RESEARCH ARTICLE
Bmp signaling mediates endoderm pouch morphogenesis by
regulating Fgf signaling in zebrafish
C. Ben Lovely*, Mary E. Swartz, Neil McCarthy, Jacqueline L. Norrie and Johann K. Eberhart
ABSTRACT
The endodermal pouches are a series of reiterated structures that segment the pharyngeal arches and help pattern the vertebrate face. Multiple pathways regulate the complex process of endodermal development, including the Bone morphogenetic protein (Bmp) pathway. However, the role of Bmp signaling in pouch morphogenesis is poorly understood. Using genetic and chemical inhibitor approaches, we show that pouch morphogenesis requires Bmp signaling from 10-18 h post-fertilization, immediately following gastrulation. Blocking Bmp signaling during this window results in morphological defects to the pouches and craniofacial skeleton. Using genetic chimeras we show that Bmp signals directly to the endoderm for proper morphogenesis. Time-lapse imaging and analysis of reporter transgenics show that Bmp signaling is necessary for pouch outpocketing via the Fibroblast growth factor (Fgf) pathway. Double loss-of-function analyses demonstrate that Bmp and Fgf signaling interact synergistically in craniofacial development. Collectively, our analyses shed light on the tissue and signaling interactions that regulate development of the vertebrate face.
KEY WORDS: Bmp signaling, Pharyngeal pouches, Endoderm morphogenesis, Fgf signaling, Zebrafish
INTRODUCTION
Formation of the adult body plan requires a diverse set of coordinated cellular behaviors and movements. These morphogenetic events shape and reshape the developing embryo as new cell types arise and diversify. Key to these events is a complex interplay between multiple cell and tissue types, requiring orchestration of many signaling pathways. An excellent system in which to explore how complex interactions shape tissues is the genesis of the vertebrate head.
In all vertebrates, cranial neural crest cells (CNCCs) give rise to the majority of the craniofacial skeleton (Gross and Hanken, 2008; Knight and Schilling, 2006). CNCCs originate in the dorsal neural tube and migrate ventrally to populate the pharyngeal arches (Knight and Schilling, 2006). The pharyngeal arches consist of a mesoderm core surrounded by CNCCs, wrapped by facial epithelia, the endoderm and ectoderm. The endoderm segments the pharyngeal arches into discrete, serial structures. Subsequently, the CNCCs in the pharyngeal arches undergo a series of morphogenetic movements to give rise to the various elements of the craniofacial skeleton (Yelick and Schilling, 2002).
Proper craniofacial development relies on highly conserved signaling interactions between CNCCs and the facial epithelia (Chai and Maxson, 2006; Cobourne and Sharpe, 2003; Swartz et al., 2012). Studies in mouse (Couly et al., 2002), chicken (Graham et al., 2005) and zebrafish (Piotrowski and Nüsslein-Volhard, 2000; David et al., 2002) show a requirement for the endoderm in the patterning and morphogenesis of the craniofacial skeleton. This requirement can be both direct (Choe and Crump, 2014; Crump et al., 2004; Swartz et al., 2012) and indirect (Haworth et al., 2007; Balczerski et al., 2012), with loss of endoderm disrupting craniofacial development. Furthermore, disruption of endoderm morphogenesis causes defects in viscerocranial development (Choe et al., 2013; Choe and Crump, 2014; Piotrowski and Nüsslein-Volhard, 2000). Overall, these studies demonstrate that endoderm morphogenesis is essential for craniofacial development.
Multiple signaling pathways are implicated in endoderm morphogenesis. Morphogenesis of the endodermal pouches and their derivatives requires Sonic hedgehog signaling (Moore-Scott and Manley, 2005; Swartz et al., 2012). Loss ofraldh2(aldh1a2) in mice and zebrafish disrupts pouch morphogenesis, potentially by disrupting Fgf signaling (Kopinke et al., 2006; Niederreither et al., 2003). Mutation intbx1causes similar pouch defects by dysregulating Fgf and Wnt signaling (Choe and Crump, 2014). Here, Fgfs, specifically Fgf8a and potentially Fgf3, act as guidance cues during pouch formation (Choe and Crump, 2014; Crump et al., 2004).
There is evidence for the involvement of Bmp signaling in endoderm specification and patterning in mouse (Paca et al., 2012; Yamamoto et al., 2009) and zebrafish (Poulain et al., 2006; Tiso et al., 2002). Definitive endoderm formation and morphogenesis, as well as migration of the anterior visceral endoderm, requireBmpr1a (Davis et al., 2004; Miura et al., 2010). However, the mechanism by which deletion of Bmpr1a causes defects to endoderm morphogenesis is unknown.
Here, we use genetic and chemical inhibitor analyses in zebrafish to show that Bmp signaling to the endoderm is crucial for proper endoderm morphogenesis and subsequent craniofacial development. We observed active Bmp signaling in pre-pouch endoderm, and loss of Bmp signaling results in disrupted pouch morphogenesis. Using genetic chimeras we show that the endoderm requires the reception of Bmp signaling for morphogenesis. We find that Bmp and Fgf signaling synergistically interact, regulating proper pouch morphogenesis. Collectively, our results provide key insights into the genetic pathways regulating endoderm morphogenesis.
