RESEARCH
Esterification of glycerol from biodiesel
production to glycerol carbonate
in non-catalytic supercritical dimethyl
carbonate
Zul Ilham
1,2*and Shiro Saka
1Abstract
Conversion of glycerol from biodiesel production to glycerol carbonate was studied by esterification with dimethyl carbonate in a non-catalytic supercritical condition. It was found that in a non-catalytic supercritical condition, glyc-erol at higher purity gave higher yield of glycglyc-erol carbonate at 98 wt% after reaction at 300 °C/20–40 MPa/15 min. The yield of glycerol carbonate was observed to increase with molar ratio, temperature, pressure and time until a certain equilibrium limit. The existence of impurities such as water and remnants of alkaline catalyst in crude glycerol will direct the reaction to produce glycidol. Although impurities might not be desirable, the non-catalytic supercritical dimethyl carbonate could be an alternative method for conversion of glycerol from biodiesel production to value-added glycerol carbonate.
Keywords: Glycerol, Biodiesel, Esterification, Glycerol carbonate, Dimethyl carbonate
© 2016 The Author(s). This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Background
In recent years, growing biodiesel industries have gen-erated a surplus of crude glycerol as the by-product. Approximately 10 wt% of triglycerides could be collected in the form of by-product glycerol from any transesteri-fication of oil with methanol to produce biodiesel (Teng et al. 2014). As the conventional biodiesel plants mostly utilize alkali-catalyzed method, the obtained glycerol is low in purity at 40–70 %, containing remnants of metha-nol, salt, catalyst residue, soap, fatty acids and glycerides (Ilham and Saka 2012). Without new applications, excess of such crude glycerol could exert impact into refined glycerol market (Yang et al. 2012) and creating a new waste, which could only be used as low-energy fuel for incineration or ruminant feed (Ciriminna et al. 2014). This has prompted the attention of researchers to explore the conversion of the low cost glycerol to value-added
products. Several new methods for biodiesel production without producing glycerol in a non-catalytic condition have also been introduced (Ilham and Saka 2010; Saka et al. 2010).
Apart from such works, some researchers also venture into converting glycerol to glycerol carbonate (Algoufi and Hameed 2014; Waghmare et al. 2015; Teng et al.
2016) and other derivatives (Szymanowska-Powalowska
2014; Santacesaria et al. 2010; Ayoub et al. 2012). A het-erogeneous alkali-catalyzed esterification of glycerol using waste coal fly ash achieved 96 % glycerol carbonate at 75 °C and 90 min. The catalyst is 4 wt% in loading and could be recycled up to four times (Algoufi and Hameed
2014). Ultrasound-assisted enzyme-catalyst esterification is also feasible but will not work in the existence of impu-rities such as water and metal ions like sodium, potas-sium and magnepotas-sium in the glycerol (Waghmare et al.
2015). In addition, microwave irradiation has been used for glycerol carbonate production using calcium oxide as catalyst. It was consequently found that 93.4 % of glycerol carbonate yield could be achieved after 5 min reaction time, 1 wt% calcium oxide, 2:1 molar ratio of dimethyl
Open Access
*Correspondence: ilham@um.edu.my
2 Institute of Biological Sciences, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
carbonate to glycerol and 65 °C (Teng et al. 2016). How-ever, although microwave irradiation technology might be effective at micro volume, it is known to have limita-tion at a larger scale.
Glycerol could be a building block to many useful deriv-atives. Physically, pure glycerol (99 %) is a clear, odorless and hygroscopic liquid under ambient condition. Its boil-ing point, meltboil-ing point and flash point are reported to be 290 °C, 18 °C and 177 °C, respectively (Ayoub and Abdul-lah 2012). The crude glycerol from biodiesel produc-tion, on the other hand, is dark brown liquid with faulty smell, being dependent greatly on the methods and the feedstocks used for its conversion (Teng et al. 2014). As purification and distillation of crude glycerol is energy-intensive and economically challenging, one way is to ensure the biodiesel production method that can produce a pure glycerol in the first place such as the supercritical methanol method (Saka and Kusdiana 2001).
