International Journal of Medical Science and Current Research (IJMSCR)
Available online at: www.ijmscr.com
Volume2, Issue 4,Page No: 581-592
July-August 2019
581
ISSN (Online): 2209-2862
PUBMED-National Library of Medicine ID-101739732
IJMSCR
Ideal Infection Control Practices – Strengthening Healthcare Associated Infection
Prevention and Control
Dr. Sanjeev Kumar
Assistant Professor, Department of Microbiology Pacific Institute of Medical sciences Udaipur, Rajasthan, India
*Corresponding Author:
Dr. Sanjeev Kumar
Assistant Professor, Department of Microbiology Pacific Institute of Medical sciences Udaipur, Rajasthan, India
Type of Publication: Review Article Conflicts of Interest: Nil
ABSTRACT
Hospital acquired infections occur at higher rates in developing countries, like India, than in developed countries. These infections are serious and they lead to increased patient’s morbidity, mortality, length of hospital stay and cost of treatment. Effective implementation of infection control practices is important to reducing the transmission of hospital acquired infections (HAIs) at hospitals worldwide. Device-associated infections (DAIs) continue to be one of the main threats to the patient safety, particularly in Intensive Care Units (ICUs) of low and middle income countries. Hand hygiene is now regarded as one of the most important element of infections control activities. Cleaning surgical instruments is critical because residual organic material, like blood, pus, tissue and bone, can inactivate disinfectants and allow potentially deadly bacteria to remain on the surfaces. Contaminated hospital linen often contains high numbers of organisms (106-108 CFU/100 cm2) from blood and body fluids. Environmental cleaning is a fundamental principle of infection prevention in healthcare settings. Contaminated hospital surfaces play an important role in the transmission of dangerous pathogens, like antibiotic resistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA), Carbapenem-resistant Enterobacteriaceae (CRE), and Vancomycin-resistant Enterococci (VRE). Hospital waste is a potential reservoir of pathogenic microorganisms and requires appropriate, safe and reliable handling. Personal protective equipment (PPE) limits the health care workers contact with all secretions or biological products. A breach in infection control practices facilitates transmission of infection from patients to health care workers, other patients and attendants.
Keywords: Infection control, HAIs, Hand hygiene, PPE, Cleaning & disinfection
INTRODUCTION
Approximately 5-10 percent of patients admitted to acute care hospitals in developed countries and more than 25 percent of such patients in developing countries have been found to acquire infections which were not present or incubating at the time of admission. Such hospital acquired infections, also known as health care associated infections (HAIs) or nosocomial infections. HAIs are among the most
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
58
2
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
Pag
e
582
caused by multi and pan-drug resistant organisms, causing therapeutic dilemma.
Device-associated infections (DAIs) such as ventilator-associated pneumonia (VAP), central line-associated blood stream infections (CLABSIs), catheter-associated urinary tract infections (CAUTIs) and surgical site infections (SSIs) together responsible for most of the HAIs across the world.[4] DAIs continue to be one of the main threats to the patient safety, particularly in Intensive Care Units (ICUs) of low and middle income countries (LMICs).[5-6]
Among the many causes of higher prevalence of HAIs in LMICs, the most important are the lack of financial resources, workforce and policies for infection control; lack of surveillance systems; poor hygiene and sanitation; insufficient of induction and continuous training for healthcare workers on infection control; limitations in sterilization/disinfection and aseptic practices; limited access to microbiology services in many hospitals and lack of regulations for use of antimicrobials.[7-8]
All the healthcare personnel are equally responsible to prevent HAIs, but nurses are the most important frontline staff. They play a critical role in controlling infection that begins with hand hygiene and good infection control practices. Healthcare workers (HCWs) should be equipped with the requisite knowledge, skill and attitude for good infection control practices for that they need continue infection control education, which is a core component of infection control programs. Education through various means imparts knowledge about the correct practices and also helps to update the existing knowledge according to the changing scenarios.[9,10]