RESULTS
Bmp signaling to the endoderm versus the CNCCs is temporally distinct
Bmp signaling regulates the development of many tissues, including the craniofacial skeleton. Work in mice and zebrafish has shown that Bmp signaling helps establish dorsal/ventral patterning of CNCCs (Medeiros and Crump, 2012). Using a
Received 3 August 2015; Accepted 12 April 2016
Molecular Biosciences, University of Texas at Austin, Austin, TX 78712, USA.
*Author for correspondence (benlovely@austin.utexas.edu)
C.B.L., 0000-0002-7838-5823
DEVEL
O
putative hypomorphic allele ofsmad5, we have shown that reduced Bmp signaling causes viscerocranial defects similar to those observed in some endoderm mutants (Swartz et al., 2011; Choe and Crump, 2014). Bmp signaling has been implicated in endoderm specification (Tiso et al., 2002), which is crucial for proper formation of the craniofacial skeleton (Balczerski et al., 2012; Couly et al., 2002; David et al., 2002). Data from a Bmp response element (BRE; derived from the X. laevis vent2 promoter) transgenic zebrafish line demonstrate that the endoderm receives Bmp signaling during pharyngeal arch development (Alexander et al., 2011). However, this work used a stabilized form of EGFP, confounding the dynamics of Bmp signaling.
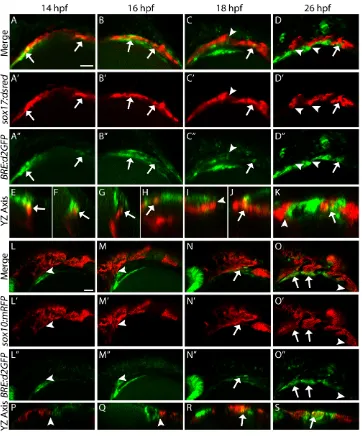
To characterize the temporal and spatial dynamics of Bmp signaling in the pharyngeal arches, we utilized a destabilized form of GFP driven by a BRE derived from the mouse Id1promoter, BRE:d2GFP (Collery and Link, 2011). Using double-transgenic embryos labeling the endoderm (sox17:dsRed) and Bmp-responsive cells (BRE:d2GFP) we found that the endoderm received canonical Bmp signaling as early as 14 hours post-fertilization (hpf) (Fig. 1A-A″, orthogonal views in Fig. 1E,F, arrows). This response persists to 26 hpf, but only in the most posterior pre-pouches (Fig. 1B-D″,
orthogonal views Fig. 1G-K, arrows). Once the pouch is fully formed, we no longer detect a BRE signal in these endodermal cells (Fig. 1C-D″, orthogonal views in Fig. 1I-K, arrowheads). This lack of a BRE signal in formed pouches is observed until at least 40 hpf (Fig. S1A-C, arrowheads). Consistent with this endodermal Bmp response, the Bmp ligands bmp2b and bmp4 are expressed in developing pharyngeal tissues. Between 12 and 16 hpf,bmp2bis expressed anteriorly and then expands posteriorly by 18 hpf, whereasbmp4is not expressed before 16 hpf (Fig. S2A-H).
[image:2.612.49.411.56.492.2]Conversely, CNCCs labeled with sox10:mRFPdo not show a BRE response before 18 hpf (Fig. 1L-M″, orthogonal views in Fig. 1P,Q, arrowheads). By 18 hpf, the first CNCCs migrating ventrally begin to exhibit a BRE response as they condense in the first pharyngeal arch (Fig. 1N-N″, orthogonal view in Fig. 1R, arrow). As subsequent arches form, the ventral-most CNCCs of each mature arch exhibit a strong BRE response (Fig. 1O-O″, orthogonal view in Fig. 1S, arrows). The posterior-most pre-arch CNCC condensation lacks a BRE response (Fig. 1O-O″, arrowhead). The BRE response persists in mature CNCCs to at least 40 hpf (Fig. S1D-F, arrows). These results demonstrate that the time window when the endoderm receives Bmp signaling is earlier Fig. 1. The reception of Bmp signaling is temporally distinct between the endoderm and CNCCs.(A-K) Confocal images of endoderm (sox17:dsred) and Bmp-responsive cells (BRE:d2GFP) at the indicated stages of zebrafish development. (E-K) Orthogonal (yz-axis) views of A (E,F), B (G,H), C (I,J) and D (K). (L-S) Confocal images of CNCCs (sox10:mRFP) and Bmp-responsive cells (BRE:d2GFP). (P-S) Orthogonal views of L-O. Arrows indicate overlap between transgene expression; arrowheads denote lack of overlap. The endoderm is Bmp responsive by 14 hpf (A-A″) and this overlap persists to 26 hpf (B-D″), but only in the most posterior cells. The ventral CNCCs do not become Bmp responsive until after 18 hpf (L-S). (A-D″,L-O″) Lateral views, anterior to the left. Scale bars: 50 µm.
