Winter Diet Composition of Himalayan goral
(Naemorhedus goral) in Kazinag National Park,
Jammu and Kashmir, India
Jahangir Ahmad Dar, Mustahson F. Fazili, Bilal A. Bhat and Riyaz Ahmad
Abstract—Understanding winter diet composition of wild ungulates in temperate habitats is of paramount importance for informing conservation measures. The winter diet composition of Himalayan goral (Naemorhedus goral), one of the least studied ungulate species in Kazinag National Park (KNP) of Jammu and Kashmir, Indiawas assessed after preparing reference photographs of various parts of 28 available plant species through micro-histological technique. On analyzing 60 faecal samples, 2454 plant fragments belonging to 17 plant species were identified after comparing reference plants. By applying Ivlev’s electivity index (IEI), it was revealed that shrubs were strongly preferred (IEI = 0.19) during winter. Among them, Indigofera heterantha (DSV =2.47) was strongly selected followed by Viburnum grandiflorum (DSV=1.64), Prunus tomentosa (DSV=1.51), Rosa macrophylla (DSV=1.40) and Lonicera obovata (DSV=1.21). Himalayan goral adopted a browser strategy during winter when availability of grasses was low as inferred from the ratio of browse to grass (59.55% : 28.88%). Chi-square goodness of fit test showed that Himalayan goral did not feed on all plant species uniformly (p< 0.05). Our findings suggest that Himalayan goral has feeding plasticity to adapt to uneven diet availability. We recommend that plant species which are the major components of winter diet of Himalayan goral during resource-lean winter be conserved and the propagation of these taxa be encouraged.
Index Terms— diet composition, himalayan goral, Kajinag National Park, resource-lean ,winter.
—————————— ——————————
1.
INTRODUCTION
Winter, a season with harsh climatic conditions, is an important period for the survival of most mountain ungulates due to limited availability of preferred forage and energetic costs associated with movements through deep snow [1], [2],[3] and [4]. Environments with heavy snowfall and steep topography tend to have strong spatial and seasonal differences in food availability for ungulates [5] and [6]. The winter snow cover is one of the most important abiotic factors affecting the selection of resources. Ungulates inhabiting such extreme habitats cope with harsh winter and deep snow cover by using a variety of strategies like restriction of movements through deep snow [7] and [5]. Information about food habits of a species is a determining component for its survival, health, and mobility [8] and [9]. The composition and selection of various food items during different seasons is a fundamental element to understand multiple aspects of ungulate ecology [10]. The behavior of mountain ungulates is mainly affected by availability of food plants and the ways in which these are obtained during different seasons. The diet of an animal species is a determining aspect of ecological niche and its quantification has been one of the most significant steps in studying basic
ecology of an animal [11] and [12]. Determination of diet composition of the wild ungulates is therefore, important for sustainable wildlife and ecosystem management [13], [14] and [15]. The Himalayan goral (Naemorhedus goral) is endemic to Himalayan stretch and is listed in Appendix I of the CITES [16]. Its status as Near Threatened [17] calls for an urgent conservation action. One of the important steps towards conservation is the identification of winter diet items. Winter a critical season with severe conditions and scarcity of food has adverse impact on the survival of wild animals and information on diet composition during this critical season is crucial to frame conservation action for maintaining viable populations in the wild [18]. The present study was undertaken with the aim to evaluate winter diet composition of one of the least studied ungulate species, Himalayan goral in Kazinag National Park (KNP) of Kashmir. The data on diet composition would be useful to conservation stakeholders for planning forage and habitat management measures
2. Materials and Methods
2.1 Study Area
The study was conducted in KNP (34°10'0"N and 74°2'0"E) with an altitudinal range of 1,800 – 4,700m, located in the North-West Himalayan Bio-geographic zone of India (2A) [19] in the valley of Kashmir. Carved out from three protected areas, Lachipora Wildlife Sanctuary, Limber Wildlife Sanctuary and Naganari Conservation Reserve, the park comprises a total area of 157 km2 (Fig.1). The vegetation in general is temperate coniferous, sub alpine and alpine type [20] dominated by Pine (Pinus wallichiana), Deodar (Cedrus deodara) and Fir (Abies pindrow) in the lower and middle elevations. At higher elevations, the subalpine forest is dominated by Birch (Betula utilis) and mixed forests where as the alpine vegetation is dominated by Juniper (Juniperus squamata) and alpine meadows. The lower areas of riverine forests are dominated by Horse ————————————————
Jahangir Ahmad Dar is currently working as a research scholar in University of Kashmir,India,PH-+917889595248.E-mail: jahangirwildlife10@gmail.com.
