Heart Disease: Findings From a Nursery
WHAT’S KNOWN ON THIS SUBJECT: The detection of critical congenital heart disease by fetal echocardiography or neonatal physical examination can have limitations. The addition of pulse oximetry screening in the newborn nursery increases the rate of diagnosis of these conditions before hospital discharge.
WHAT THIS STUDY ADDS: In a tertiary-care center with
comprehensive fetal echocardiography, nearly all newborns with critical congenital heart disease are diagnosed prenatally. Pulse oximetry will identify more infants from settings with lower prenatal detection. Improving access to and training in fetal echocardiography should also improve detection of these conditions.
abstract
BACKGROUND:Delayed diagnosis of critical congenital heart disease (CCHD) in neonates increases morbidity and mortality. The use of pulse oximetry screening is recommended to increase detection of these conditions. The contribution of pulse oximetry in a tertiary-care birthing center may be different from at other sites.
METHODS:We analyzed CCHD pulse oximetry screening for newborns $35 weeks’ gestation born at Brigham and Women’s Hospital and cared for in the well-infant nursery during 2013. We identified patients with prenatal diagnosis of CCHD. We also identified infants born at other medical centers who were transferred to Boston Children’s Hospital for CCHD and determined if the condition was diagnosed prenatally.
RESULTS: Of 6838 infants with complete pulse oximetry data, 6803 (99.5%) passed thefirst screening. One infant failed all 3 screenings and had the only echocardiogram prompted by screening that showed persistent pulmonary hypertension. There was 1 false-negative screening in an infant diagnosed with interrupted aortic arch. Of 112 infants born at Brigham and Women’s Hospital with CCHD, 111 had a prenatal diagnosis, and none was initially diagnosed by pulse oximetry. Of 81 infants transferred to Boston Children’s Hospital from other medical centers with CCHD, 35% were diagnosed prenatally.
CONCLUSIONS:In our tertiary-care setting, pulse oximetry did not detect an infant with CCHD because of effective prenatal echocardiography screening. Pulse oximetry will detect more infants in settings with a lower prenatal diagnosis rate. Improving training in complete fetal echocardiography scans should also improve timely diagnosis of CCHD.
Pediatrics2014;134:916–922
AUTHORS:Lise C. Johnson, MD,a,bEllice Lieberman, MD, DrPH,a,bEdward O’Leary, MD,b,cand Robert L. Geggel, MDb,d
aDepartment of Pediatric Newborn Medicine, Brigham and
Women’s Hospital, Boston, Massachusetts;bDepartment of
Pediatrics, Harvard Medical School, Boston, Massachusetts; and Departments ofcMedicine anddCardiology, Boston Children’s
Hospital, Boston, Massachusetts
KEY WORDS
pulse oximetry, fetal echocardiography
ABBREVIATIONS
AAP—American Academy of Pediatrics BCH—Boston Children’s Hospital BWH—Brigham and Women’s Hospital CCHD—critical congenital heart disease CICU—cardiac ICU
Dr Johnson coordinated meetings with nursing staff to initiate pulse oximetry screening program adhering to American Academy of Pediatrics guidelines, collected data from pulse oximetry screening tests, analyzed the results, and wrote preliminary methods and results section; Dr Lieberman participated in organizational meetings to construct a pulse oximeter screening program, collected data from pulse oximetry screening tests, and supervised the statistical analysis of the pulse oximetry data; Dr O’Leary collected data from pulse oximetry screening tests, assisted with the data analysis, and wrote thefirst version of the introduction; Dr Geggel proposed the current investigation to determine the contribution of pulse oximetry in the detection of critical congenital heart disease in a tertiary-care level-1 nursery, participated in meetings with the nursery staff to construct a pulse oximetry protocol, reviewed the echocardiography reports performed on infants at Brigham and Women’s Hospital in the NICU and cardiac ICU at Boston Children’s Hospital for infants born at Brigham and Women’s Hospital, and patients referred from outside medical centers to Boston Children’s Hospital for management of critical congenital heart disease during the study period, and wrote the fullfirst draft of the manuscript; and all authors approved thefinal manuscript.
www.pediatrics.org/cgi/doi/10.1542/peds.2014-1461
doi:10.1542/peds.2014-1461
Accepted for publication Aug 14, 2014
Address correspondence to Robert L. Geggel, MD, Department of Cardiology, Boston Children’s Hospital, 300 Longwood Ave, Boston, MA 02115. E-mail: robert.geggel@cardio.chboston.org
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2014 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:The authors have indicated they have nofinancial relationships relevant to this article to disclose. FUNDING:No external funding.