DEVEL
O
than for the CNCCs. Additionally, this ‘endoderm first, CNCC second’response occurs in an anterior-posterior pattern suggesting that it is a dynamic process that occurs as pharyngeal arch morphogenesis proceeds.
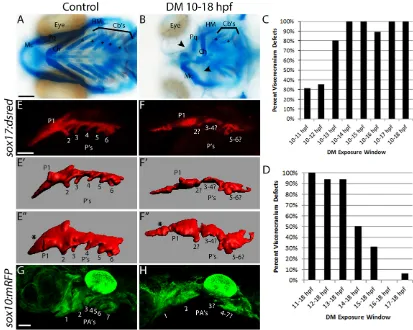
Early loss of Bmp signaling results in viscerocranial defects To precisely block Bmp signaling during defined time intervals, we utilized the small chemical inhibitor Dorsomorphin, which inhibits Bmp type I receptor phosphorylation of Smad1/5/8 (Yu et al., 2008). Our dose-response curve showed that 10 µM is the lowest effective dose to disrupt craniofacial development (data not shown). To determine the role of the Bmp pathway in craniofacial development, we blocked Bmp signaling at times when the endoderm is Bmp responsive, from 10-18 hpf. Blocking Bmp signaling from 10-18 hpf greatly reduces the BRE response at 18 hpf (Fig. S3A-C, compared with Fig. 1C-C″) resulting in severe, highly variable defects to the viscerocranium (Fig. 2B). These defects ranged from skeletal elements that were smaller and misshapen (Fig. 2B, see palatoquadrate), to the complete loss of pharyngeal cartilage structures, with Meckel’s and ceratohyal (Fig. 2B, arrowheads) and ceratobranchial (Fig. 2B, asterisks) cartilages being most sensitive. The number of ceratobranchials per side of the embryo decreased from five in untreated embryos to
fewer than two in Dorsomorphin-treated embryos (Table 1). Similar defects were observed inhs:dnBmpr1a-GFPembryos heat shocked at 10 hpf (Fig. S4B).
Because the BRE response is dynamic within the pharyngeal arches, we next determined if there were critical time points within this 10-18 hpf time window. The overall percentage of cartilage defects increased with the duration of Dorsomorphin exposure from 10 hpf, plateauing with a treatment window of 10-14 hpf (Fig. 2C, Table 1). Consistent with this finding, Dorsomorphin treatments that initiated at or following 14 hpf had substantially less impact on craniofacial development (Fig. 2D, Table 1). Hereafter, we use the term Dorsomorphin-treated embryos to represent embryos treated with Dorsomorphin from 10-18 hpf. Overall, this suggests that Bmp signaling from 10-18 hpf, when the endoderm is Bmp responsive, is crucial for normal craniofacial development.
[image:3.612.97.510.58.388.2]Proper endoderm morphology requires Bmp signaling Mutants with disrupted endoderm morphogenesis exhibit defects to the viscerocranium (Balczerski et al., 2012; Choe et al., 2013; Choe and Crump, 2014) that are reminiscent of the phenotypes in Dorsomorphin-treated embryos. To determine if Bmp signaling mediates endoderm development, we treated sox17:dsRed Fig. 2. Blocking Bmp signaling early disrupts endoderm morphology and craniofacial development.(A,B) Whole-mount images of viscerocranium at 5 days post-fertilization (dpf). Cartilage is blue and bone is red. Arrowheads point to missing cartilage elements and asterisks label the ceratobranchial cartilages. (A) No craniofacial defects are present following DMSO treatment. (B) Dorsomorphin (DM) treatment from 10-18 hpf causes severe craniofacial defects. Ventral views, anterior to the left. Cartilages: Mc, Meckel’s; Ch, ceratohyal; Cb’s, ceratobranchial; Pq, palatoquadrate; HM, hyomandibular. (C,D) The percentage of viscerocranial defects in embryos treated with Dorsomorphin over multiple time windows.nvalues for all time points are listed in Table 1. (E,F) Confocal images of control and Dorsomorphin-treatedsox17:DsRedembryos. (E′-F″) Imaris surface renderings of E,F. E″,F″are rotated 45° into the plane of view. (G,H) Confocal images of control or Dorsomorphin-treatedsox10:mRFPembryos.Compared with controls (E-E″,G), Dorsomorphin-treated embryos (F-F″,H) had severe defects to both endoderm and CNCC morphology. Lateral views (except for E″,F″, see above), anterior to the left. P, pouch; PA, pharyngeal arch. Scale bars: 100 µm in A; 50 µm in E,G.
