ARTICLE
Defect Size Determines Survival in Infants With
Congenital Diaphragmatic Hernia
The Congenital Diaphragmatic Hernia Study Group*
The authors have indicated they have no financial relationships relevant to this article to disclose.
ABSTRACT
OBJECTIVES.Congenital diaphragmatic hernia is a significant cause of neonatal mor-tality. The objective of this study was to evaluate the clinical factors associated with death in infants with congenital diaphragmatic hernia by using a large multicenter data set.
METHODS.This was a prospective cohort study of all liveborn infants with congenital diaphragmatic hernia who were cared for at tertiary referral centers belonging to the Congenital Diaphragmatic Hernia Study Group between 1995 and 2004. Factors thought to influence death included birth weight, Apgar scores, size of defect, and associated anomalies. Survival to hospital discharge, duration of me-chanical ventilation, and length of hospital stay were evaluated as end points.
RESULTS.A total of 51 centers in 8 countries contributed data on 3062 liveborn infants. The overall survival rate was 69%. Five hundred thirty-eight (18%) patients did not undergo an operation and died. The defect size was the most significant factor that affected outcome; infants with a near absence of the dia-phragm had a survival rate of 57% compared with infants having a primary repair with a survival rate of 95%. Infants without agenesis but who required a patch for repair had a survival rate of 79% compared with primary repair.
CONCLUSIONS.The size of the diaphragmatic defect seems to be the major factor influencing outcome in infants with congenital diaphragmatic hernia. It is likely that the defect size is a surrogate marker for the degree of pulmonary hypoplasia. Future research efforts should be directed to accurately quantitate the degree of pulmonary hypoplasia or defect size antenatally. Experimental therapies can then be targeted to prospectively identify high-risk patients who are more likely to benefit.
www.pediatrics.org/cgi/doi/10.1542/ peds.2006-3040
doi::10.1542/peds.2006-3040
*See “Acknowledgments” for complete listing of writing committee.
Key Words
congenital diaphragmatic hernia, pulmonary hypoplasia, neonatal surgery, ECMO
Abbreviations
CDH— congenital diaphragmatic hernia ROC—receiver operating characteristic Accepted for publication Feb 1, 2007 Address correspondence to Kevin P. Lally, MD, MS, Department of Surgery, University of Texas Health Sciences Center, 6431 Fannin St, Suite 5.258, Houston, TX 77030. E-mail: kevin.p.lally@uth.tmc.edu
C
ONGENITAL DIAPHRAGMATIC HERNIA(CDH) has a re-ported incidence of between 1 in 2500 and 1 in4000 live births.1,2 With improvements in neonatal
in-tensive care in the 1970s and 1980s, sicker patients survived to receive surgery in tertiary centers. During this time, mortality rates from CDH approximated
50%.3,4 Because of this persistently high mortality rate,
newer techniques for the management of neonatal re-spiratory failure including extracorporeal membrane ox-ygenation, high-frequency oscillatory ventilation, exog-enous surfactant, and inhaled nitric oxide were rapidly adopted. Attempts at CDH repair in utero were
pio-neered by Harrison and colleagues,5–7 but recent data
have not shown a survival advantage for patients who undergo fetal surgery. Survival to hospital discharge seems to have improved over the past 2 decades,
al-though some authors dispute these conclusions.8–11The
reported mortality rate in liveborn infants of 20% to
40% makes CDH responsible for ⬎1% of the annual
infant mortality in the United States.12 Furthermore,
CDH ranks among the most costly of neonatal
condi-tions. Metkus and colleagues13reported that CDH had an
estimated hospital cost of $250 000 per case to hospital discharge, and an estimated yearly cost of $264 000 000 in the United States (1995 dollars) for the initial hospital care alone.