Mustahson F. Fazili is currently working as Assistant Professor in University of Kashmir,India,PH-+919796932444. E-mail: drfaziliwl@yahoo.com.
Bilal A. Bhat is currently working as Assistant Professor in
University of Kashmir,India,PH-+917006767017.E-mail:bilalwildlife@yahoo.com
Chestnut (Aesculus indica) and cranberry bush (Viburnum grandiflorum). There are temperate grasslands with rolling terrain at lower elevations. Temperature varies from a minimum of –100 C in winter to a maximum of 300 C in summer. The precipitation is mainly brought by Westerly disturbances during winter and fall largely as snow, and is surely a factor of importance in determining the type of forests and the seasons in the area. The four distinct seasons in the region are: Spring (March to May), Summer (June to August), Autumn (September to November) and Winter (December to February).
Fig. 1 Map of the study area (Kazinag National Park).
2.2 Data collection
The present study was carried out during winter from December, 2017 to February, 2018. The study was based on the microscopic recognition of indigestible plant fragments mainly the epidermal features characteristic of different plant groups obtained from faecal pellets. Faecal analysis was done by the procedures outlined by Holechek
et al., [21]. The process includes preparation of reference slides of plants, collection of faecal samples, preparation of slides of faecal samples and identifications of plant fragments from the slides of faecal samples using reference slides of plants as followed by [22], [23] and [24].
2.3 Preparation of plant reference slides
Reference slides of plants were prepared for available plant species, which were determined by vegetation sampling of the area and identified from the Centre of Plant Taxonomy, University of Kashmir. For this 12 line transects of 2km each, were laid in four different winter range habitats: coniferous forest, grassland, cliffy and riverine areas. Along each transect plots of radius 10m were laid at regular intervals of 200m. The selection of these species as potential food plants was based on field observations and interviews of wildlife officials and locals. Plant samples collected were dried, shredded and placed in a test tube. Nitric acid (33%) and water in the ratio of 1:3 were added to the test tube. The test tube was heated in a water bath for five minutes. When solid material settled down, nitric acid was decanted and fresh nitric acid (33%) was added. The mixture was again boiled till the material of the test tube became transparent (chlorophyll free). The material was then thoroughly washed in running water to remove nitric acid. The sample was stained with saffarenin for two minutes to stain properly. After staining, the sample was
washed repeatedly in distilled water and dehydrated by passing through a mixture of alcohol and distilled water in the ratio of 1:3, 1:1 and 3:1 respectively. The sample was finally placed in absolute alcohol. The mounting was done in canada balsam and photograph of each sample was captured using a digital microscope (Olympus BX60).
2.4 Field collection of faecal samples
Faecal samples totaling 60 pellet samples were collected along 12 permanent transects. Pellets of one group of faeces were considered as one sample. From one sample four pellets were randomly selected for analysis [14]. The pellets of Himalayan goral were differentiated from markhor, musk deer, sheep and goat on the basis of morphological characters viz., dimension, shape and size [25]. Sampling plots were designed in a systematic manner, from randomly placed starting points. All sampling points were laid parallel to the track and almost equidistant (100 m) from one another. Wherever pellets were collected, a widely used plot size of 10m × 10m was laid around the pellets for studying dietary patterns of wild animals [26], [27], [28] and [29]. We followed Latham et al., [30] to age pellets as fresh (moist or oily texture), old (dry, slightly crusted), or aged (decaying).
2.5 Preparation of slides of faecal samples
Randomly selected oven-dried pellets from each faecal sample were grinded and passed through two successive sieves (mesh sizes: 5 and 3 mm). The course material was discarded and the fine powder was retained for further analysis. Fine material was transferred in a test tube having nitric acid (33%) and water in the ratio of 1:3. The test tube was allowed to heat in a water bath for 5 minutes. After settling, fresh nitric acid (33%) and water were added and the material was again boiled in order to obtain a fairly transparent (depigmented) material. The material was then thoroughly washed with running water repeatedly until the nitric acid is completely removed. The washed samples were used for preparing slides for analysis. Three slides were prepared for each sample giving a total of 180 (60 samples x 3 slides) for Himalayan goral in winter. While identifying the plant fragments, four fields of view (FOV) were considered giving a total of 720 FOV. Plant fragments from the pellets were identified with the help of reference slides of vegetation on the basis of characteristics such as cell wall, cell shape, trichomes and stomata [21].