DEVEL
O
transgenic embryos with Dorsomorphin and imaged the pharyngeal arches at 36 hpf. Consistent with the normal skeletal phenotypes in control embryos, the pouches are clearly formed in a segmented fashion, nearly perpendicular to midline endodermal tissue, with the third pouch just medial to the second pouch [Fig. 2E-E″ (E″ is rotated 45° into the plane of view)] (Swartz et al., 2012). In Dorsomorphin-treated embryos, the anterior-most endoderm (anterior to the first pouch) appears reduced and malformed (Fig. 2F-F″compared with E-E″, asterisks). The pouches that are
formed are hypoplastic and oriented parallel to midline endodermal tissue (Fig. 2F-F″compared with E-E″). Although pouches 1 and 2 are evident, the identity of pouches 3-6 is hard to define as these are either fused or lost (Fig. 2F-F″).
The condensation of CNCCs requires endoderm, and thus these profound endoderm defects could be accompanied by disruptions to the CNCCs. CNCC condensations appear normal in control embryos (Fig. 2G). Consistent with the endoderm defects, blocking Bmp signaling results in severe defects to the distribution of CNCCs (Fig. 2H). This result is consistent with an endoderm requirement for CNCC survival (David et al., 2002; Johnson et al., 2011), which was confirmed by TUNEL staining (data not shown). Similar defects in endoderm and CNCC morphology were observed in hs:dnBmpr1a-GFPembryos heat shocked at 10 hpf (Fig. S4D-D″,F), demonstrating specificity of the effect. These results demonstrate that Bmp signaling is crucial for CNCC and endoderm morphogenesis and that blocking Bmp signaling from 10-18 hpf disrupts overall pharyngeal arch morphology.
The endoderm must receive Bmp signaling for proper development
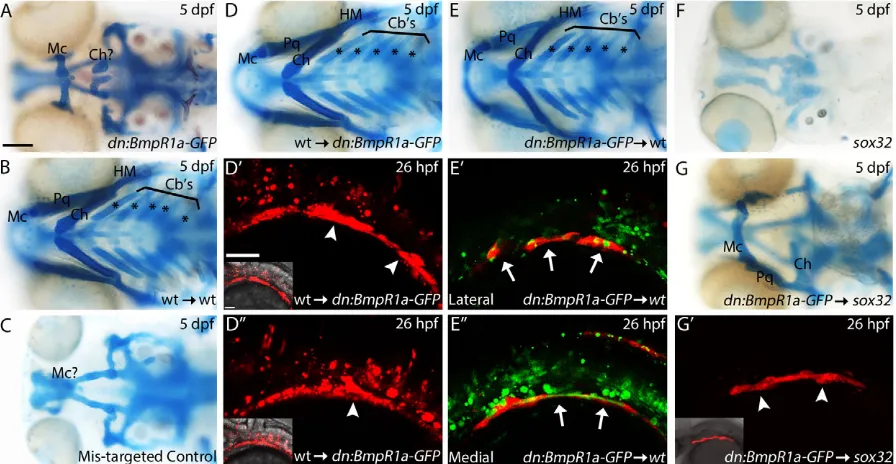
[image:4.612.48.299.79.251.2]The BRE transgenic expression suggests that Bmp signals directly to the endoderm. To test this hypothesis, we transplanted sox32 mRNA-injected wild-type cells into the margin of hs:dnBmpr1a-GFPzebrafish hosts at 4 hpf and heat shocked the chimeric embryos at 10 hpf. In these analyses, only the donor cells are able to respond to Bmp signaling following heat shock. To our surprise, craniofacial development was fully rescued in these chimeras (Fig. 3D-D″ compared with A,n=15/16). In these chimeras, wild-type endoderm Table 1. Effect of Dorsomorphin exposure window on zebrafish
craniofacial development
Window of DM exposure (hpf) n
Normal (%)
Viscerocranium defects (%)
Cb’s per side*
10-11 16 69 31 4.94
10-12 17 65 35 4.53
10-13 15 20 80 4.4
10-14 19 0 100 2.48
10-15 18 0 100 2.28
10-16 19 11 89 1.76
10-17 14 0 100 1.29
10-18 17 0 100 1.59
11-18 15 0 100 1.14
12-18 18 6 94 2.78
13-18 17 6 94 3.28
14-18 18 50 50 4.75
15-18 16 69 31 4.89
16-18 17 100 0 5
17-18 18 94 6 4.83
*The number of ceratobranchials per side of the embryo.