The relative rarity of the condition makes the conduct of well-designed clinical studies extremely difficult be-cause no single center can accrue sufficient patients to reach meaningful conclusions. Therefore, the manage-ment of CDH has evolved based largely on retrospective reviews from centers with small numbers of patients and differing treatment algorithms. A number of factors complicate interpretation of these studies. Institutions differ with respect to referral patterns and hospital re-sources such that the case mix or range of severity in patients treated may not be comparable. Some centers do not offer surgical repair for certain infants that are deemed “nonsalvageable,” whereas others do, and this
bias can clearly affect comparisons of survival rates.14,15
Stratification systems that have been reported to date, however, have been unwieldy or have not proven useful when applied to other centers or other populations of infants.16,17
The purpose of this study was to evaluate hospital-based outcomes for infants with CDH from a large num-ber of institutions and to determine clinical factors asso-ciated with a poor outcome.
METHODS
Patients
The CDH Study Group was formed in 1995 to compile data on liveborn infants with CDH at participating insti-tutions to assess therapies and outcomes. The CDH Study Group consists of tertiary referral centers that
voluntarily provide data to a central registry. Data on all infants with CDH who are born at or transferred to a participating center are entered into the database. Data were collected prospectively on all liveborn patients with CDH between 1995 and 2004 in participating hospitals and included information on delivery and subsequent hospital care (including surgery where applicable) until death or hospital discharge. Institutions were included in
this analysis if they had ⱖ4 consecutive years of data
submission. The data from the registry forms were en-tered into a Microsoft (Redmond, WA) Access database and were cross-checked against the original forms. Pa-tient demographics, birth information, Apgar scores, treatments received, and outcome were recorded. The Apgar scores were recoded into 3 categories (0 –3, 4 – 6, and 7–10) for analysis.
The size of the diaphragm defect was determined by the surgeon at the time of repair and coded as “agenesis” if the diaphragm or most of the diaphragm was absent (based on surgeons’ reports and/or operative notes with findings of “absent or missing rim of diaphragm” or repair requiring “suturing the patch to the ribs anteriorly and posteriorly”). All patients with diaphragm agenesis required a patch to repair the defect. In those patients without agenesis, either the defect could be repaired primarily, or a patch was required to close the defect. A fourth group of patients never underwent operation; most had a combination of either severe other anomalies or were thought to have fatal pulmonary hypoplasia. Central or bilateral diaphragmatic defects comprise a rare variant with a very high mortality; patients with these defects were not included in the analysis.
Significant associated anomalies, including chromo-somal anomalies, syndromes, and complex congenital heart disease, were evaluated to see whether their pres-ence with CDH affected outcomes. The most common chromosomal anomalies were trisomies 13, 18, and 21; the most common syndrome was Fryns syndrome. Com-plex congenital heart disease included hypoplastic left heart syndrome, coarctation of the aorta, and tetralogy of Fallot. An isolated ventricular septal defect or atrial septal defect was considered minor.
Statistical Analysis
Death before hospital discharge was the primary out-come variable. The length of stay in the hospital and duration of mechanical ventilation were secondary out-comes. Univariate logistic regressions were used to eval-uate the associations between clinical variables and death before hospital discharge. Those variables associ-ated with death are presented as unadjusted odds ratios and their 95% confidence intervals. The Wald test
sta-tistic was used to evaluate stasta-tistical significance. A P
value of .05 was considered significant. Patients who did not undergo repair were not included in the analysis.
clin-ical variables evaluated (inborn status, birth weight, pre-natal diagnosis, major cardiac anomalies, chromosomal anomalies, 5-minute Apgar score, and defect size) were calculated. Receiver operating characteristic (ROC) curves of these models were evaluated to determine how well they classified patients that died before discharge. The area under the ROC curve was used to summarize the classification accuracy of the logistic regression mod-els. The classification accuracies of full (including all the clinical variables) and reduced models were contrasted to identify the minimal set of clinical variables predictive of death before discharge. Kruskal-Wallis rank order 1-way analyses of variance were used to compare out-comes among patients grouped by defect size for length of hospital stay and duration of mechanical ventilation. Analysis of the database was approved by the University of Texas Institutional Review Board. Participating cen-ters filed a waiver of consent for data submission or signed a data use agreement for a limited data set. The analyses were conducted using the NCSS 2004 (NCSS, Kaysville, UT) statistical software package.