2.6 Data Analysis
The relative frequency of a plant species in faecal samples was calculated and expressed as relative importance value (RIV), which is the total number of fragments identified for a given food species divided by the total number of all counts made in the sample and expressed as a percentage [31]. Diet selection value (DSV) was calculated after Jnawali, 1995 as follows:
DSVX =
where RIVx is the RIV for species x, and reflects the
relative frequency of a plant species in the faeces. PVx is
PVx =Mx ×√fx
where Mx is the % cover of species x and fx is the frequency
of occurrence of species x in sample quadrats. Food preference of goral was determined by calculating Ivlev’s electivity index (IEI) [33] using the equation:
IEIi = ri - pi/ ri + pi
where riis the proportion of vegetation type i in the diet of Himalayan goral, and piis the proportion of vegetation type
i along all the systematically sampled quadrats (i.e., its availability in the habitat). IEI of 1.0 denotes maximum preference of a vegetation type, 0 denotes use in proportion to availability, and a value of -1.0 denotes complete avoidance [33]. The data was analyzed using statistical packages MS-Excel 2007 and MINITAB (version 13.2) at confidence level of 95 % and P<0.05 for significance.
3. Results
During winter, 28 species of plants from 28 genera and 19 families were recorded (Table 1). Species with highest prominence value, a measure of availability, included Pinus
wallaichaina ( PV= 17.27), Cyanodon dactylon (PV=12.61),
Cedrus deodara (PV=12.23), Picea smithiana (PV= 10.35),
Stipa sibirica (PV=8.85) and Indigofera heterantha
(PV=8.26).The overall availability of plant categories was different with trees having highest availability (PV=53.21) followed by grasses (PV= 51.7), shrubs (PV= 45.06) and herbs (PV=13.27). A total of 17 plant species belonging to nine different families from 2454 identified plant fragments were recorded by faecal analysis. Of these, 1463 represented browse species and 709 represented graze species (Fig. 2). Apart from the identified plants fragments, 282 unidentified fragments with a percent occurrence of 11.49 were excluded from statistical analysis. Among browse species, shrubs were most dominant with an overall occurrence of 50.93%.Grasses represented 28.88% of the identified fragments while as herbs were not recognized
during the faecal analysis. The dominant shrubs in the diet were Indigofera heterantha (RIV=20.41), Prunus tomentosa
(RIV= 10.59), Lonicera obovata (RIV= 6.19) and Rosa
macrophylla (RIV=2.56). The dominant tree species in the
diet was Pinus wallaichaina (RIV=3.87) and Poa pratensis
was the dominant grass species (RIV=12.02). We identified no tree species that goral consumed significantly in higher proportion than their availability. Plant species utilized more than their availability included Indigofera heterantha
(PV=8.26,RIV=20.41), Prunus tomentosa (PV=6.98, RIV=10.59), Rosa macrofolia (PV= 1.82, RIV=2.56), Poa
pratensis (PV=9.05,RIV=12.02), Lonicera obovata
(PV=5.11,RIV=6.19) and Viburnum grandiflorum
(PV=2.05,RIV=3.74). However, Cedrus deodara
(PV=12.23,RIV=1.95), Aesculus indica
(PV=4.52,RIV=0.93), Picea smithiana,
(PV=10.35,RIV=1.71), Abies pindrow (PV=8.00,RIV=0.16),
Pinus walliachaina (PV=17.27,RIV=3.87), Elaeagnus
umbellata (PV=8.05,RIV=5.37), Cotoneaster nummularius
(PV=2.28,RIV=2.07), Cyanodon dactylon
(PV=12.61,RIV=6.76), Bothriochola ischaemum
(PV=6.21,RIV=4.43), Themeda spp. (PV=8.05,RIV=3.34) and Stipa sibirica (PV=8.85,RIV=2.40) were utilized less than their availability. The dominant plant categories were trees and grasses in winter but were utilized relatively in lower proportions (Fig. 3). The number of identified plant fragments from pellets differed significantly at species level (χ2
=1674.36, df =16, p=0.000), at family level (χ2= 1661.37, df =8, p=0.000) and at growth form level (χ2= 573.52, df = 2, p=0.000). Himalayan goral strongly selected Indigofera
heterantha (DSV =2.47) followed by Viburnum grandiflorum
(DSV=1.64), Prunus tomentosa (DSV=1.51), Poa pratensis
(DSV=1.32) and Lonicera obovata (DSV=1.21) during winter. Ivlev’s electivity values show that Himalayan goral shows a strong preference for shrubs during winter and least preference for grasses and trees (Fig.4).