Congenital heart disease is the most common cause of infant death associ-ated with congenital malformations in the developed world.1–5Approximately
9 of 1000 live births have congenital heart disease.4,6Of these,∼25% have
critical congenital heart disease (CCHD), defined as a condition requiring surgical or catheter intervention in thefirst year of life.2,4Unfortunately, the diagnosis of
CCHD is often delayed with negative im-pact on both morbidity and mortality.7–12
Some neonates with CCHD are diagnosed only after death.3,13 This deficiency in
detection is contributed by limitations in physical examination,14,15 including
difficulty in identifying cyanosis, espe-cially in anemic or dark-pigmented neonates,7,16 early hospital discharge
in duct-dependent lesions if the duct has not yet closed,7,13,17and absence of
murmurs in many patients with CCHD due to the specific anatomy, elevated pulmonary vascular resistance, or re-duced contractility.11,15,16,18–20Other modes
of testing also have limitations. Electro-cardiography and chest radiography lack sensitivity and specificity.4Pediatric
echocardiography is expensive and not readily available at many birthing centers.4The detection of CCHD by
pre-natal ultrasound varies by lesion, expe-rience of the operator, and protocol of views used with generally,50% of cases identified.2,4,5,21–24
Multiple studies over more than a de-cade have assessed the role of pulse oximetry to improve the detection of CCHD in neonates before hospital dis-charge. The results of these studies have led the Department of Health and Human Services and the American Academy of Pediatrics (AAP) to recom-mend universal pulse oximetry screening in the newborn nursery.25,26Pulse oximetry
has a high specificity and moderate sen-sitivity in the detection of CCHD,1,4,5,7–9,20
and has a lower false-positive rate if performed after 24 hours of age.1,4
Compared with physical examination
alone, pulse oximetry increases the rate of detection of CCHD2,7,21,26–28and
contributes to improved clinical status at the time of diagnosis.
Although neonatal pulse oximetry screen-ing increases the detection of CCHD, its impact may be less in a tertiary-care setting with extensive prenatal fetal imaging.2We established a pulse oximetry
program that fulfilled AAP guidelines in such a hospital setting and evaluated thoroughness of testing, screening out-comes, and whether CCHD was detected by pulse oximetry, fetal echocardiography, or postnatal clinical presentation.
METHODS
Patient Selection at Brigham and Women’s Hospital
We examined screening among neonates of $35 weeks’ gestation born from January 1 to December 31, 2013, at Brigham and Women’s Hospital (BWH) who were cared for in the well-infant nursery during the time frame when pulse oximetry screening would have been completed, or transferred to Boston Children’s Hospital (BCH) for manage-ment of specified congenital heart dis-ease. BWH is a tertiary-care birthing center that provides extensive prenatal screening and is a referral hospital for pregnant mothers whose fetuses have been diagnosed with congenital heart disease at other medical facilities. Staff from the Department of Cardiology at BCH provides all the cardiology consul-tation on neonates born at BWH. This study was performed in accordance with a protocol approved by the Com-mittee on Clinical Investigation at both BWH and BCH.
Identification of Neonates Eligible for
Pulse Oximetry Screening
We identified all neonates of$35 weeks’ gestation by using a hospital data-base. We excluded infants who spent their entire BWH hospital stay in the NICU or who were transferred to the
NICU before 48 hours of age and had not received their CCHD screening be-fore transfer.