RESULTS
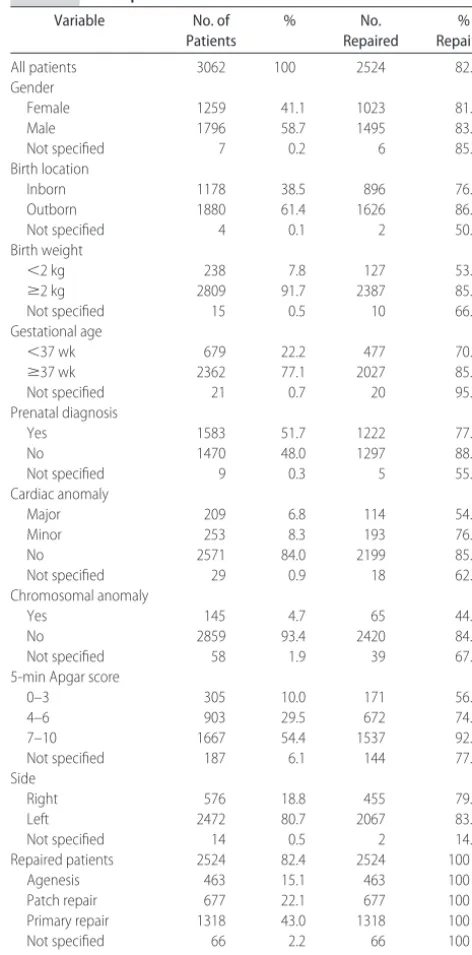
There were 3062 liveborn infants from 51 centers who met criteria for the analysis. There were 39 centers from the United States and 12 centers from 7 other countries. The overall survival until hospital discharge for all in-fants was 69%. Descriptive statistics for all patients are shown in Table 1.
Five hundred thirty-eight (18%) of the patients did not undergo operation, and all died. One hundred fifty-three of nonrepaired patients (28%) were noted to have severe anomalies (major cardiac, syndromal, or chromo-somal) compared with 165 (7%) of repaired patients. Most of the remainder of unrepaired patients was be-lieved to be unsalvageable based on hemodynamic in-stability or blood gas values. Autopsy data on defect size was only available on 50 patients, but of these, 23 (68%) had agenesis of the diaphragm, suggesting that the inci-dence of agenesis was much higher in patients thought to be nonsalvageable.
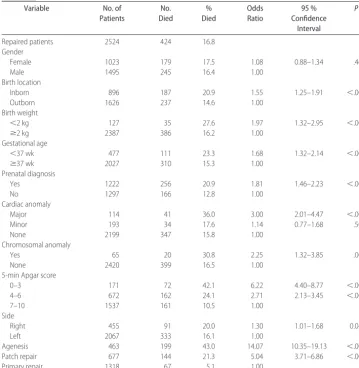
Survival in the surgically repaired patients was 2100 (83%) of 2524. A number of factors were significantly associated with death in the surgically repaired patients by univariate analysis (Table 2). Younger gestational age and lower birth weight were highly correlated, and both were associated with worse outcomes. Other significant factors included inborn status, prenatal diagnosis, pres-ence of cardiac and chromosomal anomalies, low Apgar score, and large defect size (either a patch repair or diaphragm agenesis).
A logistic regression analysis was performed by using the variables in Table 2 for the equation. The low-Apgar-score group (0 –3) and the 2 larger defect-size groups remained significant predictors in the full model. The area under the ROC curve for death in this model (full
model) was 0.803. A logistic regression model with de-fect size as the only predictor variable was calculated. Using the defect size alone, the area under the ROC curve for death was 0.764. The more complicated full model added little predictive accuracy when compared with a simple model using defect size alone. The size of the defect had a significant effect on outcome. The odds of dying for patients with diaphragm agenesis were 14.07 (95% confidence interval: 10.35–19.13) times that of patients whose defect was small enough to undergo primary repair. Patients without agenesis, but who re-quired a patch to close the diaphragm were also at increased risk for death (odds ratio: 5.04 [95%
confi-TABLE 1 Descriptive Statistics for All Patients
Variable No. of
Patients
% No.