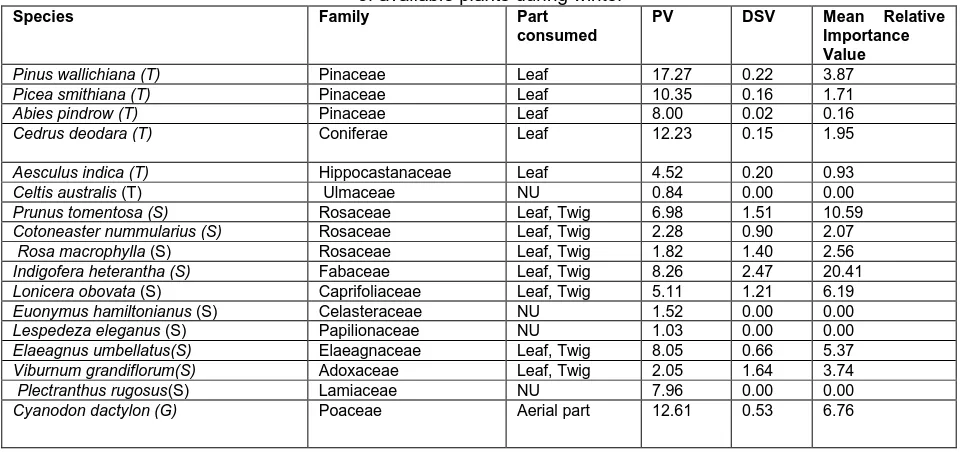
Table 1.Prominance value (PV), Diet selection value (DSV) andMean relative importance values of available plants during winter
Species Family Part consumed
PV DSV Mean Relative Importance Value
Pinus wallichiana (T) Pinaceae Leaf 17.27 0.22 3.87
Picea smithiana (T) Pinaceae Leaf 10.35 0.16 1.71
Abies pindrow (T) Pinaceae Leaf 8.00 0.02 0.16
Cedrus deodara (T) Coniferae Leaf 12.23 0.15 1.95
Aesculus indica (T) Hippocastanaceae Leaf 4.52 0.20 0.93
Celtis australis (T) Ulmaceae NU 0.84 0.00 0.00
Prunus tomentosa (S) Rosaceae Leaf, Twig 6.98 1.51 10.59
Cotoneaster nummularius (S) Rosaceae Leaf, Twig 2.28 0.90 2.07
Rosa macrophylla (S) Rosaceae Leaf, Twig 1.82 1.40 2.56
Indigofera heterantha (S) Fabaceae Leaf, Twig 8.26 2.47 20.41
Lonicera obovata (S) Caprifoliaceae Leaf, Twig 5.11 1.21 6.19
Euonymus hamiltonianus (S) Celasteraceae NU 1.52 0.00 0.00
Lespedeza eleganus (S) Papilionaceae NU 1.03 0.00 0.00
Elaeagnus umbellatus(S) Elaeagnaceae Leaf, Twig 8.05 0.66 5.37
Viburnum grandiflorum(S) Adoxaceae Leaf, Twig 2.05 1.64 3.74
Plectranthus rugosus(S) Lamiaceae NU 7.96 0.00 0.00
Poa pratensis (G) Poaceae Aerial part 9.05 1.32 12.02
Bothriochola ischaemum (G) Poaceae Aerial part 6.21 0.71 4.43
Themeda spp. (G) Poaceae Aerial part 8.05 0.41 3.34
Stipa sibirica (G) Poaceae Aerial part 8.85 0.27 2.40 Lolium perenne (G) Poaceae NU 6.68 0.00 0.00
Arabidopsis spp. (G) Brasicaceae NU 0.25 0.00 0.00
Dioscorea deltiodea (H) Cuscutaceae NU 5.01 0.00 0.00
Plantago lanceolata (H) Planaginaceae NU 2.62 0.00 0.00
Oxalis acetosella (H) Oxalidaceae NU 0.46 0.00 0.00
Rumex nepalensis (H) Polygonaceae NU 4.36 0.00 0.00
Viola odorata (H) Violaceae NU 0.82 0.00 0.00
Unidentified 11.49
Identified total 88.51
PV=Prominence value (a measure of availability); NU= Not Utilized; T=trees; S=shrubs; G=grasses and H=herbs.
Fig. 2 Number of plant fragments of various plant
categories recovered from faecal pellets of Himalayan goral
Fig. 3 Availability and utilization of different plant categories during winter in Kazinag National Park.
