Corticosteroid Pulse Combination Therapy
for Refractory Kawasaki Disease:
A Randomized Trial
WHAT’S KNOWN ON THIS SUBJECT: The efficacy of intravenous immunoglobulin and corticosteroid pulse combination therapy for refractory Kawasaki disease has been established. The Egami score can be used to predict which patients are likely to have refractory Kawasaki disease.
WHAT THIS STUDY ADDS: As a new strategy for primary treatment, intravenous immunoglobulin and corticosteroid pulse combination therapy is safe and effective for patients predicted to have refractory Kawasaki disease based on the Egami score.
abstract
OBJECTIVE: This study examined the clinical efficacy and safety of intravenous methylprednisolone-pulse plus intravenous immunoglobulin (IVIG) combination therapy (IVMP+IVIG) for the initial treatment of patients predicted to have refractory Kawasaki disease (KD).
METHODS:One hundred twenty-two patients with KD were studied at Kitasato University. Refractory KD was predicted at diagnosis using the Egami score, and the patients were randomly divided to receive either IVMP+IVIG or IVIG alone. The Egami score is used to predict refractory KD patients before treatment using the patient’s age, days of illness, platelet count, C-reactive protein, and alanine aminotransferase level (cutoff:$3 points; 78% sensitivity and 76% specificity).
RESULTS:Forty-eight patients (39.3%) were predicted to have refractory KD on the basis of the Egami score. The predicted IVIG responders (n= 74) received the standard therapy. The 48 predicted refractory KD patients were randomly assigned to a single-IVIG group (n = 26) or an IVMP+IVIG group (n= 22). Nineteen of the 22 patients (86.4%) in the IVMP+IVIG group had a prompt defervescence compared with 6 of the 26 patients (23.1%) in the single-IVIG group. The number of patients who had azscore$2.5 at 1 month was significantly higher in the single-IVIG group than in the IVMP+IVIG group. No serious adverse events were observed in either treatment group.
CONCLUSION:This study demonstrated that IVMP+IVIG therapy is safe
and effective for KD patients predicted as refractory. Pediatrics
2012;129:e17–e23 AUTHORS:Shohei Ogata, MD, Yoshihito Ogihara, MD,
Takashi Honda, MD, Shinya Kon, MD, Kazumasa Akiyama, MD, and Masahiro Ishii, MD
Department of Pediatrics, Kitasato University School of Medicine, Kanagawa-ken, Japan
KEY WORDS
Kawasaki disease, intravenous immunoglobulin, methylpredonine pulse, resistant patient, prediction score
ABBREVIATIONS
ALT—alanine aminotransferase ASA—acetylsalicylic acid
AST—aspartate aminotransferase, CAL, coronary artery lesion CRP—C-reactive protein
IQR—interquartile range IVIG—intravenous immunoglobulin IVMP—intravenous methylprednisolone-pulse KD—Kawasaki disease
LAD—proximal left anterior descending coronary artery LMT—left main trunk coronary artery
RCA—right coronary artery
This trial has been registered at http://www.umin.ac.jp/ctr/ (identifier UMIN000005021).
www.pediatrics.org/cgi/doi/10.1542/peds.2011-0148
doi:10.1542/peds.2011-0148
Accepted for publication Sep 9, 2011
Address correspondence to Masahiro Ishii, MD, PhD, Department of Pediatrics, Kitasato University School of Medicine, 1-15-1 Kitasato Mimami-ku, Sagamihara-shi, Kanagawa-ken 252-0374, Japan. E-mail: ishiim@med.kitasato-u.ac.jp
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2012 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:The authors have indicated they have nofinancial relationships relevant to this article to disclose.