Repaired
% Repaired
All patients 3062 100 2524 82.4
Gender
Female 1259 41.1 1023 81.3
Male 1796 58.7 1495 83.2
Not specified 7 0.2 6 85.7
Birth location
Inborn 1178 38.5 896 76.1
Outborn 1880 61.4 1626 86.5
Not specified 4 0.1 2 50.0
Birth weight
⬍2 kg 238 7.8 127 53.4
ⱖ2 kg 2809 91.7 2387 85.0
Not specified 15 0.5 10 66.7
Gestational age
⬍37 wk 679 22.2 477 70.3
ⱖ37 wk 2362 77.1 2027 85.8
Not specified 21 0.7 20 95.2
Prenatal diagnosis
Yes 1583 51.7 1222 77.2
No 1470 48.0 1297 88.2
Not specified 9 0.3 5 55.6
Cardiac anomaly
Major 209 6.8 114 54.5
Minor 253 8.3 193 76.3
No 2571 84.0 2199 85.5
Not specified 29 0.9 18 62.1
Chromosomal anomaly
Yes 145 4.7 65 44.8
No 2859 93.4 2420 84.6
Not specified 58 1.9 39 67.2
5-min Apgar score
0–3 305 10.0 171 56.1
4–6 903 29.5 672 74.4
7–10 1667 54.4 1537 92.2
Not specified 187 6.1 144 77.0
Side
Right 576 18.8 455 79.0
Left 2472 80.7 2067 83.6
Not specified 14 0.5 2 14.3
Repaired patients 2524 82.4 2524 100
Agenesis 463 15.1 463 100
Patch repair 677 22.1 677 100
Primary repair 1318 43.0 1318 100
Not specified 66 2.2 66 100
dence interval: 3.71– 6.86]) compared with infants who had a primary repair of the diaphragm.
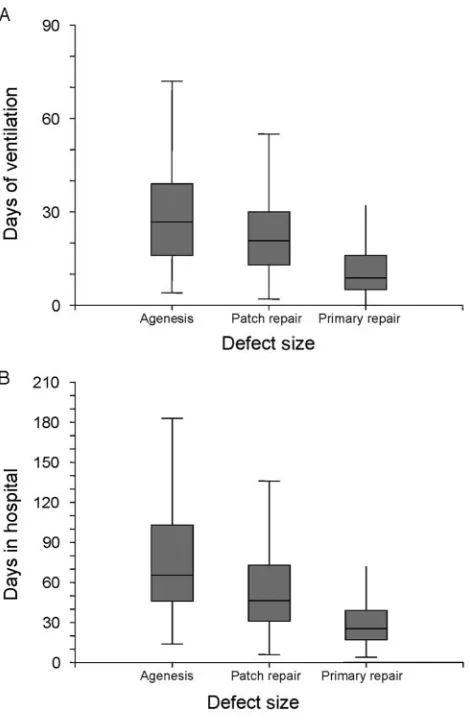
We examined the impact of defect size on length of hospitalization and duration of mechanical ventilation as well. In patients who underwent repair and survived to transfer or hospital discharge, the size of the hernia defect was strongly associated with duration of mechan-ical ventilation and length of hospital stay (Fig 1).
DISCUSSION
CDH remains an important cause of neonatal mortality despite many advances in neonatal critical care. The main determinant of survival in CDH remains the sever-ity of pulmonary hypoplasia and pulmonary hyperten-sion. Techniques to determine the degree of pulmonary
hypoplasia have not been successful to date.18Because
many infants in whom a certain preductal arterial PaO2
or PaCO2cannot be achieved do not survive, some
insti-tutions have used these measures to select candidates for
repair.8,19 Others have used measures such as birth
weight, prenatal diagnosis, associated anomalies, and
Apgar scores as predictors of outcome.16,17,20 Some of
these measures are not disease-specific; low Apgar scores, low birth weight, and presence of comorbid con-ditions such as cardiac anomalies are associated with increased morbidity and mortality in a large number of neonatal conditions.