The important components of the infection control programme are; basic measures for infection control, i.e. standard and additional precautions; education and training of health care workers; protection of health care workers, e.g. immunization; identification of hazard and minimizing risks; routine practices essential to infection control such as aseptic techniques, use of single use devices, reprocessing of instruments and equipment, antibiotic usage, management of blood/body fluid exposure, handling and use of blood and blood products, sound management of medical waste; effective work
practices and procedures, such as environmental management practices including management of hospital/clinical waste, support services (e.g., food , linen), use of therapeutic devices; surveillance; incident monitoring; outbreak investigation and research.[11]
INFECTION CONTROL PRACTICES:
Transmission of infections in health care facilities can be prevented and controlled through the application of basic infection control precautions which can be grouped into standard precautions, which must be applied to all patients at all times, regardless or diagnosis or infections status, and additional (transmission based) precautions which are specific to modes of transmission (airborne, droplet and contact).[10,12]
Standard precautions: Treating all patients in the health care facility with the same basic level of “standard” precautions involves work practices that are essential to provide a high level of protection to patients, health care workers and visitors. These include the following;
Hand washing and antisepsis (hand hygiene) Use of personal protective equipment (PPE) Appropriate handling of patient care
equipment and soiled linen
Prevention of needle stick/sharp injuries Environmental cleaning and
spills-management
Appropriate handling of Bio-medical waste HAND HYGIENE:
e
583
e
583
e583
e
583
e583
e
583
e583
e
583
e583
e
583
e
583
e
583
e
583
e583
e
583
e583
e
583
e583
e
583
e583
e
583
suspected or proven, including outbreaks of C.difficile. Otherwise, hand rubbing with an alcohol based agent is recommended. Compliance monitoring for hand hygiene among HCWs therefore had been given a higher recommendation level.[16]
USE OF PERSONAL PROTECTIVE
EQUIPMENT:
Personal protective equipment are designed to protect health care providers from serious work place injuries or illnesses.[17] Using personal protective equipment provides a physical barrier between microorganisms and the wearer. It offers protection by helping to prevent microorganisms from; contaminating hands, eyes clothing, hair and shoes; being transmitted to other patients and staff. Personal protective equipment includes: gloves, goggles, mask, apron, gown, boots/shoe covers and cap/hair cover.[10,18]
Personal protective equipment should be used by: Health care workers who provide direct care
to patients and who work in situations where
they may have contact with blood, body fluids, excretions or secretions.
Support staff including medical aides, cleaners and laundry staff in situations where they may have contact with blood, body fluids, secretions and excretions.
Laboratory staff, who handle patient specimens.
Family members who provide care to patients and are in a situation where they may have contact with blood, body fluids, secretions and excretions.
APPROPRIATE HANDLING OF PATIENT CARE EQUIPMENT AND SOILED LINEN:
PATIENT CARE EQUIPMENT:
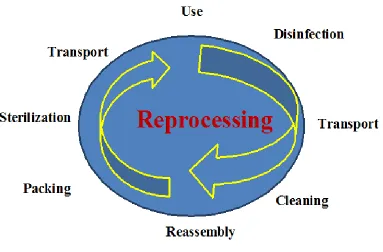
Handle patient care equipment soiled with blood, body fluids secretions or excretions with care in order to prevent exposure to skin and mucous membranes, clothing and environment. Ensure all reusable equipment is cleaned and reprocessed appropriately before being used on another patient.[Figure 1]
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
Pag
e
584
DISINFECTION AND STERILIZATION:
More than 30 years ago, Earle H. Spaulding devised a rational approach to disinfection and sterilization of patient-care items and equipment.[19] This classification scheme is so clear and logical that it has been retained, refined and successfully used by infection control professionals and others when
planning methods for disinfection or sterilization.[20,21] Spaulding believed the nature of disinfection could be understood readily if instruments and items for patient care were categorized as critical, semi-critical and noncritical according to the degree of risk for infection involved in use of the items.[Table 1]
Table 1: Classification of Devices, Processes, and Germicidal Products
Device classification Devices (examples) Spaulding process classification classification EPA product
Critical (enters sterile tissue or vascular system)
Implants, scalpels, needles, other surgical instruments, etc.