Identification of Neonates With
Prenatal or Postnatal Diagnosis of Congenital Heart Disease
Neonates born at BWH who were di-agnosed prenatally or postnatally with CCHD consisting of 1 of the 7 lesions selected to be screened by the AAP guidelines, aortic arch obstruction, or other cyanotic heart disease (see Table 1 for list of diagnoses) were identified from echocardiography databases or admissions logs from the cardiac ICU (CICU) and NICU at BCH. Maternal obstetric records in the BCH medical records were reviewed to determine if the initial prenatal detection of congenital heart disease was made at BWH or a referring medical facility. Neonates with a fetal diagnosis of CCHD were initially admitted to the BWH NICU and had an initial echo-cardiogram either at BWH or BCH.
Identification of Neonates Born at BWH
With Late Diagnosis of CCHD
The admissions logs from the CICU and NICU at BCH were reviewed for 2013 and thefirst 10 weeks of 2014 to determine any late admissions for previously un-detected CCHD among infants born at BWH and discharged from the hospital before diagnosis. With few exceptions, neonates with CCHD in our region have surgical repair at BCH.
Determination of Prenatal Versus Postnatal Diagnosis of CCHD for Neonates Born at Medical Centers Other Than BWH
Neonates with CCHD lesions listed in Table 1 born at other medical centers during the study period who were transferred to BCH were identified from admissions logs to the CICU or NICU at BCH. These patients’ records were reviewed to determine if the di-agnosis of CCHD was made before or after birth.
Pulse Oximetry Screening Protocol
We followed the pulse oximetry pro-tocol approved by AAP.5,6 Screening
was performed by nurses who had un-dergone training to obtain simultaneous preductal (right hand) and postductal (either foot) oxygen saturations. Screen-ing was targeted to occur between 24 and 48 hours of age. Infants discharged before 24 hours of age were screened as close to discharge as possible. Infants screened after 48 hours of age included
those with an intravenous line in the right hand for antibiotic administration during thefirst 48 hours of life and those infants transferred to the well-newborn nurseries after 48 hours of age. In both of these groups, screening was performed as soon as feasible. The Radical-87 pulse oximeter with reusable probe LNOP Y1 (Masimo, Irvine, CA) was used for all initial screen-ing tests in the well-newborn nurseries. The infant was considered to have passed the screening if the oxygen saturation was
2 values. Neonates failing the first screening were transferred to the NICU triage area for clinical evaluation and further testing by using a DASH pulse oximeter with disposable LNOP NeoPt probe (General Electric Medical Systems, Milwaukee, WI). Neonates with oxygen saturation values between 90% and 94% in both the right hand and foot, or.3% difference between the extremities re-quired repeat testing in 1 hour, and if similar values were obtained, a third screening was performed 1 hour later. Patients were judged to fail the screening protocol if the oxygen saturation values were in this range on all 3 occasions or if any 1 screening yielded an oxygen satu-ration in either extremity of ,90%. A failed screening protocol designation prompted a cardiology consultation.
Analysis
The characteristics of infants who failed their first CCHD screening were com-pared with those who passed. Calcu-lations of small, appropriate, or large for gestational age were made according to the 2013 Fenton curves.29The
dis-tribution of categorical variables in the 2 groups were compared by usingx2 or Fisher’s exact test. Continuous vari-ables were compared by usingttests or the Wilcoxon rank sum test for non-normally distributed variables. A value of P , .05 (2-sided) was considered significant.
RESULTS
Study Group for Pulse Oximetry Screening
During the study period, there were 7328 live births of$35 weeks’gestation at BWH, 442 of whom were excluded because pulse oximetry was not re-quired according to our protocol. Ex-cluded infants inEx-cluded those admitted to the NICU on thefirst day of life and who remained there for their hospital
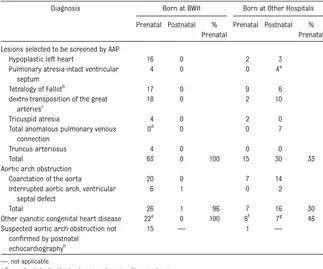
Diagnosis Born at BWH Born at Other Hospitals
Prenatal Postnatal % Prenatal
Prenatal Postnatal % Prenatal
Lesions selected to be screened by AAP
Hypoplastic left heart 16 0 2 3
Pulmonary atresia-intact ventricular septum
4 0 0 4a
Tetralogy of Fallotb
17 0 9 6
dextro-transposition of the great arteriesc
18 0 2 10
Tricuspid atresia 4 0 2 0
Total anomalous pulmonary venous connection
0d
0 0 7
Truncus arteriosus 4 0 0 0
Total 63 0 100 15 30 33
Aortic arch obstruction
Coarctation of the aorta 20 0 7 14
Interrupted aortic arch, ventricular septal defect
6 1 0 2
Total 26 1 96 7 16 30
Other cyanotic congenital heart disease 22e 0 100 6f 7g 46 Suspected aortic arch obstruction not
confirmed by postnatal echocardiographyh
15 — 1 —
—, not applicable.