This analysis shows that the size of the diaphragm defect correlates well with mortality, as well as morbid-ity in liveborn infants with CDH. Patients with dia-phragm agenesis have previously been reported to have
a high mortality.21–26Defect size is likely to be a marker
for the degree of pulmonary hypoplasia. Animal models suggest that a large defect is associated with much smaller lungs. It is possible to modify the degree of lung hypoplasia in the lamb model by the size of the defect created. In transgenic mice and toxicology models, tim-ing of the insult is a contributtim-ing factor in determintim-ing
size of the defect and outcome.27
An important criticism of this study is the accurate determination of the size of the defect. Clearly, there is some overlap between the groups defined by defect size because there is not an absolute value that defines agen-esis or a “large” defect. There are also variations in
TABLE 2 Clinical Variables Associated with Death Prior to Discharge in Patients Undergoing Repair
Variable No. of
Patients
No. Died
% Died
Odds Ratio
95 % Confidence
Interval
P
Repaired patients 2524 424 16.8
Gender
Female 1023 179 17.5 1.08 0.88–1.34 .465
Male 1495 245 16.4 1.00
Birth location
Inborn 896 187 20.9 1.55 1.25–1.91 ⬍.001
Outborn 1626 237 14.6 1.00
Birth weight
⬍2 kg 127 35 27.6 1.97 1.32–2.95 ⬍.001
ⱖ2 kg 2387 386 16.2 1.00
Gestational age
⬍37 wk 477 111 23.3 1.68 1.32–2.14 ⬍.001
ⱖ37 wk 2027 310 15.3 1.00
Prenatal diagnosis
Yes 1222 256 20.9 1.81 1.46–2.23 ⬍.001
No 1297 166 12.8 1.00
Cardiac anomaly
Major 114 41 36.0 3.00 2.01–4.47 ⬍.001
Minor 193 34 17.6 1.14 0.77–1.68 .504
None 2199 347 15.8 1.00
Chromosomal anomaly
Yes 65 20 30.8 2.25 1.32–3.85 .003
None 2420 399 16.5 1.00
5-min Apgar score
0–3 171 72 42.1 6.22 4.40–8.77 ⬍.001
4–6 672 162 24.1 2.71 2.13–3.45 ⬍.001
7–10 1537 161 10.5 1.00
Side
Right 455 91 20.0 1.30 1.01–1.68 0.045
Left 2067 333 16.1 1.00
Agenesis 463 199 43.0 14.07 10.35–19.13 ⬍.001
Patch repair 677 144 21.3 5.04 3.71–6.86 ⬍.001
surgical practice that determine whether a patch is used. However, there is a clear trend to liberal use of a patch to
repair the defect.12,28Also, it is not possible to close the
defect in a patient with agenesis or a very large defect without a patch of some kind. Similarly, the definition of agenesis is likely to vary between centers. Despite this limitation, there is a clear differentiation in outcome by defect size in this data set.
The CDH Registry was formed to allow collection and analysis of data on presentation, treatment, and out-come of CDH from a large number of centers. Registries have proven to be a good way of collecting data on rare conditions and of mitigating institutional bias in patient selection and treatment. As with any data from regis-tries, there are caveats to their interpretation. The data-base is observational, and conclusions about therapies should be interpreted cautiously. The data are collected from institutions that differ significantly in their patient recruitment and selection. These differences are espe-cially important when interpreting patients in the
non-repaired category because criteria for “nonsalvageable” patients vary between centers. Many infants with severe cardiac and chromosomal anomalies did not undergo repair making the true impact of these factors difficult to determine. Furthermore, these data only represent out-come to hospital transfer or discharge; both long-term survival and morbidity are likely to be worse.30 –33 Nonetheless, these data do represent a significant collec-tion of patients with an uncommon disease process.