Sterilization – sporicidal chemical; prolonged contact
Sterilant/disinfectant
Semi-critical (touches mucous membranes, except dental)
Flexible endoscopes, laryngoscopes,
endotracheal tubes, and other similar
instruments
High-level disinfection – sporicidal chemical; short contact
Sterilant/disinfectant
Thermometers,
hydrotherapy tanks Intermediate-level disinfection Hospital disinfectant with label claim for
tuberculocidal activity Noncritical (touches
intact skin) Stethoscopes, tabletops, bedpans, etc. Low-level disinfection Hospital disinfectant without label claim for
tuberculocidal activity Modified from Favero MS, Bond WW. Chemical disinfection of medical and surgical materials. In: Block SS. Ed. Disinfection, sterilization and preservation. 4th ed. Philadelphia: Lea &Febiger 1991:627
LINEN:
Linen is a fabric made from fibers. In general term it is used to denote clothing items. It includes all the clothing materials used in hospitals including; Mattress, blanket, bed cover, screen, pillow cover, curtains, bed sheets, doctors’ coat towel, table cover etc.,.
Fabrics, textiles and clothing used in health-care settings are disinfected during laundering and generally rendered free of vegetative pathogens (i.e., hygienically clean), but they are not sterile.[22] Laundering cycles consist of flush, main wash, bleaching, rinsing and souring.[23] Cleaned wet textiles, fabrics and clothing are then dried, pressed as needed and prepared (e.g., folded and packaged) for distribution back to the facility.
The antimicrobial action of the laundering process results from a combination of mechanical, thermal and chemical factors.[24] Dilution and agitation in
water remove substantial quantities of microorganisms. Soaps and detergents function to suspend soils and also exhibit some microbicidal properties. Hot water provides an effective means of destroying microorganisms.[25] The use of chlorine bleach assures an extra margin of safety.[26]
In the absence of microbiologic standards for laundered textiles, no rationale exists for routine microbiologic sampling of cleaned health-care textiles and fabrics.[27] Sampling may be used as part of an outbreak investigation if epidemiologic evidence suggests that textiles, fabrics or clothing are a suspected vehicle for disease transmission. Sampling techniques include aseptically macerating the fabric into pieces and adding these to broth media or using contact plates for direct surface sampling.[28]
e
585
e
585
e585
e
585
e585
e
585
e585
e
585
e585
e
585
e
585
e
585
e
585
e585
e
585
e585
e
585
e585
e
585
e585
e
585
versus reusable surgical attire and fabrics in health-care settings.[29] Regardless of the material used to manufacture gowns and drapes, these items must be resistant to liquid and microbial penetration.[30]
PREVENTION OF NEEDLE STICK/SHARP INJURIES:
Needle stick and other sharps injuries are a serious hazard in any healthcare setting. Contact with contaminated needles, scalpels, broken glass and other sharps may expose healthcare workers to blood that contains pathogens which pose a grave, potentially lethal risk.[31]
Hepatitis C (HCV) and Human immune deficiency virus (HIV), are two of the most serious of the 20 blood-borne pathogens that healthcare workers are exposed to in their daily work caring for the world’s health. Hepatitis B virus (HBV) is the most common blood borne infection and the only of the three serious viral infections for which an immunization exists. Other infections transmittable through needle sticks include syphilis, malaria and herpes.[32,33]
Healthcare workers incur 2 million needlestick injuries (NSI’s) per year that result in infections with hepatitis B & C and HIV. The WHO estimates the global burden of disease from occupational exposure to be 40% of the hepatitis B and C infections and 2.5% of the HIV infections among HCWs as attributable to exposures at work.[34] While 90% of the occupational exposures occur in the developing world.[35]
Globally, NSIs are the most common source of occupational exposures to blood and the primary cause of blood-borne infections of HCW’s.[36] The two most common causes of NSIs are two – handed recapping and the unsafe collection and disposal of sharp waste.[37]
Factors that increased risks of transmission of HIV include a deep wound, visible blood on the device, a
hollow-bore blood filled needle, use of the device to access an artery or vein and high-viral load status of the patient.[38] Post-exposure prophylactic medication has been demonstrated to reduce the risk of transmission of HIV following NSI by 80%.[39]
The most effective means of preventing the on transmission of blood-borne pathogens is to prevent exposure to NSIs. Primary prevention of NSIs is achieved through the elimination of unnecessary injections and elimination of unnecessary needles. The implementation of education, Universal precautions, elimination of needle recapping and use of sharp containers for safe disposal have reduced NSIs by 80%, with additional reductions possible through the use of safer needle devices.[40-42]
ENVIRONMENTAL CLEANING AND SPILL MANAGEMENT:
Cleaning process:
Adequate cleaning with a proper disinfectant is important as it removes all dust, bacterial flora, organic matter and other contaminants. The disinfectant used should non-carcinogenic, easily available and safe to be used by the hospital staff. Most disinfectant removes almost 90%-95% of the infectious microbes except spores. Keeping the operating complex dry would make microbes and even spores unviable.[43]
Only wet mopping of walls and floor should be employed inside the operating room complex. Use the three bucket system to mop the OT. The first two buckets contain purified water. A clean and dry mop is immersed sequentially into the buckets before the figure of eight methods of cleaning is used. The walls also cleaned with a lint free cloth using the three bucket system taking care to mop from clean to unclean area. Cleaning protocol for OT and ICU as follows [Table 2]
Table 2: Cleaning protocol for OTs and ICUs [44]
Area Disinfectant Frequency
Roof 2% Baillocid Once in 3 months
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Pag
e
586
Floor 2% Baillocid Daily twice
Refrigerator Defrost & clean with soap and water Weekly once
Sink Soap, water/Sodium hypochlorite Daily once
OT & ICU furniture Alcohol based spray Daily once
AHU & Pre-filters Water Once in 3 months
Swabs to be taken from different areas for sterility testing: [44]
OT table head end, Crash cart, Terminal HEPA filter
Phaco tray, Refrigerator, Scrub basin
Mayo trolley, Surgeon & nurse gloves, Microscopic handles
Boyle’s, Door handles/walls, Light Pendant handles
Circumstances for Fumigation:
Fumigation of high risk areas - OT/ ICU/ labour room/ isolation wards shall be done in the following circumstances:
Newly constructed / repair activity undertaken recently in that area.
In any other circumstances where fumigation is required e.g. after surgery on infectious cases or major spills of faecal matter.
Routinely: Once a month depending on the nature of civil infrastructure, number of surgical cases and movement of staff and equipment’s.
Area not used for long duration before its usage needs to be fumigated.
High occupancy areas like ICU, labour room etc. when found vacant needs to be fumigated.
Pre-fumigation procedures:
Clean the area (windows, doors, floor, walls, surgery table and all washable equipment’s) thoroughly with soap and water.
Close windows and ventilators tightly. If any openings found, seal it with cellophane tape or other material to avoid the leak of fume. Switch off all lights, Air Conditioner (AC)
and other electrical and electronic items.
Calculate the room size (surgical theatre only) in cubic feet (LxBxH) and calculate the required amount of environmental disinfectant preferably non formalin compounds like hydrogen peroxide + silver nitrate solutions as per the manufacturer’s instructions. Formaldehyde is an irritant to eyes and nose and it has also been recognized as a potential carcinogen.
SPILL MANAGEMENT OF BODY FLUIDS:
A review by Peate[45] defined a body fluid as “any fluid found in, produced by or excreted from the human body which includes blood, urine, faeces, saliva, tears, breast milk, cerebrospinal fluid (CSF), semen, vaginal fluid, amniotic fluid, pleural fluid, peritoneal fluid, bile, digestive juices, vomit and pus.” In terms of standard infection control precautions practice, body fluids are considered hazardous and should be dealt with immediately.[45-52]
The Advisory Committee for Dangerous Pathogens (ACDP) has identified body fluids that may contain blood-borne viruses (BBV): Blood, semen, CSF, breast milk, pleural fluid, amniotic fluid, peritoneal fluid, vaginal secretions, pericardial fluid, synovial fluid and any body fluids containing blood (blood stained).[47]
Spill Management:
Appropriate personal protective equipment (e.g. gloves, apron, eye/face protection) must be worn when dealing with blood and other body fluid spillage.[45,49-52]
e
587
e
587
e587
e
587
e587
e
587
e587
e
587
e587
e
587
e
587
e
587
e
587
e587
e
587
e587
e
587
e587
e
587
e587
e
587
Solutions containing sodium hypochlorite should not be prepared in hot water or mixed with anionic detergents as this can result in the release of chlorine gas. These solutions should be discarded at the end of the task or at the end of the day.[52]
Waste materials such as contaminated paper towels should be disposed of as healthcare waste (clinical waste) after use.[45,51,53,54]
The efficacy of chlorine releasing agents is reduced when in the presence of organic matter (e.g. blood), therefore it is recommended that blood spills are directly treated with a chlorine releasing agent (sodium hypochlorite) at a concentration of 10,000 parts per million available chlorine.[49,53-55]
When dealing with urine spillages it is important that these are not treated using a chlorine releasing agent as this can result in a release of chlorine gas. It is therefore suggested that these spills are first absorbed using paper towels, disposed of as healthcare waste (clinical waste) and the area washed with either detergent[45] or disinfectant,[51] however the preferred
method is not clear. Hall[51] suggests it may be useful to provide spill kits in areas where spills are most likely to occur. It is recommended that the instructions on how to deal with spillages and the associated materials required e.g. PPE, cleaning solutions; waste bags should be included in spill kits. A nominated staff member should carry out checks on these kits to ensure that all of the components are present and in date.