This study is, therefore, to report extensively on the esterification of glycerol to glycerol carbonate using non-catalytic supercritical dimethyl carbonate. It should be noted that esterification of glycerol in this study can be, instead, transesterification of dimethyl carbonate by glycerol. This is a matter of which one can be the solvent, dimethyl carbonate or glycerol. Since this work is con-cerned with the utilization of glycerol, dimethyl carbon-ate was used as the solvent for its esterification. The route for producing glycerol carbonate was also elucidated to discuss the favorable reaction condition.
Methods Materials
Pure glycerol, dimethyl carbonate, glycerol carbon-ate, sodium hydroxide (NaOH) and various authentic compounds for standards and chemicals were obtained from Nacalai Tesque Inc., Japan, all of which are of high-est purity available. Crude glycerol was also prepared by alkali-catalyzed transesterification (Lubes and Zakaria
2009) and pure glycerol from non-catalytic supercritical methanol (Saka and Kusdiana 2001) for use in this study.
Characterization of glycerol
Glycerol and methanol contents were analyzed by high performance liquid chromatography (HPLC) LC10-VP System (Column: Ultrahydrogel 120, oven temperature: 40 °C, flow-rate: 1 mL/min, mobile phase: water, detec-tor: RID 10A) (Algoufi and Hameed 2014). Water con-tent in glycerol was determined by Karl-Fischer method in accordance to EN ISO 12937, while standard titration method was used to analyze soap content in glycerol fol-lowing the American Oil Chemists’ Society (AOCS) Cc 17-95. Salt content in glycerol was determined using pre-cipitation method (Nanda et al. 2014).
Non‑catalytic supercritical dimethyl carbonate esterification of glycerol
Non-catalytic supercritical dimethyl carbonate esterifi-cation of glycerol (pure and crude) was performed in a in a batch-type supercritical biomass conversion system as reported previously (Saka and Kusdiana 2001). Pre-determined quantities of glycerol and dimethyl carbon-ate corresponding to the molar ratio of 1:10 of glycerol to dimethyl carbonate were mixed in a 5 mL Inconel-625 reaction vessel. It was, then, heated to have a reaction at supercritical conditions with dimethyl carbonate (Criti-cal Temperature (Tc): 274.9 °C/Criti(Criti-cal Pressure (Pc): 4.63 MPa) by immersing it into a molten tin bath and later quenched into a water bath to stop the reaction. All the experiments and analyses were conducted in compliance with the procedures described in our previ-ous papers (Ilham and Saka 2010, 2012) and the reaction temperature and pressure were monitored by thermo-couple and pressure gauge, respectively. Throughout the reaction, pressure was maintained by a custom designed Inconel-625 batch reactor vessel as shown in Additional file 1: Fig. S1. The obtained products were analyzed using HPLC as described beforehand. Experimental design is depicted in Fig. 1. For the calibration curve of the authen-tic standard products, glycerol and glycerol carbonate were used. All sets of experiments were made to at least triplicate for confirmation of the yields by utilizing 1:10 molar ratio of glycerol to dimethyl carbonate, although not treated statistically. The yield of glycerol carbon-ate in weight percent as presented in this study refers to the percentage of yields conversion recovered based on theoretical yield. Additional confirmation of glyc-erol carbonate formation (Additional file 2: Fig. S2) was analyzed using a Bruker AV400 1H NMR spectrometer
and referenced to the residual NMR solvent signals. 1H
NMR (300 MHz, DMSO): δ 5.25 (t, 3J
HH = 5.6 Hz, 1H),
4.83–4.76 (m, 1H), 4.49 (dd, 2J
HH = 8.3, 3JHH = 8.2 Hz,
1H), 4.28 (dd, 2J
HH = 8.2, 3JHH = 5.9 Hz, 1H), 3.66 (ddd,
2J
HH = 12.6, 3JHH = 5.3, 3JHH = 3.0 Hz, 1H), 3.50 (ddd,
2J
HH = 12.7, 3JHH = 5.4, 3JHH = 3.4 Hz, 1H). IR Neat:
1766 cm−1 (C = O).
[image:2.595.307.539.602.697.2]Conventional esterification
For comparison, the esterification of glycerol by dimethyl carbonate using homogenous NaOH catalyst was carried out in a 250 ml round bottom flask attached to a reflux condenser with a thermometer immersed in a water bath. Predetermined quantities of glycerol and dimethyl carbonate were mixed in molar ratio of 1:5 of glycerol to dimethyl carbonate and heated to 75 °C for 90 min (Ochoa-Gómez et al. 2009). The yields and conversions were determined using similar equations used by Teng et al. (2016).