tent or recrudescent fever after initial intravenous immunoglobulin (IVIG) treat-ment. IVIG resistance is a major risk factor for the development of coronary artery lesions (CALs), and the early identification of patients at risk for resistance would allow for the timely administration of ad-ditional anti-inflammatory therapies.1–4
Recent studies have reported scoring systems that predict initially IVIG-resistant patients at the diagnosis of KD.5–7There have been several reports suggesting that steroid administration can reduce fever and prevent coronary complications in KD patients without apparent adverse effects.8–14Therefore, steroid therapy for KD has been recon-sidered, and the use of steroids for IVIG-resistant cases is increasing. A previous report examined the biological mechanism underlying the effect of methylprednisolone-pulse (IVMP) ther-apy combined with initial IVIG treatment as a primary treatment of patients pre-dicted to be IVIG-resistant using a micro-array analysis and reported that the IVMP-plus-IVIG combination (IVMP+IVIG) therapy more profoundly and broadly
suppressed the inflammatory
tran-scripts than IVIG treatment alone.15 Newburger et al reported that there were no significant differences in the clinical outcome between the patients treated with IVMP+IVIG therapy or with IVIG alone. However, their report was a study of all KD patients.16The current study examined the safety and efficacy of IVMP+IVIG therapy in patients pre-dicted to be IVIG resistant.
METHODS Subjects
The study subjects were patients di-agnosed with acute KD in the Department of Pediatrics at Kitasato University Hospital, Japan, between April 2007 and November 2010. All consecutive patients diagnosed with KD were approached for
fulfilled the Criteria for Diagnostic Guidelines for KD (5th revision) pub-lished by the Kawasaki Disease Research Committee in Japan.17 The eligibility criteria included having 5 of the 6 clical criteria. The exclusion criteria in-cluded a previous diagnosis of KD and the presence of a CAL before initial treatment. In addition, any patients who received steroid therapy before being diagnosed with KD were also excluded from the study. The Ethics Board of Kitasato University Hospital approved the study, and we obtained informed consent in writing from the parents of all subjects according to the guidelines of the institutional review board.
Protocol
The study protocol is shown in Fig 1. The KD patients were predicted to be either IVIG responsive or resistant by the Egami scoring system at the time of diagnosis. We decided to use the Egami score because we evaluated the tran-script abundance between the patients who received IVMP+IVIG therapy or IVIG alone in a previous report.15The cutoff
ficity; Table 1).5The patients who were predicted to be IVIG responsive received either IVIG alone (2 g/kg over 24 hours) or aspirin alone. The patients with pre-dicted IVIG resistance were randomly assigned to receive either IVMP+IVIG therapy (IVMP+IVIG group) or IVIG alone (single-IVIG group), using a random number list. Both treatment groups re-ceived the IVIG at 2 g/kg for 24 hours. The IVMP+IVIG group received the IVMP therapy (30 mg/kg, 1 dose) over 2 hours before receiving IVIG treatment. Heparin (10 U/kg/h, continuous infusion) was used concomitantly from 2 hours before the start of IVMP therapy and was continued for 24 hours. In addition, all patients received a standard dose of 30 mg/kg acetylsalicylic acid (ASA) ev-ery 8 hours until they were afebrile for 36 hours, then received 5 mg/kg/day as a single dose. We started each treat-ment course at the time of KD diagnosis.
Outcome Measures
Treatment resistance was defined as
persistent or recrudescent fever (axil-lary temperature$37.5°C) at 36 hours
FIGURE 1
after completion of the initial treat-ments. The patients who showed a de-cline in fever at 36 hours were discharged from the hospital at that time. All initial treatment-resistant patients received additional IVIG treatment (2 g/kg over 24 hours) at 36 hours after completion of the initial treatment. If these patients did not respond effectively to the second course of therapy, they were treated with infliximab or IVMP+IVIG therapy as third-line therapy. Laboratory tests and echocardiograms were performed on all subjects before treatment, at 36 hours and at 1 month after the end of
therapy.2 Echocardiograms were read
by a reviewer blinded to the treatment assignment but not to the time point. The presence of coronary arterial
lesions was diagnosed based on thez
scores of the left main trunk coronary artery (LMT), proximal left anterior descending coronary artery (LAD), and right coronary artery (RCA).18
The major endpoint of the efficacy of treatment was that patients were afe-brile (,37.5°C) at least 36 hours after completion of the initial treatment. In addition, the duration of fever (total du-ration of fever and calculated numb-er of days aftnumb-er finishing the initial treatment), laboratory measurements of vasculitic markers (white blood cell count, percent neutrophils, platelet count, albumin concentration, aspartate aminotransferase [AST], alanine amino-transferase [ALT], total bilirubin, and C-reactive protein [CRP] levels), and the
zscores of the coronary arteries were compared among the treatment groups.