APPROPRIATE HANDLING OF
BIO-MEDICAL WASTE:
The term ‘Bio-medical waste’ includes any waste which is generated during the diagnosis, treatment or immunization of human beings or animals or research activities pertaining there on, or in the production or testing of biological or in health camps.[56] The management of waste starts from waste minimization, segregation at source till its final treatment and disposal options. The important component that should be kept in mind throughout the cycle approach is that of worker safety, patient safety and environment safety. [Figure 2]
Figure 2: Bio-Medical waste flow chart
The major salient features of BMW management rules, 2016 along with Bio-Medical Waste Management (Amendment) Rules, 2018.[57]
1. The scope of the rules has been expanded to include vaccination camps, blood donation camps, surgical camps or any other healthcare activity.
2. Phase-out the use of chlorinated plastic bags, gloves and blood bags within two years of
notification of BMW management 2016 rules i.e. by 27th March, 2018. But as per the Bio-Medical waste management (Amendment) rules, 2018, use of chlorinated plastic bags (excluding blood bags) and gloves has to be phased out by the 27th March, 2019.
Pag
e
588
Pag
e
588
Pag
e
58
8
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
Pag
e
588
on-site in the manner as prescribed by WHO or NACO.
4. Provide training to all its health care workers and immunize all health workers regularly against disease like tetanus and Hepatitis B. 5. Establish Bar-code system for bags or
containers containing bio-medical waste for disposal within one year of notification of rules i.e 27th March, 2017. But as per the Bio-Medical waste management (Amendment) rules, 2018, bar-code system has to be established in accordance with the guidelines issued by the central pollution control board by 27th March, 2019.
6. Report major accidents like needle stick injuries, broken mercury thermometer, accidents caused by fire, blasts during handling of bio-medical waste and the remedial action taken and record the same.
7. Procedure to get authorization is simplified. 8. The new rules prescribe more stringent
standards for incinerator to reduce the emission of pollutants in environment.
9. No hospital/healthcare facility (occupier) shall establish on-site treatment and disposal facility, if a service of “common bio-medical waste treatment facility” (CBMWTF) is available at seventy-five kilometers.
10.Operator of a common bio-medical waste treatment and disposal facility to ensure the timely collection of bio-medical waste from the healthcare facility and assist the healthcare facility in conducting training.
Bio-medical waste has been classified into 4 categories [Table 3] instead of 10 categories as per Biomedical waste (Management & Handling) Rules, 1998 to improve the segregation of waste at source.
Table 3: Classification of Bio-Medical waste as per BMWM rules 2018 [58]
COLOUR CODING
Type of bag / container used
TYPE OF WASTE Treatment / disposal options
Yellow
Non- chlorinated plastic bags
a. Human anatomical waste
Incineration or Plasma pyrolysis or deep burial b. Animal anatomical waste
c. Soiled waste
d. Expired or discarded medicines
e. Chemical waste f. Chemical liquid waste
g. Discarded linen,
mattresses, beddings contaminated with blood or body fluid
h. Microbiology,
biotechnology, blood bags and other clinical laboratory waste. Also routine mask & gown as per BMW rules, 2018.
Red
Non-chlorinated plastic bags or
Contaminated waste (recyclable) – Vacutainers, tubing, bottles, intravenous tubes and sets,
e
589
e
589
e589
e
589
e589
e
589
e589
e
589
e589
e
589
e
589
e
589
e
589
e589
e
589
e589
e
589
e589
e
589
e589
e
589
containers catheters, urine bags, syringes (withour needles) and gloves
landfill
White Translucent
puncture, leak,
tamper proof
containers
Waste sharps including metals (Hypodermic needles, auto-disabled syringes, scalpels, knives, blades, lumbar puncture needles and intravenous needles).