Results and discussion
Properties of pure and crude glycerol
In this study, two grades of glycerol produced by two dif-ferent biodiesel production methods were used and the properties are shown in Table 1. Properties of commer-cial glycerol are included just for comparison.
The properties of crude glycerol produced as by-product from alkali-catalyzed method varied greatly. However, the glycerol produced from non-catalytic supercritical methanol method did not vary, showing similar properties with the commercial glycerol (99 % purity) with no traces of water, salt and soap detectable. This is due to the fact that no catalyst is used in non-cat-alytic supercritical methanol method (Saka and Kusdiana
2001). On the other hand, crude glycerol from alkali-cat-alyzed method contains significant amount of water, salt and soap with 70 % purity. Hereinafter in this study, glyc-erol from alkali-catalyzed method will be referred to as crude glycerol and glycerol from non-catalytic supercriti-cal methanol will be referred to as pure glycerol.
Glycerol carbonate formation from pure and crude glycerol
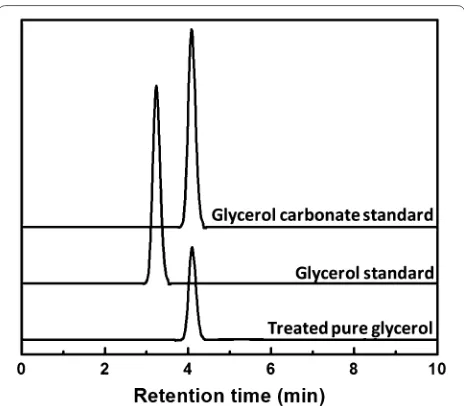
In a trial run, pure glycerol was treated in non-catalytic supercritical dimethyl carbonate at 300 °C/12 MPa for 15 min. When analyzed and compared to several authen-tic compounds, the resulted compound was found to be glycerol carbonate (4-hydroxymethyl-1,3-dioxolan-2-one), a value-added derivative of glycerol, as depicted at around 4 min retention time in the chromatogram of Fig. 2.
[image:3.595.306.538.86.289.2]Previously, conversion of glycerol to glycerol carbon-ate in non-catalytic supercritical dimethyl carboncarbon-ate was shown to proceed at 300 °C/15 MPa after 15 min with 90 wt% yield (Ilham and Saka 2010). Temperature higher than 300 °C and longer reaction time is not favorable due to the possibility of thermal decomposition (Ilham and Saka 2012). However, Fig. 3 shows the yield of pure glycerol as treated in supercritical dimethyl carbonate at 300 °C with different reaction pressures. It could be seen from the graph that the trend shows higher formation of glycerol carbonate at higher reaction pressure. HPLC plot
Table 1 Properties of glycerol as by-product from differ-ent biodiesel production methods
n.d. not detected
Glycerol from different
methods Purity Water (wt%) Salt (wt%) Soap (ppm)
Alkali-catalyzed glycerol 70 9.82 20.18 35,100 Supercritical methanol
glycerol >98 n.d. n.d. n.d.
Commercial glycerol 99 n.d. n.d. n.d.
Fig. 2 HPLC chromatogram of pure glycerol after treatment in supercritical dimethyl carbonate at 300 °C/20 MPa/15 min without any catalyst applied. Standards of glycerol and glycerol carbonate are shown as authentic compounds
0 10 20 30 40 50 60
5MPa 20MPa 40MPa
300°C
Glycerol carbonate (wt%
)
Reaction time (min)
[image:3.595.305.540.520.688.2] [image:3.595.55.292.630.709.2]for partial conversion could be seen in Additional file 3: Fig. S3. Similar trends at lower reaction pressures have been reported (Ilham and Saka 2012). This is in agree-ment with previous findings in supercritical fluid behav-ior study where higher reaction pressure always leads to higher yield (Abbott et al. 2005; Warabi et al. 2004).