aTwo patients had critical pulmonary stenosis with near atresia.
bEighteen with pulmonary stenosis, 11 with pulmonary atresia, 2 with absent pulmonary valve, 1 with complete atrioven-tricular canal.
cTwenty-one with intact ventricular septum, 3 with ventricular septal defect, 6 with ventricular septal defect and pulmonary stenosis. dTotal anomalous pulmonary venous connection did occur in 3 patients with heterotaxy and atrioventricular canal defect that was detected prenatally, and are listed in the“Other cyanotic congenital heart disease”section.
eHeterotaxy (total 7) with atrioventricular canal and total anomalous pulmonary venous connection (3), unbalanced atrioventricular canal and hypoplastic left ventricle (2), or atrioventricular canal, double outlet right ventricle, pulmonary stenosis (2); complex congenital heart disease with single ventricle physiology (5); critical aortic stenosis with fetal intervention (4); unbalanced complete atrioventricular canal (2); Ebstein anomaly of the tricuspid valve (1); pulmonary atresia with large apical muscular ventricular septal defect (1); hypoplastic right ventricle, ventricular septal defect, pulmonary stenosis (1); double outlet right ventricle, superior-inferior ventricles, crisscross atrioventricular valves (1). fHeterotaxy (total 2) with double outlet right ventricle, ventricular septal defect, crisscross atrioventricular valves, pulmo-nary stenosis (1), or tetralogy of Fallot and malposed atrial septum (1); Ebstein anomaly of the tricuspid valve (1); dysplastic tricuspid valve (1); right dominant unbalanced atrioventricular canal, small left ventricle, coarctation of the aorta (1); double outlet right ventricle, transposition of the great arteries, pulmonary stenosis (1).
gCritical aortic stenosis (1); arterial tortuosity syndrome and midaortic syndrome (1); pericardial teratoma compressing airway (1); pulmonary glycogenosis and pulmonary vein stenosis (1); vascular ring (right aortic arch with aberrant left subclavian artery compressing airway and multiple ventricular septal defects) (1); Ebstein anomaly of the tricuspid valve (1); arcade mitral valve associated with severe mitral regurgitation (1).
stay (n= 293), infants with prenatal di-agnosis of CCHD (n= 126, Table 1), and those transferred to the NICU between 24 and 48 hours of life before pulse oximetry screening was performed (n= 23). Of the remaining 6886 infants, 48 (0.7%) were missing CCHD screening data (13 forms with passing oximetry values had no patient identifying infor-mation, 14 forms had uninterpretable or missing oximetry values, and 21 forms were missing from the medical record). The study group comprised the remaining 6838 infants (99.3% of eligi-ble newborns).
Pulse Oximetry Screening Results
Of the infants who had complete pulse oximetry data, 6803 (99.5%) passed the first screening. One of these infants represented the only false-negative result in the study. This infant had a normal fetal cardiac examination as part of a routine ultrasound fetal survey at 18 weeks’gestation at BWH. On pulse oximetry screening at 29 hours of age, pre- and postductal oxygen saturations were 97% and 96%, respectively. He developed a new murmur 10 hours and cyanosis 12 hours after the first ox-imetry screening (oxygen saturation 94% in the right hand, 85% in the leg), and was diagnosed to have interrupted aortic arch with ventricular septal defect. He underwent surgical repair with an excellent outcome.