Auto or dry heat sterilization followed by shredding or mutilation or encapsulation
Blue
Puncture proof and leak proof boxes or containers
a. Broken/discarded Glass ware
Disinfection or autoclaving, microwaving, hydroclaving and then sent for recycling b. Metallic body implants
Source: Bio-medical waste Management Rules, 2018, Ministry of Environment, Forest and Climate Change, Government of India.
ADDITIONAL (TRANSMISSION BASED)
PRECAUTIONS; are taken while ensuring standard precautions are maintained. These include;[59]
Airborne precautions – Diseases which spread by this mode include open/active pulmonary tuberculosis (TB), measles, chicken pox, pulmonary plague and haemorrhagic fever with pneumonia. Precautions need to be taken are; place patient in a single room that has a monitored negative airflow pressure, and is often referred to as a “negative pressure room”. The air should be discharged to the outdoors or specially filtered before it is circulated to other areas of the health care facility; keep door closed; anyone who enters the room must wear a special, high filtration particulate respirator (N95) mask.
Droplet precautions – Diseases, which are transmitted by this route, include pneumonias, pertussis, diphtheria, influenza type B, mumps and meningitis. Precautions need to be taken are; place patient in a single room; wear a surgical mask when working within 1-2 meters of the patient; place a surgical mask on the patient if transport is necessary; special air handling and ventilation are not required to prevent droplet transmission of infection. Contact precautions – Diseases which are
transmitted by this route include colonization or infection with multiple antibiotic resistant organisms, enteric infections and skin infections. Precautions need to be taken are;
place patient in a single room; wear clean gloves when entering the room; wear a clean gown when entering the room if substantial contact with the patient, environmental surfaces or items in the patient’s room is anticipated.
Infections can develop in a health care facility through various sources, namely, patients, visitors, staff, as well as objects. Comprehensive infection prevention and control practices are required to effectively prevent, identify, monitor, and control the spread of infections in all health care facilities/environment.
References:
1. Sarma JB, Ahmed GU. Infection control with limited resources: Why and how to make it possible?. Indian Journal of Medical Microbiology, 2010;28(1):11-16.
2. World alliance for patient safety/Global patient safety challenge: 2005-2006. World Helth Organization 2005. Available from: http:// www.who.int/patientsafety/ events/05/GPSC_Launch_ENGLISH_FINAL /pdf.
3. Healthcare-Associated infection working group of the joint public policy committee, Essentials of public reporting of healthcare-associated infections: A tool kit. Centers for disease control and prevention. Web site. http://www.cdc.gov/ncidod/dhqp/pdf/ar
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
Pag
e
590
4. Mathur P. Prevention of Healthcare-Associated infections in low and middle income countries: The ‘Bundle Approach’. Indian Journal of Medical Microbiology, 2018;36(2):155-162.
5. Rosenthal VD, Maki DG, Salomao R, Moreno CA, Mehta Y, Higuera F, et al. Device-associated nosocomial infections in 55 Intensive care units of 8 developing countries. Ann Intern Med 2006;145:582-91.
6. Mathur P, Tak V, Gunjiyal J, Nair SA, Lalwani S, Kumar s, et al. Device-associated infections at a level-1 trauma centre of a developing nation: Impact of automated surveillance, training and feedbacks. Indian J Med Microbiol 2015;33:51-62.
7. McFee RB. Nosocomial or Hospital-acquired infections: An overview. Dis Mon 2009;55:422-38.
8. Allegranzi B, Pittet D. Healthcare-associated infections in developing countries: Simple solutions to meet complex challenges. Infect Control Hosp Epidemiol 2007;28:1323-7. 9. Christopher S. Implementation of a Need
Based Participatory Training Program on Hospital Infection Control: A clinical Practice Improvement Project, Infection Control – Updates, 2012.
10.WHO. Practical guidelines for infection control in health care facilities. WHO, SEARO regional publication; 2004: 41. 11.WHO. Prevention of hospital-acquired
infections-A practical guide 2nd Edn. Web site.http://www.who.int/emc. 2002.