When the reaction condition was maintained at 300 °C/20–40 MPa, the highest yield of glycerol carbon-ate based on the theoretical value at 98 wt% could be achieved after 20 min reaction. However, when treated at 300 °C/5 MPa, yield of glycerol carbonate is decreas-ing particularly after 20 min reaction, indicatdecreas-ing possible decomposition of glycerol carbonate under low reaction pressures.
The reaction scheme for the conversion could be expected to proceed in a non-catalytic manner as depicted in the graphical abstract. Glycerol undergoes esterification with dimethyl carbonate, leading to the for-mation of thermodynamically stable five-member cyclic glycerol carbonate. Methanol, formed from this reaction, was removed by evaporation. The scheme is in agreement with several previous studies describing a similar method in a catalytic manner (Teng et al. 2016; Waghmare et al.
2015; Algoufi and Hameed 2014; Ochoa-Gómez et al.
2009).
Effect of impurities on glycerol carbonate formation
The pure glycerol from supercritical methanol method and crude glycerol from alkali-catalyzed method were both treated in non-catalytic supercritical dimethyl car-bonate (300 °C/20 MPa/15 min). As depicted in Fig. 4, pure glycerol showed high conversion to glycerol carbon-ate, while crude glycerol showed significantly lower yield. To confirm the effect of impurities in glycerol from alkali-catalyzed method on reducing the yield of glycerol
carbonate, a thorough analysis was done on the HPLC chromatogram of products obtained from crude glycerol of alkali-catalyzed method. The chromatogram showed an additional peak. When analyzed and compared with several authentic compounds, this was determined to be glycidol (Ilham and Saka 2012).
Based on these findings, decomposition occurred in the presence of water and salts (impurities in alkali-cat-alyzed glycerol) to partly decompose glycerol carbonate into glycidol. The proposed scheme for this decomposi-tion pathway is presented in Fig. 5. As discussed before-hand (Fig. 3), low reaction pressures in supercritical condition may also lead to glycidol formation, following a similar pathway as described (Ilham and Saka 2012; Abbott et al. 2005). A research on glycerol carbonates synthesis by coalfly ash catalyst by Algoufi and Hameed (2014) is also in agreement with this finding. Rathore et al. (2014) described the formation of glycerol dicarbo-nate in their study using dimethyl and diethyl carbodicarbo-nate as reactant but no such intermediate was detected in our study.
Other methods for glycerol carbonate production
According to Ochoa-Gómez et al. (2009), conventional transesterification by homogeneous NaOH, a range of yield from 35 to 98 wt% could be achieved after 90 min reaction at 75 °C. This might be attractive from an indus-trial standpoint as a lower reaction time could be used without affecting the yield. However, separation is inevita-ble and in the case of biodiesel, huge amount of water is needed to wash the dissolved NaOH from the product. In glycerol carbonate production, it is even more complicated as water could not be used. It should be noted that water and glycerol carbonate are miscible. On the other hand, no heterogenous catalyst and acid catalyst could give a high yield of glycerol carbonate (Ochoa-Gómez et al. 2009).
Other possible methods for glycerol carbonate pro-duction are ultrasound-assisted lipase-catalyzed method (Waghmare et al. 2015) and microwave-assisted trans-esterification with calcium oxide (CaO) as catalyst (Teng et al. 2016). Lipase-catalyzed method, similar to other biocatalysis, showed advantages in terms of environ-mentally-friendly as well as high in yield (>97 wt%), but enzyme is not economical, especially when coupled with high cost ultrasound equipment (Waghmare et al. 2015). Microwave-assisted conversion has been reported to show fast reaction, low operating temperature and effective for crude glycerol but hard to penetrate large volume of sam-ple (Teng et al. 2014). In addition, the use of CaO catalyst is very much dependent on whether or not, it was ther-mally pre-treated beforehand (Ochoa-Gómez et al. 2009).
Based on the evidence presented beforehand, the non-cat-alytic supercritical dimethyl carbonate showed a potential as
10 20 30 40 50 60
0 20 40 60 80 100
Reaction time (min)
Glycerol carbonate (wt%
)
Pure glycerol Crude glycerol
[image:4.595.56.290.535.687.2]an alternative method for conversion of glycerol to glycerol carbonate. It was found that the supercritical dimethyl car-bonate could esterified pure glycerol to glycerol carcar-bonate in high yield without any catalyst applied. When crude glycerol with impurities is used, glycerol carbonate yield decreases in the compensation of glycidol formation. Glycerol carbon-ate is a stable, colorless liquid currently used industrially as solvent and surfactant (Herseczki et al. 2009), while glycidol is important in the production of epoxy resins and polyure-thanes. Its high functionality, together with the versatile and well-investigated reactivity of its hydroxyl functions could supply as a basis for a variety of derivatives (Pagliaro et al.