Fig. 3. Endoderm requires reception of Bmp signaling for proper development.(A-G) Whole-mount images (ventral view) of 5 dpf viscerocranium. Asterisks label the ceratobranchial cartilages. (D′-E″,G′) Confocal images (lateral view, anterior to the left) of 26 hpf genetic chimeras labeling donor cells with either Alexa 568 dextran (D′,D″), Alexa 488 dextran (E′,E″) or thesox17:dsredtransgene (G′). Arrowheads indicate endodermal pouch contribution, whereas arrows indicate endoderm failing to contribute to the pouches; insets show transgene expression with bright-field image. All embryos were heat shocked at 39°C for 1 h at 10 hpf. (A) Heat-shockedhs:dnBmpr1a-GFPembryos have severe craniofacial defects. (B) Control wild-type endoderm transplants do not disrupt craniofacial development. (D-D″) Transplanting wild-type endoderm into a heat-shockedhs:dnBmpr1a-GFPembryo completely rescues craniofacial development (n=15/16). (E-E″, arrows) Heat-shockedhs:dnBmpr1a-GFPdonor cells are excluded from the pouches and fail to induce viscerocranial defects in wild-type embryos. (C) No rescue occurs when donor cells fail to populate the endoderm (n=6/6). (F)sox32mutants lack endoderm and lose all of the viscerocranium. (G,G′) Heat-shocked
hs:dnBmpr1a-GFPendoderm does not rescuesox32mutants (n=6/6). Cartilages: Mc, Meckel’s; Ch, ceratohyal; Cb’s, ceratobranchial; Pq, palatoquadrate; HM,
hyomandibular. Scale bars: 100 µm in A-G; 50 µm in D′-E″,G′.
DEVEL
O
[image:4.612.83.531.402.634.2]readily contributed to the endodermal pouches on both sides of the embryo (Fig. 3D,D″, arrowheads). There was no rescue of craniofacial development in transplants where donor cells failed to populate the endoderm, instead populating mesoderm (Fig. 3C, n=6/6), demonstrating that this effect is not due to some other aspect of the manipulation. In reciprocal transplants, we failed to induce viscerocranial defects in wild-type embryos containing dnBmpr1a-GFP-expressing endoderm donor cells (Fig. 3E-E″,n=23/24). In addition, the dnBmpr1a-GFP-expressing endoderm donor cells were largely excluded from the endodermal pouches, instead populating the medial endoderm (Fig. 3E′,E″, arrows). These results suggest that a mechanism exists within the embryo that segregates non-Bmp-responsive cells away from the pouches in the mosaic embryos. Thus, the presence of wild-type, Bmp-responsive endodermal pouch cells is sufficient for proper endoderm and craniofacial development, although determining exactly how many wild-type cells are needed will require much more detailed genetic analyses.
In order to determine the phenotypes of embryos lacking any wild-type endoderm, we transplantedhs:dnBmpr1a-GFPendoderm cells into sox32 mutant hosts. These mutants fail to generate endoderm and lack the viscerocranium, a defect that can be rescued by transplantation of endoderm (David et al., 2002). In these chimeras, any endoderm present will be fromhs:dnBmpr1a-GFP donors and will lack reception of Bmp signaling. Even with extensive donor contributions in host embryos (n=6/6), hs: dnBmpr1a-GFP endoderm does not rescue craniofacial development (Fig. 3F-G′,n=5/6 with any degree of viscerocranial cartilage formation,n=1/6 failed to generate the viscerocranium). Viscerocranial structures that did form show the same malformation as observed in hs:dnBmpr1a-GFP and Dorsomorphin-treated embryos. The embryo displaying the most extensive cartilage is presented in Fig. 3G, as compared with Fig. 3A and Fig. 2B. All embryos failed to form ceratobranchials (n=6/6), with three embryos forming part or all of the Meckel’s, palatoquadrate and ceratohyal cartilages. Virtually no viscerocranial structures were formed in three embryos, whereas one embryo formed a single Meckel’s cartilage element (data not shown). This suggests that the endoderm requires Bmp signaling autonomously for proper endoderm morphogenesis and subsequent craniofacial development.
Bmp signaling is necessary for proper endoderm morphogenesis
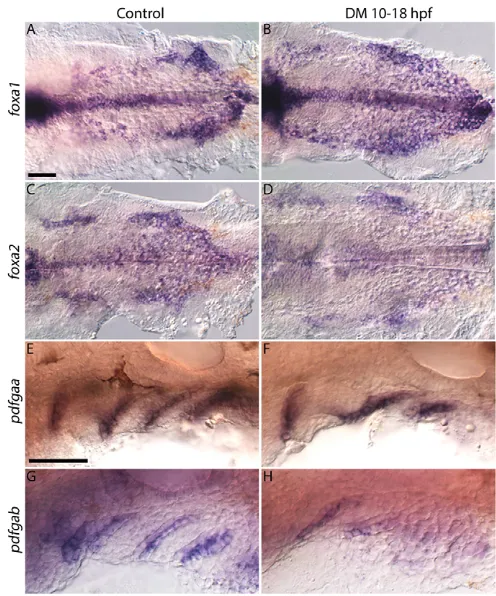
These defects in endoderm morphology could be due to defects in endoderm generation, proliferation/survival or morphogenesis. Bmp signaling is an important regulator of endoderm specification, acting to restrict expression of endoderm initiation factors, in particular sox17 (Poulain et al., 2006). Therefore, expression of thesox17:dsRedtransgene suggests that endoderm is properly specified in Dorsomorphin-treated embryos. Furthermore, we observed no difference in GFP fluorescence in theher5:EGFP transgenic fish line (data not shown). Dorsomorphin-treated embryos express early markers of endoderm, foxa1 and foxa2 (Alexander et al., 1999), although their expression domains are altered (Fig. 4A-D). Thus, reduced Bmp signaling does not disrupt early genesis of the endoderm.