Thirty-five infants (0.5%) failed thefirst pulse oximetry screening. Of these, 34 infants passed the second screening, 30 of whom had an uneventful subsequent hospital course, 3 of whom had sub-sequent desaturation associated with laryngomalacia (length of stay 5 days), neonatal stroke (length of stay 5 days), or unknown cause (length of stay 7 days), and 1 who had apnea of unknown cause (length of stay 7 days). One infant failed all 3 screenings and was diag-nosed by using echocardiography to have persistent pulmonary hypertension
of the newborn (length of stay 3 days); this was the only echocardiogram per-formed during the study period for a failed CCHD screening test.
Infants who failed the first screening did not differ from those who passed the first screening in terms of race, gestational age, or method of delivery (vaginal versus cesarean delivery). Both being large for gestational age (1.4% [7/520] vs 0.4% [28/6318],P= .006) and having a birth weight .4 kg (1.2% [7/599] vs 0.5% [28/6239], P = .02) were associated with failing the first CCHD screening. There was a trend to-ward a higher risk of failing the first screening among male infants com-pared with female infants (0.66% vs 0.36%,P= .08).
There were no infants born at BWH during the study period who were ad-mitted to the BCH CICU within 10 weeks of their birth with previously unrecog-nized CCHD.
Prenatal Diagnosis of CCHD
During the study period, 112 infants (∼1.5% of all live births) born at BWH had CCHD, 111 (99%) of whom had a prenatal diagnosis; the only postnatal diagnosis occurred in the 1 patient with interrupted aortic arch described in the previous section. Of these infants, 63 (56%) had 1 of the 7 lesions selected to be screened by AAP, 27 (24%) had coarctation or interrupted aortic arch, and 22 (20%) had other complex congenital heart dis-ease (Table 1). Thirteen mothers had detection of CCHD by prenatal ultrasound performed at BWH, and 98 mothers (42 from other Massachusetts medical centers, 29 from the other 5 New England states, 24 from other regions of the United States, and 3 from foreign nations) had ultrasound performed locally and were referred with con-cern about congenital heart disease that was confirmed or fully defined by staff at BWH or the Advanced Fetal Care Center at BCH. Prenatal
screen-ing raised concern about aortic arch obstruction in 15 other patients be-cause of ventricular size discrepancy or aortic arch hypoplasia but postnatal echocardiography demonstrated normal cardiac anatomy and function.
During the study period, 81 infants born at other medical centers were trans-ferred to the BCH CICU for management of CCHD. Of this group, 28 (35%) had a prenatal diagnosis and 53 (65%) had a postnatal diagnosis; 1 additional pa-tient did not have postnatal confi rma-tion of suspected aortic obstrucrma-tion (Table 1). Thirteen of the patients with prenatal diagnosis had fetal echo-cardiography performed at BCH, 8 of whom were born at another tertiary-care birthing center 1 block from BCH and 5 of whom were born at other medical centers. Referral medical re-cords documented a failed pulse ox-imetry screen in 4 infants; we could not determine how many of the referral centers had a neonatal pulse oximetry screening program. Five patients with postnatal diagnosis had been dis-charged from the hospital after birth and developed severe respiratory dis-tress or profound cyanosis (2 infants with infradiaphragmatic total anoma-lous pulmonary venous connection) or shock (3 infants with coarctation) before diagnosis. Another infant with postnatal diagnosis developed severe acidosis at the birth hospital (dextro-transposition of the great arteries with restrictive atrial septum) before diagnosis.
DISCUSSION
We were able to establish a pulse oxim-etry screening program that success-fully tested.99% of neonates cared for in the well-infant nursery. During the study period,∼1.5% of infants born at BWH had CCHD, a value ∼7 times the expected incidence and associated with the tertiary referral status of our med-ical center. Of the patients diagnosed with CCHD, 56% had 1 of the 7 lesions
interrupted aortic arch), and 20% had other cyanotic heart disease.
In our tertiary-care setting, each of the neonates with 1 of the 7 targeted lesions was diagnosed before birth by fetal echocardiography and none was initially detected by postnatal pulse oximetry. This finding is in contrast to infants born at other hospitals and referred for cardiac care, approximately two-thirds of whom had a postnatal diagnosis. Some of these neonates presented after hos-pital discharge in a compromised state and likely may have benefited from pulse oximetry screening. Previous studies also have noted a higher pre-natal detection rate at tertiary-care centers.5,24 Approximately one-third
of infants whose prenatal echocar-diogram raised concern about aortic arch obstruction did not have the diagnosis confirmed after birth, so that some echocardiograms in healthy neonates were prompted by the fetal study.
