Impact of Chlorinated Swimming Pool Attendance on the Respiratory Health of Adolescents
Full text
Figure
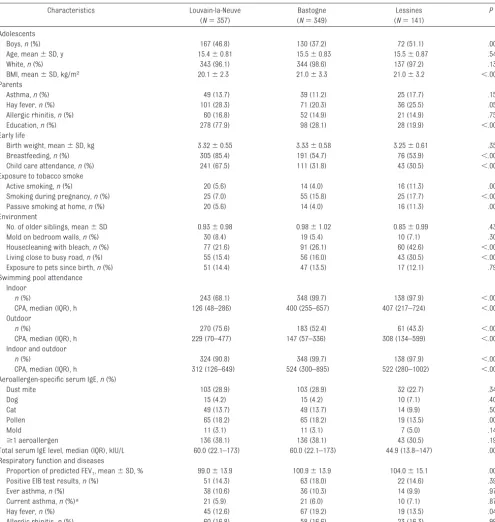
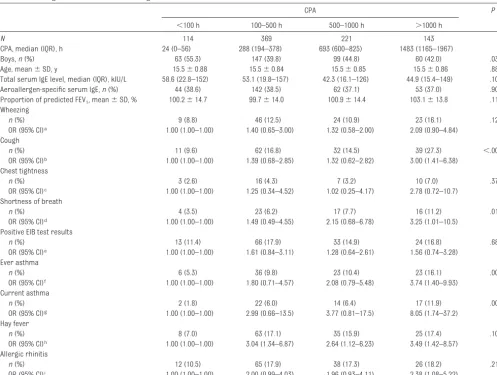
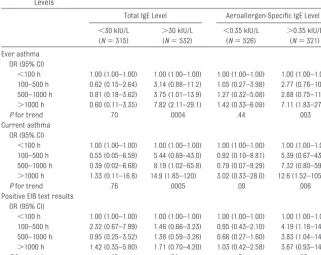
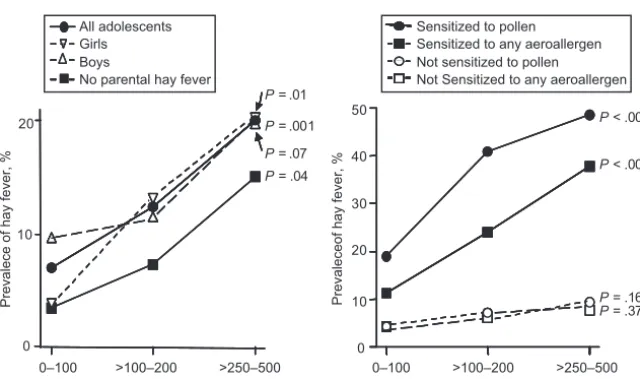
Related documents
As this debate continues, it is important to distinguish between recommending a vaccine (as the Advisory Committee on Immunization Practices [ACIP] does), deciding whether state
Factors examined included: recall of a doctor telling the respondent that their child was overweight or gaining weight too fast (doctor concern); perception that their child was
Find out the total deformation of pump impeller for lowest natural frequency of vibration and in case of result section compared it for all four vane outlet angle 20 , 24 , 30
A study to determine whether Radiological Impact Assessment (RIA) is needed for landfill disposal of treated sludge (slag) from oil and gas industries has been carried
• GCOS SC are invited to recognise the ISTI databank as a GCOS data archive for land surface temperatures and other data and to help provide links between data sources and
The study aimed to gather expert opinion on pain management in cognitively impaired hospitalised older people.. Methods: Consultant Geriatricians listed as dementia leads in
Methods: We propose a nonparametric spatial scan statistic based on the Wilcoxon rank-sum test statistic and com- pared the performance of the method with parametric models via