The disruption of pouch morphology could be due to a loss of pouch identity. Platelet-derived growth factor ligands pdgfaa and pdgfablabel pouches by 30 hpf (Fig. 4E,G) (Eberhart et al., 2008). Both ligands are expressed in Dorsomorphin-treated embryos (Fig. 4F,H), althoughpdgfabexpression may be reduced. For both
ligands, in Dorsomorphin-treated embryos, pouch morphology is disrupted, consistent with the endoderm morphology observed in the sox17:dsRedtransgenic (Fig. 2F-F″). Collectively, these results show that markers of pouch identity are expressed, but pouch morphology is disrupted when Bmp signaling is blocked from 10-18 hpf.
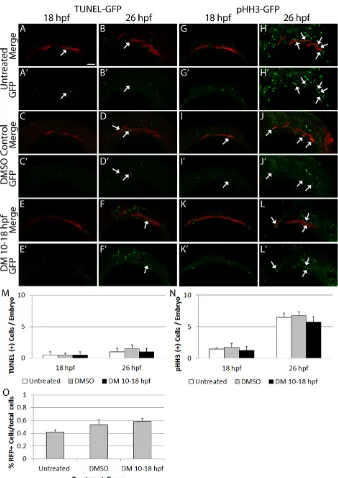
[image:5.612.312.560.56.354.2]Elevated cell death or reduced cell proliferation could lead to the morphological defects of the endoderm. Using a TUNEL assay in sox17:dsRedtransgenics, we found no increase in the number of apoptotic endoderm cells in Dorsomorphin-treated embryos compared with untreated or DMSO-treated control embryos at either 18 hpf (one-way ANOVA, f=0, P=1) or 26 hpf (one-way ANOVA, f=0.3, P=0.6) (Fig. 5A-F′,M, n=4 embryos). We did detect an increase in cell death in non-sox17-labeled tissue, which was likely to be in CNCCs as previously described (David et al., 2002). Similarly, we observed no change in proliferating phospho-histone H3-positive cells at either 18 hpf (one-way ANOVA,f=0.2, P=0.8) or 26 hpf (one-way ANOVA,f=0.1,P=0.8) (Fig. 5G-L′,N, n=4 embryos). We used FACS to count the total number ofsox17: dsRed-labeled cells to further identify any changes in endoderm cell number. Dorsomorphin treatment did not result in a significant change in the number ofsox17:dsRed-labeled cells when compared with untreated and DMSO-treated embryos (Fig. 5O, one-way ANOVA, f=1.8, P=0.2, n=8 samples, 10 embryos per sample). These data suggest that Bmp signaling between 10 and 26 hpf does not play a major role in the production or maintenance of endoderm cells. Overall, this implies that the main role of Bmp signaling to the endoderm is for proper morphogenesis.
Fig. 4. Endoderm specification is maintained in Dorsomorphin-treated embryos.Images of 26 hpf control or Dorsomorphin-treated embryos. (A,B)foxa1, (C,D)foxa2, (E,F)pdgfaaand (G,H)pdgfabare all expressed in Dorsomorphin-treated embryos.foxa1expression is expanded, whereasfoxa2
andpdgfabexpression is reduced, andpdgfaaexpression is normal. Pouch morphology is disrupted in all Dorsomorphin-treated embryos. (A-D) ventral views, (E-H) lateral views, anterior to the left. Scale bars: 50 µm.