Fig. 4 Ivlev’s electivity index of plant categories identified from faecal pellets of Himalayan goral
4. Discussion
The composition and selection of food by ungulates is a fundamental element to understand dietary habits [10]. Knowledge regarding such habits of ungulates is of crucial importance for conservation, as food and food choice affect growth, development, vigour, disease resistance and reproductive success of an animal [34], [35] and [36]. The cryptic and sensitive nature of ungulates makes it difficult to obtain direct information on their feeding ecology. Therefore, pellet group analysis using micro-histological technique has become the most admissible indirect method to study their food components and feeding ecology [37]. The goral is believed to be principally a grazer, yet the magnitude of the grazing and the browsing diversify with the season and the area [38] and [39]. Availability of different forage categories is a function of habitat type. Himalayan goral usually prefers to forage in the early morning and late in the evening [40]. However such preferences change during winter and feeding continues throughout the day with short intervals as reported for Markhor [41].
At Kazinag National Park, shrubs and grasses were found to be important components of the goral diet during winter. The ratio of browse to graze (59.55% : 28.88%) obtained during present study clearly indicates that Himalayan goral shows a browsing strategy during winter and rely less on grazing. The reason behind such changed strategy of feeding during winter could be the environmental conditions as reported for grey goral in Pakistan [42]. The present findings are also supported by a study at Primorsky Krai
Trees, 212
Shrubs, 1251
Unidentifi ed, 282
Grasses, 709
Herbs, 0
53.21
45.06
51.7
13.27 8.62
50.93
28.88
0
Trees Shrubs Grasses Herbs
Availability Utilization
-0.8 -0.6 -0.4 -0.2 0 0.2 0.4
(Russia) where gorals are frequently regarded as both grazers and browsers, however, the extent of grazing and browsing changes with the season [43]. Similarly, bighorns (sheep) in British Columbia mostly browsed during the winter and shrubs contributed the greatest proportion of the diet [44]. The present study clearly reveals that goral strongly prefers shrubs (Fig.4) during winter with Indigofera
heterantha and Prunus tomentosa alone contributing
35.05% of the total diet. This is in agreement with the findings of Ashraf et al.,[24] who reported that the shrubs formed the largest component of the grey goral’s diet during winter with Berberis vulgaris and Viburnum nervosum as the most common dietary shrubs. However, Abbas [42] reported that shrubs contributed the lowest (< 1%) part in the total food of the grey goral based on the analysis of 15 faecal samples. But findings of several workers are in line with our study which show that ungulates heavily consumed shrubs during winter [45], [46] and [47].
Grasses were available in the goral habitat during winter but their proportion in the diet of Himalayan goral was relatively low. In contrast, a higher proportion of grasses was reported in several studies [48], [49] and [50]. Kazinag National Park receives precipitation mainly in the form of heavy snowfall during winter and could be the reason for low utilization of grasses as most of grasses remain under it. However, grasses in low snow areas were available to Himalayan goral but not in a sufficient quantity due to over grazing by livestock of herders and locals during rest of the seasons. The negative impact of livestock grazing on forage availability in winter was also speculated in Kunlun Mountains of Qinghai, China [14]. The herbs were also available in the habitat during winter but didn’t appear in pellets during analysis. The underestimation of herbs in the diet may be attributed to their higher digestibility [51] Five species of trees (Cedrus deodara, Aesculus indica, Picea smithiana, Abies pindrow and Pinus walliachaina) were found in the winter diet of Himalayan goral. The consumption of species viz., Picea smithiana, Cedrus
deodara and Abies pindrowby grey goral in Pakistan during
winter season has been reported [24]. The trees were abundant (53.21%) in the habitat, but contributed only 8.26% to the diet, owing their utilization to availability rather than selection which is evident from diet selection values of trees (Table 1). The consumption of gymnosperms by mountain ungulates indicates diet adjustments when forage availability is very limited [52] and serves as an important emergency winter forage when deep snow makes other forages unavailable [53]. The consumption of leaves by Himalayan goral was supported by the explanation that leaves are preferred by ungulates in in winter to enhance the quality of food when grasses are indecline [54].
Our findings reveal that the winter diet of Himalayan goral is dominated by shrubs and trees which contributed 59.55% to the total consumption. These findings were in confirmity with Kufeld et al., [55] who reported that winter diet of mule deer was dominated by browse species (74%) followed by graze species (26%) in western North America. Markhor consumes primarily grasses and forbs during spring and summer and shifts to browsing mode in winter for nourishment [56]. The dietary shift of himalayan goral to browsing mode may be due to decline in availability of graze species in winter with increasing snow depth. A shift
from a graminoid to browse diet due to reduced forage availability in winter have been reported in bharal [57]. Carpenter et al., [58] also found that there occurs an increased shrub use and decreased forb /grass consumption during winter with increased snow pack.