The safety of the treatment was as-sessed by the occurrence of prospectively
defined adverse events that were
documented in the daily medical record reviews by nurses. A body temperature ,36°C (axillary temperature) was
de-fined as hypothermia, a heart rate,60 beats per min (monitored electrocar-diographically) as bradycardia, and
systolic blood pressure $120 mm Hg
(pediatrics manometer) as hyperten-sion. All data were expressed as the comparison of the IVMP+IVIG versus the single-IVIG group.
Statistical Analysis
The statistical analyses were conducted using the SPSS statistical software package, version 16.0J (SPSS Japan, Tokyo, Japan). The data are presented
as the medians6interquartile range
(IQR) or the means6SD for
continu-ous variables. The statistical analysis for differences in all values was per-formed with the Wilcoxon rank sum test. The associations between pairs of categorical variables were compared by the 2-sidedx2test. Thezscore and the value of the IQR from a standard-ized coronary artery dimension were calculated from the surface area.18All
Pvalues were 2-tailed. A value of P,
.05 was considered statistically signif-icant. The sample size was calculated by the PASS 2008 software program (NCSS, UT). On the basis of previous reports, a 25% response rate was pre-dicted to be observed when patients were treated with single IVIG and 80% to 100% after administration of IVMP +IVIG.11,15In this study, to achieve a 55% difference between the response rates of the 2 groups with a power of 90%, and a significance level of .01, 22 patients were needed in each group. We used the uncorrected 2-sidedx2test to evaluate the null hypothesis.
RESULTS
Patient Population
One hundred twenty-two KD patients were enrolled in the study. The patient
cohort included 74 boys and 48 girls
with a median6IQR age of 3.460.5
years. Forty-eight KD patients were assigned to the predicted IVIG-resistant group (39.3%) based on the Egami score at the time of diagnosis. Sixty-eight patients in the predicted IVIG-responsive group received IVIG treatment, and 6 patients were treated with ASA alone (treatment with ASA alone was per-formed for patients whose guardians did not consent to IVIG treatment or whose fever decreased before treat-ment). Patients in the predicted IVIG-resistant group were randomized to treatment with either IVMP+IVIG (n= 22) or single IVIG (n = 26; Fig 1). None of the patients were treated with steroid 1therapy before being diagnosed with KD.
The patients in the predicted IVIG-resistant group had a shorter mean duration of illness at the time of diag-nosis (predicted IVIG-responsive vs pre-dicted IVIG-resistant group, median 6 IQR: 5.061.0 vs 4.060.5, mean6SD: 4.861.6 vs 3.961.2 days;P= .001), a higher neutrophil sequestration (66.66 12.8 vs 66.9614.6%,P= .001), a lower mean serum albumin level (3.860.2 vs 3.660.4 mg/dL,P= .03), a higher se-rum AST level (35.5611.6 vs 59.566.3 IU/L,P= .001), serum ALT level (18.56 11.9 vs 90.5686.4 IU/L,P= .001), total bilirubin (0.460.1 vs 0.960.6 mg/dL,
P= .001), and CRP level (5.563.3 vs
9.263.3 mg/dL, P= .002) compared
with the patients in the predicted IVIG-responsive group (Table 2). The
coronary artery dimension z score
of the predicted IVIG-resistant group was higher in some segments than that in the predicted IVIG-responsive group (predicted IVIG-responsive vs pre-dicted IVIG-resistant group, median 6 IQR: LMT 0.760.5 vs 0.861.0,P= .037, LAD 1.260.8 vs 1.661.3,P= .038, RCA 1.460.6 vs 1.861.0,P,.001; mean6
SD: LMT 0.76 0.1 vs 1.26 0.2, LAD
1.260.2 vs 2.260.4, RCA 1.460.1 vs 2.460.4).