DEVEL
O
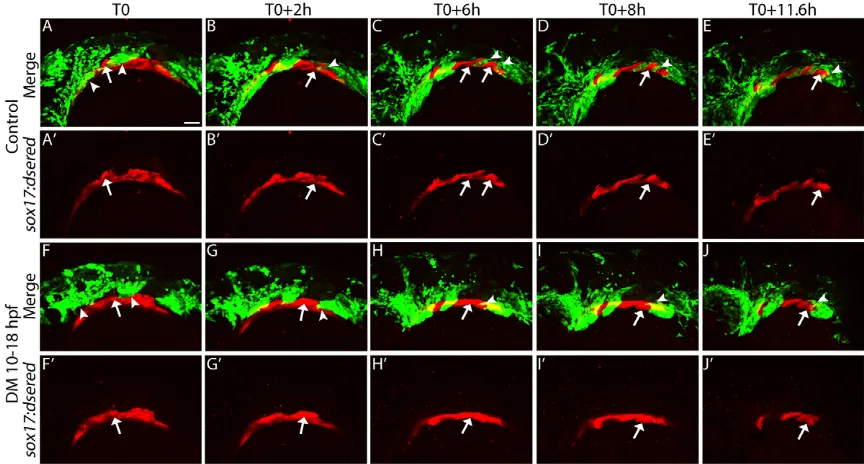
Endodermal morphogenesis occurs in a segmented fashion, with each new pouch forming posterior to the previous pouch every∼4 hours (Choe et al., 2013; Choe and Crump, 2014). To directly analyze pouch morphogenesis we used 4D confocal analysis (McGurk et al., 2014) in sox10:EGFP; sox17:dsRed double-transgenic embryos. We began the time-lapse analysis at 18 hpf, after Dorsomorphin washout and before morphogenesis of the pouches most sensitive to loss of Bmp signaling. At the initiation of the movie (T0), the first pouch is hypoplastic in the Dorsomorphin-treated embryo compared with the control embryo (Fig. 6F,F′ compared with A,A′, arrows; Movies 1-4). In addition, the CNCC condensations in the first and second pharyngeal arches appear malformed (Fig. 6F,F′compared with A,A′, arrowheads). At T0 +2h, the second pouch begins to form in the control embryo (Fig. 6B,B′, arrow); concomitantly, the third arch forms (Fig. 6B,B′, arrowhead) segmenting from the posterior pre-arch condensation. In the Dorsomorphin-treated embryo both processes fail [Fig. 6G,G′,
arrow (endoderm), arrowhead (CNCC)]. By T0+6h, the third pouch and fourth arch begin to form in the control embryo, but not in the Dorsomorphin-treated embryo [Fig. 6C,C′,H,H′, arrow (endoderm), arrowhead (CNCC)]. The segmentation of the pharyngeal structures continues to occur at a regular interval of every∼4 h in the control embryo (Fig. 6D-E′) (Choe et al., 2013; Choe and Crump, 2014). In the Dorsomorphin-treated embryo, pouches 2-6 and arches 3-7 fail to properly segment [Fig. 6I-J′, arrow (endoderm), arrowhead (CNCC)]. Collectively, these results demonstrate that Bmp signaling to the endoderm is required for proper morphogenesis of the pouches and subsequent craniofacial skeleton.
[image:6.612.50.388.55.533.2]Fgf-dependent pouch segmentation requires Bmp signaling Similar to Bmp signaling, Fgf signaling to the endoderm is necessary for pouch outgrowth (Choe and Crump, 2014; Crump et al., 2004). To determine if Bmp signaling is needed for this Fig. 5. Blocking Bmp signaling does not alter endodermal cell number.(A-F′,M) Confocal images ofsox17:dsredtransgenic embryos labeled for cell death at 18 and 26 hpf. Similar, low levels of apoptosis are present across all groups (18 hpf,f=0,P=1; 26 hpf,f=0.3,P=0.6, one-way ANOVA,n=4 embryos). (G-L′,N) Confocal images ofsox17:dsredtransgenic embryos labeled for cell proliferation at 18 and 26 hpf. No differences in the level of proliferating endoderm cells are apparent (18 hpf,f=0.2,P=0.8; 26 hpf,f=0.1,P=0.8, one-way ANOVA,n=4 embryos). Arrows indicate overlap between the transgene and marker expression. Lateral views, anterior to the left. (O) Total endoderm cell numbers were counted via FACS and no statistically significant differences between 26 hpf Dorsomorphin-treated, untreated or DMSO-treated embryos were observed (f=1.8,
P=0.2, one-way ANOVA,n=8 samples, 10 embryos per sample). Error bars indicate mean+ s.e.m. Scale bar: 50 µm.
DEVEL
O
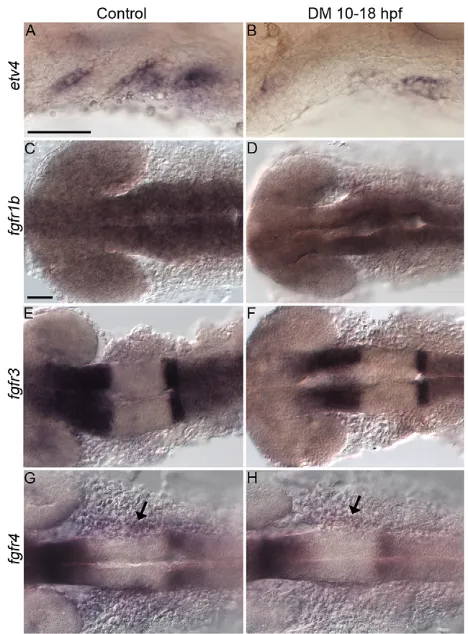
Fgf response, we analyzed the expression of the Fgf signaling target etv4. Dorsomorphin treatment greatly reduced the overall expression of etv4 (Fig. 7A,B). Similar results were obtained in heat-shocked dnBmpr1a-GFP embryos (Fig. S5A,B), ruling out off-target effects of Dorsomorphin on the Fgf pathway. The autonomous requirement of the endoderm for Bmp signaling suggests that it regulates the reception of Fgf signaling. There are five Fgf receptors in zebrafish andfgfr1aandfgfr2are not expressed before 24 hpf (Larbuisson et al., 2013). The expression offgfr1b, fgfr3andfgfr4has not been examined. Onlyfgfr4is expressed in the pharyngeal tissues and Dorsomorphin treatment reduced its expression (Fig. 7C-H, arrows). Expression of fgfr4 is also reduced in sox32 mutants (Fig. S5C,D), suggesting that the endoderm is required forfgfr4expression. In addition,fgfr4is not expressed in pharyngeal tissues before 18 hpf, which is later than expression of the Bmp ligands is observed (Fig. S2I-L). This suggests thatfgfr4is a target of Bmp signaling in the endoderm.