Although we do not have data on how many of the infants born at other med-ical centers had fetal echocardiography, prenatal ultrasound is performed in the vast majority of pregnancies in our re-gion. The detection of congenital heart disease by prenatal ultrasonography can be limited by fetal gestational age or position, maternal obesity,30and operator
experience.2,4,5,21–23Lesions involving
ven-tricular outflow tracts (transposition of the great arteries, tetralogy of Fallot, double outlet right ventricle), total anomalous pulmonary venous connec-tion, and left-sided obstructive lesions other than hypoplastic left heart syn-drome (coarctation, interrupted aortic arch) have lower detection rates.5,24
Use of a fetal ultrasound protocol that includes multiple echocardiographic views, especially of the ventricular outflow tracts and aorta, improves detection.23,24,30
and does not replace the utility of pre-natal screening, physical examination, and continued observation.20,27 The
importance of multiple diagnostic ap-proaches is demonstrated in the 1 neonate in our study who had a false-negative initial pulse oximetry screening, developed new physical examina-tionfindings of murmur and cyanosis, and was subsequently diagnosed to have interrupted aortic arch. False-negative pulse oximetry screenings for CCHD are uncommon4 and have
been mainly reported in patients with coarctation,4,8,9some of which may not
be duct dependent.31False-negative tests
also have been reported in patients with interrupted aortic arch,7
trans-position of the great arteries, total anomalous pulmonary venous connec-tion, truncus arteriosus, and double-outlet right ventricle.4,16,18,20,32
Our study confirmed a low false-positive rate associated with pulse oximetry screening performed after 24 hours of age.1As noted in previous studies, a
false-positive result often has clinical utility in detecting noncardiac causes of cyanosis that require treatment, in-cluding sepsis, pulmonary hypertension, pneumothorax, transient tachypnea of the newborn, meconium aspiration, and seizures secondary to intraventricular hemorrhage.1,2,7–9,20,27,28,33In our study,
1 echocardiogram was performed be-cause of the false-positive results and identified a patient with pulmonary hy-pertension. Analysis of results of pre-vious studies when pulse oximetry was performed after 24 hours of age has estimated that 1 additional echocar-diogram is obtained per 3000 births.4
We compared the characteristics of in-fants who failed with those who passed the initial screening and found that larger infants were more likely to fail. Given the small number of failures in our study, we were unable to determine
characteristics of the pregnancy or perinatal period. Whatever the under-lying reason, even among large infants, the proportion who failed the initial screening was very low.
Our study has some potential limitations. The false-negative rate of screening may have been underestimated. We did not review records from the regional Med-ical Examiner’s Offices for out-of-hospital infant death secondary to undiagnosed CCHD. In addition, some infants born at BWH may have been admitted and treated for CCHD at other medical centers. However, in our region, with few exceptions, infants with CCHD are referred to and have surgical proce-dures at BCH. The prenatal detection of CCHD at other medical centers was underestimated, because some women transferred their care to BWH after CCHD was diagnosed at the facility where they initiated care.
CONCLUSIONS
in thorough fetal echocardiography protocols during routine fetal surveys also should improve timely diagnosis of CCHD. Finally, approximately one-quarter of patients with CCHD had aortic
arch obstruction (coarctation or inter-rupted aortic arch) and consideration should be made for adding these di-agnoses to the list of target lesions to be screened.
ACKNOWLEDGMENTS
We thank Dr Zhigang Lu for assis-tance with computer programming and Rachel Dabek for assistance with data collection.