TABLE 1 The Egami Scoring System
Risk Factor Points
Age at diagnosis (mo) ,6 1 Illness days at diagnosis (days) ,4 1 Platelet count,3104/mL ,30 1 CRP, mg/dL $8 1 ALT, IU/L .80 2
Cutoff:$3 points; 78% sensitivity and 76% specificity.
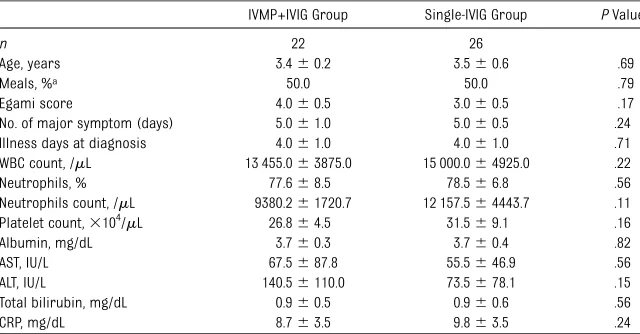
There were no significant differences in any of the examined parameters between the patients randomized to the IVMP+IVIG and the single-IVIG groups within the predicted IVIG-resistant group at diagnosis (Table 3). There were also no differences in thezscores between the 2 groups at diagnosis (median6IQR: LMT 0.961.0 vs 1.06 1.0,P= .58, LAD 1.460.9 vs 1.461.2,P= .28, RCA 1.960.8 vs 2.061.0,P= .11; mean6SD: LMT 1.161.4 vs 1.361.5, LAD 1.461.3 vs 1.561.2, RCA 1.561.2 vs 2.261.3).
Efficacy of Treatment
The major endpoint for examining the efficacy of treatment was that the patients were afebrile (,37.5°C) for at least 36 hours after completion of the initial treatment. Sixty-two of the 68 patients who were predicted to be IVIG responders by the Egami score responded to the initial IVIG treatment, and 20 of 26 patients who were pre-dicted to be IVIG nonresponders were resistant to the initial IVIG treatment (sensitivity 77.0%, specificity 91.1%; Fig 1). The treatment was effective in 19 of
(23.1%) in the single-IVIG group in the predicted IVIG-resistant patients (P,
.001). Twenty patients in the single-IVIG group (76.9%) were nonresponders to initial IVIG and had to receive a sec-ond course of treatment (IVIG: 16 cases; IVMP+IVIG: 2 cases; IVMP pulse for 3 days: 2 cases). Sixteen of these patients (80.0%) required third-line therapy (IVIG: 1 case; IVMP+IVIG: 1 case; IVMP pulse for 3 days: 7 cases; single-dose Infliximab therapy: 7 cases). The me-dian duration of fever after the initia-tion of therapy was 1.060.5 days in the IVMP+IVIG group, compared with 7.063.3 days in the single-IVIG group (P,.001). The IVMP+IVIG group also had a shorter total duration of fever compared with the single-IVIG group (6.061.5 vs 12.062.8 days,P,.001). The median of the minimum tempera-ture at 36 hours after initial treatment in the IVMP+IVIG group was 36.360.5°
C in comparison with 37.76 0.5°C in
the single-IVIG group (P,.001).
At 36 hours after the initial treatment, the patients in the IVMP+IVIG group had significantly lower neutrophil counts (sequestration: 49.1617.2 vs 59.86 14.3%,P= .05; Count: 4291.062284.9 vs 7749.962848.2/mL,P= .04), a higher serum albumin level (3.260.3 vs 2.96 0.3 mg/dL,P= .001), and a lower CRP level (2.461.4 vs 6.361.9 mg/dL,P= .003) than those in the single-IVIG group. The total bilirubin, AST, and ALT levels in the IVMP+IVIG group were normalized promptly after the treatment (Table 4).