12.Health Canada, Laboratory centre for disease control. Infection control guidelines. Routine practices and additional precautions for preventing the transmission of infection in health care. Canada Communicable Disease report. 1999 Jul;25(4):1-155.
13.Mathur P. Hand hygiene: Back to the basics of infection control. Indian J Med Res, 2011;611-620.
14.Boyce JM, Pittet D. Guideline for Hand Hygiene in Health-care settings: Recommendations of the healthcare infection control practices advisory committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene
Task Force. Morbid Mortal Wkly Rep. 2002;51:1-45.
15.WHO guidelines on hand hygiene in health care: A summary. 2014. Mar 10, Available from:
http://www.whqlibdoc.who.int/hq/2009/WHO _IER_PSP_2009.07_eng.pdf.
16.Mehta Y, Gupta A, Todi S, Myatra SN, Samaddar DP, Patil V, Bhattacharya PK, et al,. Guidelines for prevention of hospital acquired infections. Indian J Crit Care Med, 2014;18(3):149-163.
17.Aguwa EN, Arinze-Onyia SU, Ndu A. Use of personal protective equipment among health workers in a Tertiary health institution, South East Nigeria: Pre-Ebola period. IJHSR, 2016;6(8):12-18.
18.Lakshmi A PA, et al. A study on personal protective equipment use among health care providers, Tamil Nadu. Int J Community Med Public Health, 2018;5(5):1771-74. 19.Spaulding EH. Chemical disinfection of
medical and surgical materials. In: Lawrence C, Block SS, eds. Disinfection, sterilization, and preservation. Philadelphia: Lea & Febiger, 1968:517-31.
20.Favero MS, Bond WW. Chemical disinfection of medical and surgical materials. In: Block SS, ed. Disinfection, sterilization, and preservation. Philadelphia: Lippincott Williams & Wilkins, 2001:881-917.
21.Rutala WA, 1994, 1995, and 1996 APIC Guidelines Committee. APIC guideline for selection and use of disinfectants. Association for Professionals in Infection Control and Epidemiology, Inc. Am. J. Infect. Control 1996;24:313-42.
22.Barrie D. How hospital linen and laundry services are provided. J Hosp Infect, 1994;27:219-35.
23.Riggs CH, Sherrill JC. Textile laundering technology. Hallendale FL: Textile Rental Service Association, 1999;92-7.
24.Walter WG, Schillinger JE. Bacterial survival in laundered fabrics. Appl Microbiol, 1975;29:368-73.
e
591
e
591
e591
e
591
e591
e
591
e591
e
591
e591
e
591
e
591
e
591
e
591
e591
e
591
e591
e
591
e591
e
591
e591
e
591
26.Belkin NL. Aseptic and aesthetics of chlorine bleach: can its use in laundering be safely abandoned?. Am J Infect Control, 1998;26:149-51.
27.Ayliffe GAJ, Collins BJ, Taylor LJ. Laundering In: Wright PSG, ed. Hospital-acquired infection: principles and prevention. Bristol, UK: 1982;101-6.
28.Mangram AJ, Horan Tc, Pearson ML, Silver LC, Jarvis WR. Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol 1999;20:247-80.
29.DiGacomo JC, Odom JW, Ritoto PC, Swan KC. Cost containment in the operating room: use of reusables versus disposable clothing. Am Surg, 1992;58:654-6.
30.American Society for testing materials. Standards test method for resistance of materials used in protective clothing to penetration by synthetic blood. ASTM, 1998;F1670-98.
31.CDC. NIOSH Fast Facts. Home Healthcare workers. DHHS (NIOSH) publication, 2012-123.pdf.
32.Centers for Disease Control and Prevention. Guidelines for infection control in health care personnel. Infect Control Hosp Epidemiol. 1998;19:4-45.
33.Centers for Disease Control and Prevention, Division of Health-care Quality promotion. Surveillance of Healthcare Personnel with HIV/AIDS, as of December 2001-2003. 34.World Health Organization. The World
Health Report, Box 4.4. 2002. Geneva, Switzerland:
http://www.who.int/whr2002/chapter 4/en/index8.html.