5. Conclusion
Within Indian limits Himalayan goral is a least studied ungulate species and detailed data on its ecological aspects is thus needed to design long term conservation planning. The information yielded on winter forage availability and diet composition of Himalayan goral in temperate climatic conditions of KNP is urgently needed for planning winter forage and habitat conditions during lean period of winter. There is a persistent need to evaluate the quality of winter food items for ascertaining the nutritional importance of each item in relation to survival of Himalayan goral.
6. Acknowledgments:
Our sincere thanks are due to SERB-DST, Govt of India for financing the work under the research project No. CRG/2019/002369. We are highly thankful to the Head of the Zoology Department, University of Kashmir for providing laboratory facilities. We are also thankful to the Department of Wildlife Protection, Jammu and Kashmir for permitting us to carry out this work in KNP.
7. References
[1] D.W. Burles and M. Hoefs, “Winter mortality of Dall sheep, Ovis dalli dalli, in Kluane National Park, Yukon,”
Can. Field-Nat., vol.98, pp.479–484, 1984.
[2] K.L.Parker, C.T. Robbins, T.A. Hanley, “Energy expenditures for locomotion by mule deer and elk,” J. Wildl. Manage. Vol.48, pp.474–488. 1984. doi:10.2307/ 3801180.
[3] T.V. Dailey and N.T. Hobbs, “Travel in alpine terrain: energy expenditures for locomotion by mountain goats and bighorn sheep,” Can. J. Zool., vol.67, pp.2368– 2375. 1989. doi:10.1139/z89-335.
[4] G.G. Cotterill, P.C. Cross, E.K. Cole, R.K. Fuda, J.D. Rogerson, B.M. Scurlock and J.T. du Toit, “Winter feeding of elk in the Greater Yellowstone Ecosystem and its effects on disease dynamics,” Philosophical Transactions of the Royal Society B., pp. 373, 2018. [5] B. Zweifel-Schielly, M. Kreuzer, K.C. Ewald and W.
Suter, “Habitat selection by an Alpine ungulate: the significance of forage characteristics varies with scale and season,” Ecography, vol. 32, pp.103–113, 2009.
[6] D.C. Stoner, J.O. Sexton, J. Nagol, H.H. Bernales and T.C, Edwards Jr., “Ungulate reproductive
parameters track satellite observations of plant phenology across latitude and climatological regimes,” PLoS One, vol. 11, pp.1–19, 2016.
[7] A. Mysterud, C, Iversen and G. Austrheim, “Effects of density, season and weather on use of an altitudinal gradient by sheep,” Appl. Anim. Behav. Sci., vol.108, pp.104–113, 2007.
environs,” PhD thesis, Department of botany, Harisingh Gaur Vishwavidyalaya, Sagar, India, 249, 1990. [9] A.M. Hein, C. Hou and A.F. Gillooly, “Energetic and
biomechanical constraints onanimal migration distance,” Ecol. Lett., vol.15, pp.104–110, 2012. [10] T. Bhattacharya, T. Bashir, K. Poudyal, S.
Sathyakumar and G.K. Saha, “Distribution, occupancy and activity patterns of goral (Nemorhaedus goral) and serow (Capricornis thar) in Khangchendzonga Biosphere Reserve, Sikkim, India,” Mamm.Study, vol. 37, pp.173–181, 2012.
[11] A. Sih and B. Christensen, “Optimal diet theory: when does it work, and when and why does it fail?,” Animal Behaviour, vol.61,pp.379–390. 2001.
[12] J. D. Scasta, J. L. Beck and C, J. Angwin, “Meta-analysis of diet composition and potential conflict of wild horses with livestock and wild ungulates on western rangelands of North America,” Rangeland Ecology & Management, vol.69, pp.310–318, 2016. [13] G. Caughley and A. R. E. Sinclair, “Wildlife Ecology
and Management,” Cambridge: Blackwell Science, pp.305–324, 1994.
[14] R.B. Harris and D.J. Miller, “Overlap in summer habitats and diets of Tibetan plateau ungulates,” Mammalia, vol. 59,pp.197–212. 1995.
[15] Z. Q. Li, Z. G. Jiang and C. W. Li, “Dietary overlap of Przewalski's gazelle, Tibetan gazelle, and Tibetan sheep on the Qinghai–Tibet Platea,”. Journal of Wildlife Management, vol.72, pp.944–948, 2008.
[16] J.W. Duckworth and J. Mackinnon, “Naemorhedus goral. In: IUCN 2011. IUCN Red List of Threatened Species. Version 2011.1,” 2008.