Coronary Artery Outcomes
The coronary artery dimensionzscores at diagnosis were not significantly dif-ferent between the single-IVIG and the IVMP+IVIG groups within the predicted IVIG-resistant patients (Fig 2). Follow-up evaluations by echocardiography 36 hours after completion of the initial
treatment showed that the z scores
Predicted IVIG-Responsive Group
Predicted IVIG-Resistant Group
PValue
n 74 48
Age, years 3.563.5 3.463.0 .73 Meals, %a 60.8 60.4 .97 Egami score 1.060.6 4.060.5 .001 No. of major symptom 5.060.5 4.060.5 .88 Illness days at diagnosis (days) 5.061.0 4.060.5 .001 WBC count, /mL 13 500.062675.0 14 400.063737.5 .51 Neutrophils, % 66.6612.8 66.9614.6 .001 Neutrophils count,mL 8629.162440.1 10 449.663346.4 .02 Platelet count,3104/mL 33.166.4 29.865.7 .04 Albumin, mg/dL 3.860.2 3.660.4 .03 AST, IU/L 35.5611.6 59.566.3 .001 ALT, IU/L 18.5611.9 90.5686.4 .001 Total bilirubin, mg/dL 0.460.1 0.960.6 .001 CRP, mg/dL 5.563.3 9.263.3 .002
Presented as the median6IQR or as a percentage. Wilcoxon rank sum test except as noted. WBC, white blood cell. a2-sidedx2test.
TABLE 3 Patient Demographic Data at Diagnosis in the Predicted IVIG-Resistant Group
IVMP+IVIG Group Single-IVIG Group PValue
n 22 26
Age, years 3.460.2 3.560.6 .69 Meals, %a 50.0 50.0 .79 Egami score 4.060.5 3.060.5 .17 No. of major symptom (days) 5.061.0 5.060.5 .24 Illness days at diagnosis 4.061.0 4.061.0 .71 WBC count, /mL 13 455.063875.0 15 000.064925.0 .22 Neutrophils, % 77.668.5 78.566.8 .56 Neutrophils count, /mL 9380.261720.7 12 157.564443.7 .11 Platelet count,3104/mL 26.864.5 31.569.1 .16 Albumin, mg/dL 3.760.3 3.760.4 .82 AST, IU/L 67.5687.8 55.5646.9 .56 ALT, IU/L 140.56110.0 73.5678.1 .15 Total bilirubin, mg/dL 0.960.5 0.960.6 .56 CRP, mg/dL 8.763.5 9.863.5 .24
at the LMT and RCA were significantly smaller in the IVMP+IVIG group than in
the single-IVIG group (median6 IQR:
LMT 0.360.8 vs 1.261.6,P= .039; LAD 1.460.6 vs 1.561.7; RCA 1.160.8 vs
2.761.6,P= .016; mean 6SD: LMT
0.661.2 vs 2.463.4, LAD 1.161.2 vs 3.264.4, RCA 1.461.2 vs 3.864.5).
Thez score at the LMT was also
sig-nificantly smaller in the IVIMP+IVIG group than in the single-IVIG group at 1 month after the end of initial treatment
(median6IQR: LMT 0.560.3 vs 0.86 0.8,P= .043; LAD 0.660.3 vs 1.161.3; RCA 1.460.6 vs 1.961.2; mean6SD: LMT 0.460.6 vs 1.562.3, LAD 0.760.5 vs 2.363.5, RCA 1.460.9 vs 2.963.7). There were no significance differences in the LAD or RCAzscores at 1 month after treatment between the 2 groups.
The number of patients who had z
scores $2.5 at 1 month was 2 of 22
patients (9.1%) in the IVMP+IVIG group and 10 of 26 patients (38.5%) in the
single-IVIG group (P= .04). In addition, 10 of 20 patients (50%) who showed resistance to the initial IVIG treatment in the single-IVIG group had z scores $2.5 at 1 month.
Adverse Events Related to IVMP +IVIG Therapy
Two patients in the IVMP+IVIG group had recurrent fever 24 hours after re-ceiving the IVMP+IVIG therapy. However, these patients did not receive any ad-ditional treatment because their fever decreased within the next 6 hours. Three patients had recurrent fever 36 hours after receiving the IVMP+IVIG therapy (13.6%). These patients were judged to be resistant to the IVMP+IVIG therapy, and they received additional IVIG treatment. None of these patients developed CALs.