CONCLUSIONS
Approximately two thirds of liveborn infants with CDH will survive to hospital discharge. Outcome for these infants is largely dependent on the size of the diaphragm defect. Future efforts should be directed to accurately determine defect size preoperatively or optimally prena-tally to correlate the defect size with actual lung hyp-oplasia. Ultimately, it would be ideal to stage patients prenatally by the severity of lung hypoplasia using a system validated across multiple institutions. Additional characterization of the patients who do not undergo diaphragmatic hernia repair would also be useful. Iden-tifying patients with small defects before intervention will allow those patients to be treated with less invasive therapies, reserving the high-risk interventions for those with more severe disease. Improved accuracy in catego-rizing patients will also help in counseling families about survival, expected lengths of stay in the hospital, and long-term outcomes.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants K24RR17050 and M01RR002558.
The centers that contributed to the CDH Registry were Arnold Palmer Hospital for Women and Children (Orlando, FL), Astrid Lindgren Children’s Hospital (Stockholm, Sweden), Central Hospital Aichi Prefectural Colony (Kasugai Aichi, Japan), Children’s Hospital at Carolinas Medical Center (Charlotte, NC), Children’s Hospital Boston (Boston, MA), Children’s Hospital of Akron (Akron, OH), Children’s Hospital of Alabama (Birmingham, AL), Children’s Hospital of Illinois (Peo-ria, IL), Children’s Hospital of Los Angeles (Los Angeles, CA), Children’s Hospital of Minneapolis (Minneapolis, MN), Children’s Hospital of Oakland (Oakland, CA), Children’s Hospital of Oklahoma (Oklahoma City, OK), Children’s Hospital of Wisconsin (Milwaukee, WI), Cin-cinnati Children’s Hospital Medical Center (CinCin-cinnati, OH), Cleveland Clinic Foundation-Children’s Hospital (Cleveland, OH), Columbus Children’s Hospital, Colum-bus, OH), DeVos Children’s Hospital (Grand Rapids, MI), Emory University (Atlanta, GA), Hershey Medical Cen-ter (Hershey, PA), James Whitcomb Riley Children’s Hospital (Indianapolis, IN), Kosair Children’s Hospital (Louisville, KY), Legacy Emanuel Children’s Hospital (Portland, OR), Loma Linda University Children’s
Hos-FIGURE 1
pital (Loma Linda, CA), Lucile Salter Packard Children’s Hospital (Palo Alto, CA), Mattel Children’s Hospital at UCLA (Los Angeles, CA), Medical College of Georgia (Augusta, GA), Children’s Memorial Hermann Hospital (Houston, TX), Miami Valley Hospital (Dayton, OH), National Center for Child Health and Development (To-kyo, Japan), Oespedale Pediatrico Bambino Gesu (Rome, Italy), Oespedale Riunite Bergamo (Bergamo, Italy), Osaka University Graduate School of Medicine (Osaka, Japan), Phoenix Children’s Hospital (Phoenix, AZ), Rainbow Babies & Children’s Hospital (Cleveland, OH), Royal Alexandra Hospital (Edmonton, Alberta, Canada), Royal Children’s Hospital Parkville (Victoria, Australia), Royal Hospital for Sick Children (Glasgow, Yorkhill, Scotland), San Diego Children’s Hospital (San Diego, CA), Santa Rosa Children’s Hospital (San Anto-nio, TX), Shands Children’s Hospital/University of Flor-ida (Gainesville, FL), Sophia Children’s Hospital (Rotter-dam, Netherlands), St Francis Children’s Hospital (Tulsa, OK), St Joseph’s Hospital and Medical Center (Phoenix, AZ), Strong Children’s Hospital (Rochester, NY), Sydney Children’s Hospital (Randwick NWS, Australia), Hospital for Sick Children (Toronto, Ontario, Canada), University of Michigan Medical Center (Ann Arbor, MI), University of Nebraska Medical Center (Omaha, NE), University of Texas Medical Branch (Galveston, TX), University of Virginia Health System (Charlottesville, VA), and Vanderbilt Children’s Hospital (Nashville, TN).