35.Sagoe CM, Pearson JD, Perry J, Jagger J. Risks to health care workers in developing countries. N Engl J Med, 2001;345:538-9. 36.Centers for Disease Control and Prevention.
National Institute of Occupational Safety and Health (NIOSH) NIOSH Alert: Preventing Needlestick Injuries in Health care settings, 1999;2000-108.
37.World Health Organization. Aide-Memoire for a Strategy to protect health workers from
Infection with Bloodborne viruses. Geneva, Switzerland:WHO, November 2003.
38.Ippolito G, Puro V, Heptonstall J, Jagger J, De Carli G, Petrosillo N. Occupational human immunodeficiency virus infection in health care workers: worldwide cases through September 1997. Clin Infect Dis. 1999;28:365-83.
39. Centers for Disease Control and Prevention. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV,HCV and HIV and recommendations for post-exposure prophylaxis, MMWR 2001;50(RR-11):1-42. 40.American Nurse Association. Needlestick
Prevention Guide 2002;p13.
41.Foley M, Leyden AM. American Nurses Association Independent study Module Needlestick Safety and Prevention, 2003. 42.Susan Q, Wilburn BSN MPH, Gerry E MD.
Preventing Needlestick injuries among Healthcare workers. Int J Occup Environ Health, 2004;10(4):451-456.
43.Fredrick TN, Kumaran M. Operation theaters and sterilization requirements – Design consideration and standards for infection control. TNOA J Ophthalmic Sci Res, 2018; 56:84-90.
44.Sharma S, Bansal AK, Gyanchand R. Asepsis in the ophthalmic operating room. Indian J Opthalmol 1996;44:173-77.
45.Peate I. Body fluids, part 1: infection control. British Journal of Healthcare Assistants 2008 Jan;2(1):6-10.
46.Health and Safety Executive. Blood borne viruses - methods of decontamination. 2015 http://www.hse.gov.uk/biosafety/blood-borne-viruses/methods-of-decontamination.htm 47.Health and Safety Executive. Advisory
Committee on Dangerous Pathogens. Protection against blood-borne infections in the workplace: HIV and hepatitis. 2008. 48.Health Facilities Scotland. The NHSScotland
national cleaning services specification. Glasgow: Health Facilities Scotland; 2009 Apr.
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Pag
e
592
Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recommendations and Reports 2003 Jun 6;52(RR-10):1-42.
50.UK Health Departments. Guidance for clinical health care workers: protection against infection with blood borne viruses, Recommendations of the Expert Advisory Group on AIDS and the Advisory Group on hepatitis. Department of Health: London; 1998.
51.Hall S. Infection control: legal aspects and audit tools. Practice Nurse 2007 Feb;33(4):52-8.
52.Fraise AP, Bradley C. Decontamination of equipment, the environment and the skin. In: Fraise AP, Bradley C, editors. Ayliffe's control of healthcare-associated infection. 5th ed. London: Edward Arnold (Publishers) Ltd; 2009. p. 107-49.
53.Chitnis V, Chitnis S, Patil S, Chitnis D. Practical limitations of disinfection of body fluid spills with 10,000 ppm sodium hypochlorite (NaOCl). American Journal of Infection Control 2004 Aug;32(5):306-8. 54.Rutala WA, Weber DJ, Healthcare Infection
Control Practices Advisory Committee
(HICPAC). Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Atlanta: Centers for Disease Control and Prevention; 2008.
55. Ward D. Implementing evidence-based practice in infection control. British Journal of Nursing (BJN) 2000 Mar 9;9(5):267-71. 56.Healthcare-waste.org.(2017). Health care
waste management. Available at: http://www.healthcare-waste .org/ [Accessed 9 Jun.2017].
57.Biospectrumindia.com.(2018). New Bio-Medical waste Management Rules notified.
[online] Available at
http://www.biospectrumindia.com/news/73/5 937/new-bio-medical -waste-management-rules-notified.html.
58.UNIDO. Training manual on Bio-Medical waste management for doctors, nurses, nodal officers and waste managers. United Nations Industrial Development Organization, 2018;29-31.


![Table 3: Classification of Bio-Medical waste as per BMWM rules 2018 [58]](https://thumb-us.123doks.com/thumbv2/123dok_us/8447099.1703641/8.612.66.550.359.746/table-classification-bio-medical-waste-bmwm-rules.webp)