REFERENCES
1. Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screen-ing for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis.Lancet. 2012;379 (9835):2459–2464
2. Ewer AK, Middleton LJ, Furmston AT, et al;
PulseOx Study Group. Pulse oximetry
screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study.Lancet. 2011;378(9793):785–794
3. Abu-Harb M, Hey E, Wren C. Death in infancy from unrecognised congenital heart disease.
Arch Dis Child. 1994;71(1):3–7
4. Mahle WT, Newburger JW, Matherne GP, et al; American Heart Association Con-genital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research; American Academy of Pediatrics Section on Cardiology and Cardiac Surgery, and Committee on Fetus and Newborn. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics.Circulation. 2009;120 (5):447–458
5. Friedberg MK, Silverman NH, Moon-Grady AJ, et al. Prenatal detection of congenital heart disease.J Pediatr. 2009;155(1):26–31, 31.e1
6. Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects.Pediatrics. 2001;107(3). Avail-able at: www.pediatrics.org/cgi/content/full/ 107/3/e32
7. de-Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on the detection of duct dependent con-genital heart disease: a Swedish prospec-tive screening study in 39,821 newborns.
BMJ. 2009;338:a3037
8. Koppel RI, Druschel CM, Carter T, et al. Ef-fectiveness of pulse oximetry screening for congenital heart disease in asymptomatic newborns.Pediatrics. 2003;111(3):451–455
9. Richmond S, Reay G, Abu Harb M. Routine pulse oximetry in the asymptomatic newborn.
Arch Dis Child Fetal Neonatal Ed. 2002;87(2): F83–F88
10. Liske MR, Greeley CS, Law DJ, et al; Tennessee Task Force on Screening Newborn Infants for Critical Congenital Heart Disease. Report of the Tennessee task force on screening newborn infants for critical congenital heart disease. Pediatrics. 2006;118(4). Available at: www.pediatrics.org/cgi/content/full/118/ 4/e1250
11. Brown KL, Ridout DA, Hoskote A, Verhulst L, Ricci M, Bull C. Delayed diagnosis of con-genital heart disease worsens preoperative condition and outcome of surgery in neo-nates.Heart. 2006;92(9):1298–1302
12. Schultz AH, Localio AR, Clark BJ, Ravishankar C, Videon N, Kimmel SE. Epidemiologic features of the presentation of critical congenital heart disease: implications for screening.Pediatrics. 2008;121(4):751–757
13. Kuehl KS, Loffredo CA, Ferencz C. Failure to diagnose congenital heart disease in infancy.
Pediatrics. 1999;103(4 pt 1):743–747
14. Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life-threatening neo-natal cardiovascular malformations.Arch Dis Child Fetal Neonatal Ed. 2008;93(1):F33–F35
15. Wren C, Richmond S, Donaldson L. Presen-tation of congenital heart disease in in-fancy: implications for routine examination.
Arch Dis Child Fetal Neonatal Ed. 1999;80 (1):F49–F53
16. Hoffman JIE. It is time for routine neonatal screening by pulse oximetry.Neonatology. 2011;99(1):1–9
17. Chang R-KR, Gurvitz M, Rodriguez S. Missed diagnosis of critical congenital heart dis-ease.Arch Pediatr Adolesc Med. 2008;162 (10):969–974
18. Reich JD, Miller S, Brogdon B, et al. The use of pulse oximetry to detect congenital heart disease.J Pediatr. 2003;142(3):268–272
19. Ainsworth S, Wyllie JP, Wren C. Prevalence and clinical significance of cardiac murmurs in neonates.Arch Dis Child Fetal Neonatal Ed. 1999;80(1):F43–F45
20. Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for de-tection of critical congenital heart disease
in daily clinical routine—results from a prospective multicenter study.Eur J Pediatr. 2010;169(8):975–981
21. Meberg A, Andreassen A, Brunvand L, et al. Pulse oximetry screening as a complemen-tary strategy to detect critical congenital heart defects. Acta Paediatr. 2009;98(4): 682–686
22. Acharya G, Sitras V, Maltau JM, et al. Major congenital heart disease in Northern Norway: shortcomings of pre- and postnatal diag-nosis. Acta Obstet Gynecol Scand. 2004;83 (12):1124–1129
23. Hunter S, Heads A, Wyllie J, Robson S. Prenatal diagnosis of congenital heart disease in the northern region of England: benefits of a training programme for ob-stetric ultrasonographers.Heart. 2000;84(3): 294–298
24. Levy DJ, Pretorius DH, Rothman A, et al. Improved prenatal detection of congenital heart disease in an integrated health care system. Pediatr Cardiol. 2013;34(3):670– 679
25. Kemper AR, Mahle WT, Martin GR, et al. Strategies for implementing screening for critical congenital heart disease.Pediatrics. 2011;128(5). Available at: www.pediatrics. org/cgi/content/full/128/5/e1259
26. Mahle WT, Martin GR, Beekman RH III, Morrow WR; Section on Cardiology and Cardiac Surgery Executive Committee. Endorsement of Health and Human Services recommendation for pulse oximetry screen-ing for critical congenital heart disease.