[17] IUCN, “The IUCN Red List of Threatened Species.
Version 2016-1,” 2016. Available at:
www.iucnredlist.org. (Accessed: 30 June 2016). [18] A. A. Formosov, “Snow cover as an integral factor of
the environment and its importance in the ecology of mammals and birds,” 1946. (English translation, 176 pp. Alberta, 1964).
[19] W.A. Rodgers and H.S. Panwar, “Planning a protected area network in India. Vol.1.Wildlife Institute of India, Dehra Dun,” 1988.
[20] H. Champion and S. Seth, “Forest types of India. Dehra Dun: Forest Research Institut,” 1968..
[21] J.L. Holecheck and B.D. Gross, “Training needed for quantifying simulated diets from fragmented range plants,” Journal of Range Management, vol. 35, pp.644-647, 1982.
[22] A. Haleem, , O. Ilyas, Z. Sayed, S.K. Arya and E. Imam, “Distribution, status and aspects of ecology of mammalian species in Kedarnath wildlife Sanctuary, Uttarakhand Himalayas, India,” J. Mater. Environ. Sci., vol. 5, pp.683-692, 2014.
[23] B.K. Nayak and A.K. Patra,” Food and feeding habits of Indian Bison, (Smith, 1827) in Kuldiha Wildlife Sanctuary, Balasore, Odisha, India and its conservation,” Int. Res. J. Biol. Sci., vol. 4,pp.73–79, 2015.
[24] N. Ashraf, M. Anwar, M. K. Oli, W. E. Pine, M. Sarwar, I. Hussain and M.S. Awan, “Seasonal variation in the diet of the grey goral (Naemorhedus goral) in Machiara
National Park (MNP), Azad Jammu and Kashmir, Pakistan,” Mammalia., 2016.
[25] N. Ashraf, M. Anwar, I. Hussain and M.A. Nawaz, “Competition for Food between the markhor and domestic goat in Chitral Pakistan,” Turk. J. Zool., Vol.38, pp.191-198, 2014.
[26] S.D. Schemnitz, “Wildlife management technique manual,” 4th edition. Washington, D.C.: Wildlife Society., 1980.
[27] S. Panthi, “Feeding ecology, habitat preference and distribution of red panda (Ailurus fulgens fulgens) in Dhopatan Hunting Reserve, Nepal,” BSc thesis, Tribhuvan University, Instrtute of Forestry, Pokhara, Nepal., 2011.
[28] S. Panthi, A. Aryal, D. Raubenheimer, J. Lord, and B. Adhikari, “Summer diet and distribution of the red panda (Ailurus fulgens fulgens) in Dhorpatan hunting reserve, Nepal,” Zoological Studies, vol.51, pp.701-709, 2012.
[29] A. Aryal, S. Panthi, R.K. Barraclough, R. Bencini, B. Adhikari, W. Ji and D. Raubenheimer, “Habitat selection and feeding ecology of dhole (Cuon alpinus) in the Himalayas,” Journal of Mammalogy, vol. 96,pp.47-53 2015a.
[30] A.D.M. Latham, M.C. Latham, K.H. Knopff, M. Hebblewhite and S. Boutin, “Wolves, white-tailed deer, and beaver:implications of seasonal prey switching for
woodland caribou declines,” Ecography,
vol.36,pp.1276–1290. 2013.
[31] S.R. Jnawali, “Population ecology of greater one-horned rhinoceros (Rhinoceros unicornis) with particular emphasis on habitat preference, food ecology, and ranging behavior of a reintroduced population in Royal Bardia National Park in lowland Nepal(thesis),” Aas, Norway: Agricultural University of Norway, 129, 1995.
[32] R.A. Koirala, R. Shrestha and P. Wegge, “Grasslands in the Damodar Kunda Region of Upper Mustang, Nepal. In Grassland ecology and management in protected areas of Nepal,” Technical and status papers on grasslands of mountain protected areas, pp. 53–6, 2000.
[33] V.S. Ivlev,” Experimental ecology of the feeding of fishes,” Yale University Press, New Haven, Connecticut, USA, 1961.
[34] S.H. Berwick, “The Gir Forest: An endangered ecosystem,” Am. Sci.,vol. 64, pp. 28–40, 1976.
[35] C. Martin, “Status and ecology of the barasingha (Cervus duvauceli branderi) in Kanha National Park. (India),” J. Bom. Nat. His. Soc., vol.74, pp.60-132, 1977.