The rate of adverse events in the IVMP +IVIG group was significantly higher than that in the single-IVIG group (ad-verse event rate: 27.3% vs 8.5%,P= .04). Six patients developed hypothermia (axillary temperature: 35.0–36.0°C, 27.3%), and 2 of these patients (9.1%) had bradycardia (,60 beats per min) and hypertension (systolic pressure
.120 mm Hg) during the IVMP+IVIG
therapy. These adverse events were transient and disappeared within 36 hours. No other serious adverse events (eg, infusion reaction) occurred in any of the patients.
DISCUSSION
Approximately 15% to 20% of patients with KD are not responsive to initial IVIG treatment, and these patients are at a higher risk for CALs.4,5It is important to identify these patients because they
might benefit from more aggressive
initial treatment. IVIG-resistant patients can be identified at the diagnosis of KD using the Egami scoring system. We evaluated the efficacy and safety of IVMP+IVIG therapy as the primary TABLE 4 Laboratory Data at 36 Hours After Initial Treatment in the Predicted IVIG-Resistant Group
IVMP+IVIG Group Single-IVIG Group PValue
n 22 26
Duration of fever, days (after initial treatment) 1.060.5 7.063.3 .001 Body temperature, °C 36.360.5 37.760.5 .001 Body temperature responsive rate, % 23.1 86.3 .001 WBC count, /mL 9300.063000.0 12 100.062550.0 .17 Neutrophils, % 49.1617.2 59.8614.3 .05 Neutrophils count, /mL 4291.062284.9 7749.962848.2 .04 Platelet count,3104/mL 34.2610.3 33.169.5 .92 Albumin, mg/dL 3.260.3 2.960.3 .001 AST, IU/L 36.568.4 31.069.6 .56 ALT, IU/L 56.5626.0 47.5633.0 .17 Total bilirubin, mg/dL 0.360.1 0.360.3 .14 CRP, mg/dL 2.461.4 6.361.9 .003
Presented as the median6IQR or as a percentage. Wilcoxon rank sum test except as noted. WBC, white blood cell. a2-sidedx2test.
FIGURE 2
Comparison of the maximumzscores. A, The coronary artery dimensionzscores at 36 hours after the initial treatments. B, The coronary artery dimensionzscores at 1 month after the initial treatment. The LMT and RCAzscores in the IVMP+IVIG group were significantly smaller than those in the single-IVIG group at 36 hours after the initial treatments. The LMTzscores was also significantly smaller in the IVMP+IVIG group at 1 month after treatment. The central horizontal line in the box represents the median value, and the bottom and top edges of the box are located at the 25th and 75th percentiles, respectively. The central vertical lines extend from the box to the 90th or 10th percentiles. The sta-tistically significant differences between the IVMP+IVIG and single-IVIG group was determined by the Wilcoxon rank sum test. *P,.05 between the IVMP+IVIG and the single-IVIG group within the predicted IVIG-resistant group.
The pulse dose of methylprednisolone
used in this study was specifically
chosen to avoid potential toxicity while
inhibiting many of the inflammatory
responses thought to mediate the vasculitis associated with KD. Although a previous study indicated that oral prednisolone was less effective in KD patients than ASA,19 a single dose of methylprednisolone pulse therapy was administered in the current study be-cause the single-pulsed dose of meth-ylprednisolone results in a brief serum half-life but provides profound and prolonged anti-inflammatory effects.14 In addition, the effects and molecular mechanism of the IVMP+IVIG therapy for the predicted IVIG-resistant patients were previously evaluated in a micro-array study, and that study showed this treatment to be effective for decreasing the expression of inflammation-related transcripts.15
The IVMP+IVIG patients had a shorter duration of fever and more rapid nor-malization of neutrophils, serum al-bumin levels, and serum CRP levels than the single-IVIG group in the cur-rent study. The improvement in the neutrophils and the CRP level may indi-cate that the inflammatory mediators were well controlled by the treatment. The disruption of vascular endothelial function by active neutrophils is central to the pathogenesis of acute KD and may
also reported that IVIG treatment sup-presses the activity of neutrophils and regulates their infiltration into endo-thelial tissues.23,24The normalization of the serum albumin level indicated the resolution of vascular permeability.25 Therefore, the results of this study in-dicated that IVMP+IVIG therapy induces more rapid inhibition of the infl amma-tion of vascular endothelial cells than single-IVIG treatment. The IVMP+IVIG
group also had smaller z scores at
several coronary arteries compared with the single-IVIG group after treat-ment (Fig 2). Because vasculitis is re-lated to the development of CALs, IVMP+IVIG therapy may lead to more rapid improvement of the vasculitis, making it more effective for prevent-ing the developmental of CALs.