Pediatrics. 2012;129(1):190–192
27. Valmari P. Should pulse oximetry be used to screen for congenital heart disease?
Arch Dis Child Fetal Neonatal Ed. 2007;92 (3):F219–F224
28. Garg LF, Van Naarden Braun K, Knapp MM, et al. Results from the New Jersey statewide critical congenital heart defects screening program. Pediatrics. 2013;132(2). Available at: www.pediatrics.org/cgi/content/full/132/ 2/e314
29. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59–71
2013;32(6):1067–1082
31. Rosati E, Chitano G, Dipaola L, De Felice C, Latini G. Indications and limitations for a neonatal pulse oximetry screening of
E, Godi E, Bührer C. Failed detection of complex congenital heart disease (in-cluding double outlet right ventricle and total anomalous pulmonary venous return)
of routine predischarge pulse oximetry screening in a regional neonatal unit.Arch Dis Child Fetal Neonatal Ed. 2014;99:F297– F302
FIRST CONTACT:Mycobacterium tuberculosisis a common and deadly infection of humans. Up to one-third of the world’s population may be infected, and the World Health Organization estimates that the bacterium was responsible for 1.3 million deaths in 2012. Evidence of tuberculosis has even been found in Egyptian mummies, demonstrating that the bacterium has been infecting humans for quite some time–but exactly how long is a matter of intense debate. Most sci-entists believe thatM. tuberculosishas infected humans for at least 10,000–and as many as 70,000 –years. This is based on finding molecules produced by
M. tuberculosisin 9,000 year old human remains found in Israel, and in 17,000 year old bison bones. However, a new study has questioned traditional assumptions. As reported in The New York Times (Science: August 20, 2014), researchers studying bacterial DNA from 1,000-year-old Peruvian mummies report that
M. tuberculosismost likely began to infect humans less than 5,000 years ago. The scientists found so muchM. tuberculosisDNA in the mummies that they were able to recreate the entire genome. They then measured the number of mutations (which tend to accumulate at a steady rate) to calculate the age of the common ancestor of all theM. tuberculosisstrains in their study. Their results were quite surprising, for two reasons. First, the mutational analysis suggested that tu-berculosis arose approximately 5,000 years ago. Secondly, theM. tuberculosis
strains found in the human mummies were closely related to strains that infect seals.
The scientists hypothesize that tuberculosis arose in humans in Africa and spread to animals, including seals. The seals then carried the organism to the New World, where hunters butchering or eating the seals became infected. However, it is not known whether seals or humans carried the organism to North America. Not all scientists agree with the results, and vigorous intellectual debate is ongoing. However, regardless of whenM. tuberculosisfirst infected humans,first contact was not good for humans and the organism remains a terrible pathogen.
DOI: 10.1542/peds.2014-1461 originally published online October 6, 2014;
2014;134;916
Pediatrics
Lise C. Johnson, Ellice Lieberman, Edward O'Leary and Robert L. Geggel
From a Nursery
Prenatal and Newborn Screening for Critical Congenital Heart Disease: Findings
Services
Updated Information &
http://pediatrics.aappublications.org/content/134/5/916
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/134/5/916#BIBL
This article cites 33 articles, 19 of which you can access for free at:
Subspecialty Collections
ers_sub
http://www.aappublications.org/cgi/collection/cardiovascular_disord Cardiovascular Disorders
http://www.aappublications.org/cgi/collection/cardiology_sub Cardiology
http://www.aappublications.org/cgi/collection/birth_defects_sub Birth Defects
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_ Fetus/Newborn Infant
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2014-1461 originally published online October 6, 2014;
2014;134;916
Pediatrics
http://pediatrics.aappublications.org/content/134/5/916
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.