[36] T. M. Martinez, “The feeding strategy of Spanish ibex (Capra pyrenaica) in the northern Sierra de Gredos (central Spain),” Folia Zoologica, vol. 50, pp.257-270, 2001.
[37] O. Ilyas, “Status and conservation of ungulates in the Kumaon Himalaya with special reference to barking deer (Muntiacus muntjac) and goral (Nemorhaedus goral),” PhD thesis, A.M.U. Aligarh, pp.280, 2001. [38] J.I. Mead, “Naemorhedus goral,” Mamm. Species,
[39] T.J. Roberts, “Mammals of Pakistan,” Revised edition, Oxford University Press, Karachi, Pakistan, 525. 1997. [40] C. Zhang, “Nemorhaedus canbrooki Hayman In: Soma,
H.H.(ed) The biology and management of Capricorns & related mountain antelopes,” Croom Helm, London, pp.213-223, 1987.
[41] T. J. Roberts, “The mammals of Pakistan,” London, Ernest Benn., pp. 361, 1977.
[42] F. Abbas, “A study on ecobiology of grey goral (Naemorhedus goral) with reference to Pakistan,” (Unpublished) Ph.D.Thesis. University of the Punjab, Lahore, Pakistan, 179, 2006.
[43] Z.G. Valva, “Food plants of the long tailed goral in the Primorsky Krai,” Rastitelnye Resursy., Vol.14, pp.446– 454, 1979.
[44] B.M. Wikeem and M.D. Pitt, “Diet of California bighorn sheep (Ovis canadensis californiana) in British Columbia: assessing optimal foraging habitat,” Can. Field Nat., vol.106, pp.327–335, 1992.
[45] G. Zofia, “Food of the roe deer (Capreolus capreolus) and red deer (Cervus elaphus) in the Bialowieza Primeval Forest, Poland,” Acta Theriol, vol. 25, pp.487 – 500, 1980.
[46] G.D. Wagner and J.M. Peek, “Bighorn sheep diet selection and forage quality in Central Idaho,” Northwest Sci., vol.80, pp.246–258, 2006.
[47] G.M. Shah, M.Y. Qadri and A.R. Yousuf, “Winter diets of hangul deer (Cervus elaphus hanglu, wagner) at Dachigam national Park, Kashmir,” J. Indian Inst. Sci., vol. 64,pp.129-136, 1983.
[48] C. Mishra, “Habitat Use by goral (Nemorheaedus goral bedfordi ) in Majhatal harsang Wildlife Sanctuary, Himachal Pradesh, India,” M.Sc. Thesis on Wildlife Science: Wildlife Institute of India, Dehradun, 1993.
[49] C. Mishra and A.J.T. Johnsingh, “On habitat selection by the goral (Naemorhedus goral bedfordi),” J. Zool. ,vol. 240, pp.573-580, 1996.
[50] O. Ilyas and J.A. Khan, “Food habits of barking deer (Muntiacus muntjak) and goral (Naemorhedus goral) in Binsar Wildlife Sanctuary India,” Mammalia, 2004 [51] S. Galina and C. Chargoy, “Forage quality and load
capacity of the native vegetation of the La Michilla Biosphere Reserve for deer and cattle,” 1987.
[52] R. Ahmad, C. Mishra, N. J. Singh, R. Kaul, Y. V. Bhatnagar, “Forage and security trade-offs by markhor Capra falconeri mothers,” Current Science, vol.110, pp.1559–1564, 2016.
[53] L.G. Adams and J.A. Bailey, “Winter forages of mountain goats in central Colorado,” Journal of Wildlife Management, vol.47, pp.1237-1243, 1983. [54] K. Ligi and T. Randveer, “Pre winter diet composition of
red deer (Cervus elaphus L.) in Estonia,” Balt. For. vol.18, pp.150–155, 2012.
[55] R.C. Kufeld, O.C. Wallmo and C. Feddema, “Foods of the Rocky Mountain mule deer,” U.S. Dep. Agric. For. Serv. Res., Paper RM–1 11, 1973.
[56] M.K. Ranjitsinh, C.M. Seth, R. Ahmad, Y.V. Bhatnagar and S.S. Kyarong, “A rapid assessment of the Pir Panjal Markhor in Jammu and Kashmir: Distribution, Status and Threats,” A Schaller Conservation Survey. Wildlife Trust of India, 2007.
[57] K.R. Suryawanshi, Y.V. Bhatnagar and C. Mishra, “Why should a grazer browse?:Livestock impact on winter resource use by blue sheep Pseudois nayaur,” Oecologia, vol.162, pp.453–462, 2010.