A Validated Stability-indicating RP-HPLC Method for Analysis of Azelaic Acid in Pharmaceuticals
Full text
Figure


Related documents
Isopropyl Vinyl Ether See Vinyl Isopropyl Ether. lsovalerone See Diisobutyl Ketone.. Density BOILING METHODS IDENTIFICATION POINT TEMP. Percent by Vol.. See Hazardous
For EIG, significantly higher scores were observed in the edgewise appliance compared to Invisalign in all three variables (intensity of pain, number of days, and discom- fort
Although this problem does not pack graphs, the matching algorithm relates to our problem of packing because it is packing as many edges in a graph with the constraint that each
This systematic review aims to identify correlates of screen time (specifically smart- phones, electronic tablets, handheld computers, personal digital assistants (PDA) and any
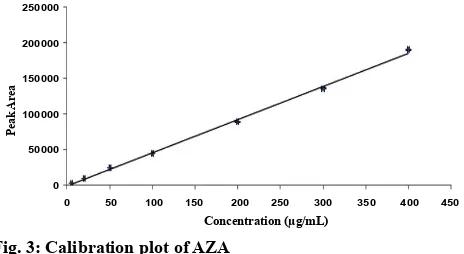
The objective of the study is to develop simple, precise, selective and stability indicating high-performance liquid chromatography (HPLC) method for determination of
In this present study the mechanical behavior were predicted through statistical analysis of the measured micro hardness at different conditions and find the effects of
Three meth- ods of random assignment to the control therapies are listed: (1) subjects may be assigned at random to the treatment and control groups (6A1); (2) subjects may be used
This case reports an incidence of culture negative endocarditis (CNE) in obstetric practice derived from intravenous heroin abuse and how important multidiscipline collaboration