, N., Butler, J. P. and Ingber, D. E. (1993). Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127.