The adverse events related to the IVMP+IVIG therapy were confirmed to be hypothermia (27.3%), bradycardia, and hypertension (9.1%). However these adverse events were transient, and no serious adverse events were observed. Three patients in the IVMP+IVIG group were recurrent fever cases, and 2 of these patients experienced spontane-ous improvement without additional treatment. None of these patients de-veloped CALs.
This pilot study has several limitations. The Egami score is highly sensitive and specific in Japanese patients, but it is
at 36 hours after completion of the initial treatment as the endpoint for efficacy in this study. Sleeper et al reported there was a steroid versus placebo difference in the z scores at each time point and that this was not different for the predicted high- and low-risk groups.26Although this study had low power to detect the de-velopment of CALs, it could detect clinically relevant differences in these outcomes. The current study was not a blind multicenter study. Therefore, a multicenter, double-blind, placebo-controlled study will be necessary to confirm the safety and efficacy of this treatment regimen.
CONCLUSION
This study demonstrated that IVMP+IVIG combination therapy, as a new strategy for primary treatment, is safe and ef-fective for patients predicted to have refractory KD based on the Egami score.
ACKNOWLEDGMENTS
This work was supported by a grant-in-aid for Scientific Research (C 21591397, C 9022950) from the Ministry of Educa-tion, Culture, Sports, Science and Tech-nology; a Parents’Association Grant at Kitasato University School of Medicine; and a Grant-in-aid from the Kawasaki Disease Research Center in Japan.
REFERENCES
1. Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan.Pediatrics. 1974; 54(3):271–276
2. Newburger JW, Takahashi M, Gerber MA, et al; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health
professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovas-cular Disease in the Young, American Heart Association.Pediatrics. 2004;114(6): 1708–1733
3. Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP; US/Canadian Kawasaki Syndrome Study Group. Intrave-nous gamma-globulin treatment and retreat-ment in Kawasaki disease. Pediatr Infect Dis J. 1998;17(12):1144–1148
4. Tremoulet AH, Best BM, Song S, et al. Re-sistance to intravenous immunoglobulin in children with Kawasaki disease.J Pediatr. 2008;153(1):117–121
5. Egami K, Muta H, Ishii M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki dis-ease.J Pediatr. 2006;149(2):237–240
7. Sano T, Kurotobi S, Matsuzaki K, et al. Prediction of non-responsiveness to stan-dard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166(2):131–137
8. Ogata S, Bando Y, Kimura S, et al. The strat-egy of immune globulin resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy.J Cardiol. 2009;53(1):15–19
9. Hashino K, Ishii M, Iemura M, Akagi T, Kato H. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy.Pediatr Int. 2001;43(3):211–217
10. Wright DA, Newburger JW, Baker A, Sundel RP. Treatment of immune globulin-resistant Kawasaki disease with pulsed doses of cor-ticosteroids.J Pediatr. 1996;128(1):146–149
11. Okada K, Hara J, Maki I, et al; Osaka Kawasaki Disease Study Group. Pulse methylprednisolone with gammaglobulin as an initial treatment for acute Kawasaki disease.Eur J Pediatr. 2009;168(2):181–185
12. Furukawa T, Kishiro M, Akimoto K, Nagata S, Shimizu T, Yamashiro Y. Effects of steroid pulse therapy on immunoglobulin-resistant Kawasaki disease.Arch Dis Child. 2008;93(2): 142–146