The Congenital Diaphragmatic Hernia Study Group writing committee is as follows: Kevin P. Lally, MD, MS (University of Texas Medical School and Children’s Me-morial Hermann Hospital), Pamela A. Lally, MD (Uni-versity of Texas Medical School and Children’s Memorial Hermann Hospital), Robert E. Lasky, PhD (University of Texas Medical School and Children’s Memorial Her-mann Hospital), Dick Tibboel, MD (Sophia Children’s Hospital), Tom Jaksic, MD, PhD (Children’s Hospital Boston), Jay M. Wilson, MD (Children’s Hospital Bos-ton), Bjorn Frenckner, MD (Astrid Lindgren Children’s Hospital), Krista P. Van Meurs, MD (Stanford University School of Medicine and Lucile Packard Children’s Hos-pital), Desmond J. Bohn, MD (Hospital for Sick dren), Carl F. Davis, MD (Royal Hospital for Sick Chil-dren), and Ronald B. Hirschl, MD (University of Michigan).
REFERENCES
1. Wenstrom KD, Weiner CP, Hanson JW. A five-year statewide experience with congenital diaphragmatic hernia.Am J Obstet Gynecol.1991;165:838 – 842
2. Langham MR Jr, Kays DW, Ledbetter DJ, Frentzen B, Sanford LL, Richards DS. Congenital diaphragmatic hernia: epidemiol-ogy and outcome.Clin Perinatol.1996;23:671– 688
3. Wilson JM, Lund DP, Lillehei CW, Vacanti JP. Congenital diaphragmatic hernia: a tale of two cities: the Boston experi-ence.J Pediatr Surg.1997;32:401– 405
4. Mishalany HG, Nakada K, Woolley MM. Congenital
diaphrag-matic hernias: eleven years’ experience.Arch Surg.1979;114: 1118 –1123
5. Harrison MR, Adzick NS, Longaker MT, et al. Successful repair in utero of a fetal diaphragmatic hernia after removal of her-niated viscera from the left thorax. N Engl J Med.1990;322: 1582–1584
6. Harrison MR, Keller RL, Hawgood SB, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia.N Engl J Med.2003;349:1916 –1924 7. Cass DL. Fetal surgery for congenital diaphragmatic hernia: the
North American experience.Semin Perinatol.2005;29:104 –111 8. Boloker J, Bateman DA, Wung JT, Stolar CJ. Congenital dia-phragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective re-pair.J Pediatr Surg.2002;37:357–366
9. Kays DW, Langham MR, Jr, Ledbetter DJ, Talbert JL. Detri-mental effects of standard medical therapy in congenital dia-phragmatic hernia.Ann Surg.1999;230:340 –348
10. Javid PJ, Jaksic T, Skarsgard ED, Lee S; Canadian Neonatal Network. Survival rate in congenital diaphragmatic hernia: the experience of the Canadian Neonatal Network.J Pediatr Surg. 2004;39:657– 660
11. Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia.Pediatrics.2003; 112:532–535
12. Clark RH, Hardin WD Jr, Hirschl RB, et al. Current surgical management of congenital diaphragmatic hernia: a report from the congenital diaphragmatic study group.J Pediatr Surg.1998; 33:1004 –1009
13. Metkus AP, Esserman L, Sola A, Harrison MR, Adzick NS. Cost per anomaly: what does a diaphragmatic hernia cost?J Pediatr Surg.1995;30:226 –230
14. Newman KD, Anderson KD, Van Meurs K, Parson S, Loe W, Short B. Extracorporeal membrane oxygenation and congeni-tal diaphragmatic hernia: should any infant be excluded?J Pe-diatr Surg.1990;25:1048 –1052
15. Stolar C, Dillon P, Reyes C. Selective use of extracorporeal membrane oxygenation in the management of congenital di-aphragmatic hernia.J Pediatr Surg.1998;23:207–211 16. Skari H, Bjornland K, Frenckner B, et al. Congenital
diaphrag-matic hernia in Scandinavia from 1995 to 1998: predictors of mortality.J Pediatr Surg.2002;37:1269 –1275