2007). For comparison, Table 2 shows glycerol carbonate yield and remarks on various glycerol carbonate production technologies.
Conclusions
The results showed that pure glycerol could be converted to value-added glycerol carbonate with a yield of 98 wt% using supercritical dimethyl carbonate (300 °C/20– 40 MPa/15 min) without any catalyst applied. On the other hand, when crude glycerol with impurities was used, glycerol carbonate could decompose to glycidol. The high reaction pressure (>10 MPa) must also be main-tained in supercritical dimethyl carbonate as low reaction pressure would lead to the decomposition. The formation of value-added chemicals, glycerol carbonate and glycidol from the non-catalytic supercritical dimethyl carbon-ate method showed the potential of this method to be an alternative way to reduce glycerol glut from biodiesel production.
Fig. 5 Proposed pathways for glycerol carbonate formation and partial glycerol carbonate decomposition to glycidol and carbon dioxide
Table 2 Glycerol carbonate yield and remarks on various production technologies
Method Yield (wt%) Remark
Alkali-catalyzed 35–98 Separation and purification is needed. Water could not be used as it is miscible with glycerol carbonate and thus, yield decreases
Ultrasound-assisted lipase-catalyzed >97 Enzyme is costly especially when coupled with ultrasound equipment
Microwave-assisted CaO-catalyzed 93.4 Fast reaction but hard to penetrate large volumes. CaO needs thermal pre-treatment Non-catalytic supercritical dimethyl carbonate 98 Pure glycerol is needed for high yield but no complicated separation and purification.
[image:5.595.56.540.86.361.2] [image:5.595.58.538.619.709.2]Abbreviations
HPLC: high performance liquid chromatography; AOCS: American Oil Chem-ists’ Society; DMSO: dimethyl sulfoxide; NMR: nuclear magnetic resonance.
Authors’ contributions
ZI is the main author and researcher in this project, who executes most of the experimental works and SS is the principle advisor of the project and co-author of this manuscript. Both authors read and approved the final manuscript.
Author details
1 Graduate School of Energy Science, Kyoto University, Yoshida-honmachi, Sakyo-ku, Kyoto 606-8501, Japan. 2 Institute of Biological Sciences, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia.
Acknowledgements
This work was partially supported by JICA Project for AUN/SEED-Net Short Term Research Program in Japan 2015 and University of Malaya (RP004B-13BIO) of which the authors highly acknowledged.
Competing interests
The authors declare that they have no competing interests.
Received: 13 March 2016 Accepted: 21 June 2016
References
Abbott AP, Corr S, Durling NE, Hope EG (2005) Pressure effects on Friedel– Crafts alkylation reactions in supercritical difluoromethane. Chem Phys Chem 6:466–472. doi:10.1002/cphc.200400363
Algoufi YT, Hameed BH (2014) Synthesis of glycerol carbonate by trans-esterification of glycerol with dimethyl carbonate over K-zeolite derived from coalfly ash. Fuel Process Technol 126:5–11. doi:10.1016/j. fuproc.2014.04.004
Ayoub M, Abdullah AZ (2012) Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew Sustain Energy Rev 16:2671–2686. doi:10.1016/j.biortech.2012.02.103
Ayoub M, Khayoon MS, Abdullah AZ (2012) Synthesis of oxygenated fuel additives via the solventless etherification of glycerol. Bioresour Technol 112:308–312. doi:10.1016/j.biortech.2012.02.103
Additional files
Additional file 1: Fig. S1. Custom designed 5-mL Inconel-625 batch reactor vessel with pressure controller and detector for use into molten tin bath.
Additional file 2: Fig. S2. 1H NMR spectrum of glycerol carbonate (4-hydroxymethyl-1,3-dioxolan-2-one).
Additional file 3: Fig. S3. HPLC plot for partial conversion of pure glyc-erol in supercritical dimethyl carbonate treatment at 300 °C/20 MPa from 2 min to 20 min reaction time.