13. Miura M, Kohno K, Ohki H, Yoshiba S, Sugaya A, Satoh M. Effects of methylprednisolone pulse on cytokine levels in Kawasaki disease
patients unresponsive to intravenous im-munoglobulin. Eur J Pediatr. 2008;167(10): 1119–1123
14. Sundel RP, Baker AL, Fulton DR, Newburger JW. Corticosteroids in the initial treatment of Kawasaki disease: report of a random-ized trial.J Pediatr. 2003;142(6):611–616
15. Ogata S, Ogihara Y, Nomoto K, et al. Clinical score and transcript abundance patterns identify Kawasaki disease patients who may benefit from addition of methylpred-nisolone.Pediatr Res. 2009;66(5):577–584
16. Newburger JW, Sleeper LA, McCrindle BW, et al; Pediatric Heart Network Inves-tigators. Randomized trial of pulsed corti-costeroid therapy for primary treatment of Kawasaki disease.N Engl J Med. 2007;356 (7):663–675
17. Ayusawa M, Sonobe T, Uemura S, et al; Kawasaki Disease Research Committee. Revi-sion of diagnostic guidelines for Kawasaki disease. 5th revised ed.Pediatr Int. 2005; 47(2):232–234
18. de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as nor-mal in Kawasaki disease.J Pediatr. 1998; 133(2):254–258
19. Kato H, Koike S, Yokoyama T. Kawasaki dis-ease: effect of treatment on coronary artery involvement.Pediatrics. 1979;63(2):175–179
20. Niwa Y, Sohmiya K. Enhanced neutrophilic functions in mucocutaneous lymph node
syndrome, with special reference to the possible role of increased oxygen interme-diate generation in the pathogenesis of cor-onary thromboarteritis.J Pediatr. 1984;104(1): 56–60
21. Takahashi K, Oharaseki T, Naoe S, Wakayama M, Yokouchi Y. Neutrophilic involvement in the damage to coronary arteries in acute stage of Kawasaki disease.Pediatr Int. 2005; 47(3):305–310
22. Abe J, Ebata R, Jibiki T, Yasukawa K, Saito H, Terai M. Elevated granulocyte colony-stimulating factor levels predict treatment failure in patients with Kawasaki disease. J Allergy Clin Immunol. 2008;122(5):1008– 1013.e8.
23. Clynes R. IVIG therapy: interfering with interferon-gamma.Immunity. 2007;26(1):4–6
24. Gill V, Doig C, Knight D, Love E, Kubes P. Targeting adhesion molecules as a poten-tial mechanism of action for intravenous immunoglobulin. Circulation. 2005;112(13): 2031–2039
25. Terai M, Honda T, Yasukawa K, Higashi K, Hamada H, Kohno Y. Prognostic impact of vascular leakage in acute Kawasaki dis-ease.Circulation. 2003;108(3):325–330
26. Sleeper LA, Minich LL, McCrindle BM, et al; Pediatric Heart Network Investigators. Evalu-ation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. 2011;158(5):831– 835.e3
DOI: 10.1542/peds.2011-0148 originally published online December 5, 2011;
2012;129;e17
Pediatrics
and Masahiro Ishii
Shohei Ogata, Yoshihito Ogihara, Takashi Honda, Shinya Kon, Kazumasa Akiyama
Services
Updated Information &
http://pediatrics.aappublications.org/content/129/1/e17
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/129/1/e17#BIBL
This article cites 25 articles, 7 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/therapeutics_sub Therapeutics
http://www.aappublications.org/cgi/collection/pharmacology_sub Pharmacology
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2011-0148 originally published online December 5, 2011;
2012;129;e17
Pediatrics
and Masahiro Ishii
Shohei Ogata, Yoshihito Ogihara, Takashi Honda, Shinya Kon, Kazumasa Akiyama
Randomized Trial
Corticosteroid Pulse Combination Therapy for Refractory Kawasaki Disease: A
http://pediatrics.aappublications.org/content/129/1/e17
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.