17. The Congenital Diaphragmatic Hernia Study Group. Estimat-ing disease severity of congenital diaphragmatic hernia in the first 5 minutes of life.J Pediatr Surg.2001;36:141–145 18. Holt PD, Arkovitz MS, Berdon WE, Stolar CJ. Newborns with
diaphragmatic hernia: initial chest radiography does not have a role in predicting clinical outcome. Pediatr Radiol. 2004;34: 462– 464
19. Boix-Ochoa J, Peguero G, Seijo G, Natal A, Canals J. Acid-base balance and blood gases in prognosis and therapy of congenital diaphragmatic hernia.J Pediatr Surg.1974;9:49 –57
20. Skari H, Bjornland, Haugen G, Egeland T, Emblem R. Congen-ital diaphragmatic hernia: a meta-analysis of mortality factors. J Pediatr Surg.2000;35:1187–1197
21. Singh SJ, Cummins GE, Cohen RC, et al. Adverse outcome of congenital diaphragmatic hernia is determined by diaphrag-matic agenesis, not by antenatal diagnosis.J Pediatr Surg.1999; 34:1740 –1742
22. Tsang TM, Tam PK, Dudley NE, Stevens J. Diaphragmatic agenesis as a distinct clinical entity.J Pediatr Surg.1994;29: 1439 –1441
23. Baglaj M, Spicer R, Ashworth M. Unilateral agenesis of the diaphragm: a separate entity or an extremely large defect? Pediatr Surg Int.1999;15:206 –209
and physiologic consequences. J Pediatr Surg. 1980;15: 395–397
25. Fumino S, Shimotake T, Kume Y, et al. A clinical analysis of prognostic parameters of survival in children with congen-ital diaphragmatic hernia. Eur J Pediatr Surg. 2005;15: 399 – 403
26. Lally KP, Lally PA, Van Meurs KP, et al. Treatment evolution in high-risk congenital diaphragmatic hernia: ten years’ experi-ence with diaphragmatic agenesis. Ann Surg. 2006;244: 505–513
27. Keijzer R, Liu J, Deimling J, Tibboel D, Post M. Dual-hit hy-pothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol. 2000;156: 1299 –1306
28. Loff S, Wirth H, Jester I, et al. Implantation of a cone-shaped double-fixed patch increases abdominal space and prevents
recurrence of large defects in congenital diaphragmatic hernia. J Pediatr Surg.2005;40:1701–1705
29. Davis PJ, Firmin RK, Manktelow B, et al. Long-term outcome following extracorporeal membrane oxygenation for congeni-tal diaphragmatic hernia: the UK experience.J Pediatr.2004; 144:309 –315
30. Cortes RA, Keller RL, Townsend T, et al. Survival of severe congenital diaphragmatic hernia has morbid consequences. J Pediatr Surg.2005;40:36 – 45
31. Trachsel D, Selvadurai H, Bohn D, Langer JC, Coates AL. Long-term pulmonary morbidity in survivors of congenital diaphragmatic hernia.Pediatr Pulmonol.2005;39:433– 439 32. Chiu PP, Sauer C, Mihailovic A, et al. The price of success in the
DOI: 10.1542/peds.2006-3040
2007;120;e651
Pediatrics
The Congenital Diaphragmatic Hernia Study Group
Hernia
Defect Size Determines Survival in Infants With Congenital Diaphragmatic
Services
Updated Information &
http://pediatrics.aappublications.org/content/120/3/e651
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/120/3/e651#BIBL
This article cites 32 articles, 1 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/surgery_sub
Surgery
http://www.aappublications.org/cgi/collection/neonatology_sub
Neonatology
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_
Fetus/Newborn Infant following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2006-3040
2007;120;e651
Pediatrics
The Congenital Diaphragmatic Hernia Study Group
Hernia
Defect Size Determines Survival in Infants With Congenital Diaphragmatic
http://pediatrics.aappublications.org/content/120/3/e651
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.