Ciriminna R, Pina CD, Rossi M, Pagliaro M (2014) Understanding the glycerol market. Eur J Lipid Sci Technol 116:1432–1439. doi:10.1002/ ejlt.201400229
Herseczki Z, Varga T, Marton G (2009) Synthesis of glycerol carbonate from glycerol, a by-product of biodiesel production. Int J Chem Reactor Eng 7:1–14. doi:10.2202/1542-6580.2168
Ilham Z, Saka S (2010) Two-step supercritical dimethyl carbonate method for biodiesel production from Jatropha curcas oil. Bioresour Technol 101:2735–2740. doi:10.1016/j.biortech.2009.10.053
Ilham Z, Saka S (2012) Conversion of glycerol as by-product from biodiesel production to value-added glycerol carbonate. In: Zero-carbon energy Kyoto 2011, pp 127–133. Springer, Tokyo
Lubes ZIZ, Zakaria M (2009) Analysis of parameters for fatty acid methyl esters production from refined palm oil for use as biodiesel in the single-and twostage processes. Malays J Biochem Mol Biol 17:5–9
Nanda MR, Yuan Z, Qin W, Poirier MA, Chunbao X (2014) Purification of crude glycerol using acidification: effects of acid types and product characteri-zation. Austin J Chem Eng 1:1–7
Ochoa-Gómez JR, Gómez-Jiménez-Aberasturi O, Maestro-Madurga B, Pesquera-Rodríguez A, Ramírez-López C, Lorenzo-Ibarreta L, Torrecilla-Soria J, Villarán-Velasco MC (2009) Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: catalyst screening and reaction optimization. Appl Catal A Gen 366:315–324. doi:10.1016/j. apcata.2009.07.020
Pagliaro M, Ciriminna R, Kimura H, Rossi M, DellaPina C (2007) From glycerol to value-added products. Angew Chem Int Ed 46:4434–4440. doi:10.1002/ anie.200604694
Rathore V, Tyagi S, Newalkar B, Badoni RP (2014) Glycerin-free synthesis of jatropha and pongamia biodiesel in supercritical dimethyl and diethyl carbonate. Ind Eng Chem Res 53:10525–10533. doi:10.1021/ie5011614
Saka S, Kusdiana D (2001) Biodiesel fuel from rapeseed oil as pre-pared in supercritical methanol. Fuel 80:225–231. doi:10.1016/ S0016-2361(00)00083-1
Saka S, Isayama Y, Ilham Z, Jiayu X (2010) New process for catalyst-free bio-diesel production using subcritical acetic acid and supercritical methanol. Fuel 89:1442–1446. doi:10.1016/j.fuel.2009.10.018
Santacesaria E, Tesser R, Di Serio M, Verde D (2010) New process for producing epichlorohydrin via glycerol chlorination. Ind Eng Chem Res 49:964–970. doi:10.1021/ie900650x
Szymanowska-Powalowska D (2014) 1,3-Propanediol production from crude glycerol by Clostridium butyricum DSP1 in repeated batch. Electron J Biotechnol 17:322–328. doi:10.1016/j.ejbt.2014.10.001
Teng WK, Ngoh GC, Yusoff R, Aroua MK (2014) A review on the performance of glycerol carbonate production via catalytic transesterification: effects of influencing parameters. Energy Convers Manag 88:484–497. doi:10.1016/j.enconman.2014.08.036
Teng WK, Ngoh GC, Yusoff R, Aroua MK (2016) Microwave-assisted transesteri-fication of industrial grade crude glycerol for the production of glycerol carbonate. Chem Eng J 284:469–477. doi:10.1016/j.cej.2015.08.108
Waghmare GV, Vetal MD, Rathod VK (2015) Ultrasound assisted enzyme cata-lyzed synthesis of glycerol carbonate from glycerol and dimethyl carbon-ate. Ultrason Sonochem 22:311–316. doi:10.1016/j.ultsonch.2014.06.018
Warabi Y, Kusdiana D, Saka S (2004) Biodiesel fuel from vegetable oil by vari-ous supercritical alcohols. Appl Biochem Biotechnol 113–116:793–801. doi:10.1385/ABAB:115:1-3:0793