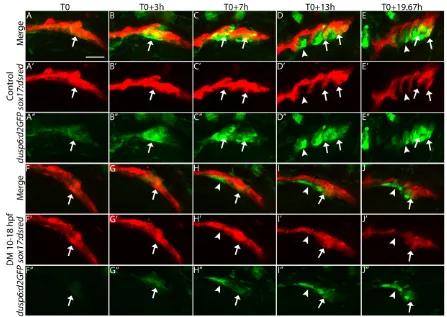
To determine if Bmp signaling modulates endodermal Fgf signaling kinetics, we used 4D confocal analysis ondusp6:d2GFP transgenics, where a destabilized form of GFP is driven by the promoter of the Fgf target genedusp6 (Molina et al., 2007). We created dusp6:d2GFP; sox17:dsRed double transgenics to characterize the endodermal Fgf response beginning at 18 hpf as described above. At initiation of the movie (T0), the first pouch has formed and the Fgf response is low in this mature pouch in the control embryo (Fig. S6A,A′, Movies 5-7). The presumptive pre-pouch endoderm, posterior to the first pre-pouch, which will form pouches 2-6, is actively responding to Fgf signaling (Fig. 8A-A″, arrows; magnified view of Fig. S6A-A″). At this time, the second pouch is beginning to form and segment from this presumptive pre-pouch endoderm (Fig. 8A-A″, arrows). At T0+3h, the second pouch has a strong Fgf response and is segmenting from the presumptive pre-pouch endoderm in the control embryo (Fig. 8B-B″, arrows). Simultaneously, the third pouch is beginning to segment from the presumptive pre-pouch endoderm and a new discrete Fgf response
domain is upregulated in this presumptive pouch. As the second pouch matures, the Fgf response is downregulated specifically in endodermal cells (Fig. 8C-C″). This pattern continues through the rest of the movie. As a new pouch begins to form from the presumptive pre-pouch endoderm, a strong Fgf response is upregulated in the forming pouch. Once the pouch matures, the Fgf response is downregulated specifically in the mature pouch (Fig. 8D-E″, arrows). Although the Fgf response is downregulated in mature pouch cells, the Fgf response is maintained in what are likely to be CNCCs (Fig. 8C-E″). Interestingly, only two forming pouches appear to be actively responding to Fgf signaling at any given time [Fig. 8C-E″, arrows (active response), arrowheads (reduced response)].
At T0 in the Dorsomorphin-treated embryo, the presumptive pre-pouch is still responding to Fgf signaling, but the response is substantially reduced (Fig. 8F-F″, arrows; Movies 8-10). At T0+3h, the Fgf response is still muted and the second pouch has yet to form (Fig. 8G-G″, arrows). In fact, at all subsequent time points the Fgf response is reduced and the pouches fail to segment properly (Fig. 8F-J″, arrows). Not until T0+19.67h do the most posterior pouches, presumably 5 and 6, start to segment. This segmentation is poorly orchestrated and the subsequent pouches are severely hypoplastic [Fig. 8J-J″, arrows (active response), arrowheads (reduced response)]. Expanded views (Fig. S6F-J′compared with S6A-E′) show that transgene expression is reduced in the pharyngeal arches, whereas other tissues, such as the ear (arrowheads) and brain, have similar expression between control and Dorsomorphin-treated embryos. Even prior to the initiation of the movies, at 14 hpf, we find that the endodermal Fgf response requires Bmp (data not shown). Our results suggest that Bmp signaling to the endoderm is required for establishing an endodermal Fgf signaling response.
[image:7.612.89.521.58.290.2]If alteration to the Fgf response causes craniofacial defects in Dorsomorphin-treated embryos then we might expect to observe synergistic interactions between the two pathways. To test if Bmp and Fgf signaling interact for proper craniofacial development, we Fig. 6. Bmp signaling regulates pouch morphogenesis.Still images from confocal time-lapse movies ofsox17:dsred; sox10:EGFPdouble-transgenic embryos. (A,A′,F,F′) 18 hpf embryos at the initiation of the movie (T0). (A-E′) Pouches (arrows) form in a sequential fashion to segregate the pharyngeal arches (arrowheads) in control embryos. (F-J′) In Dorsomorphin-treated embryos, pouch formation and arch segmentation fail. Lateral views, anterior to the left. Scale bar: 50 µm.
DEVEL
O
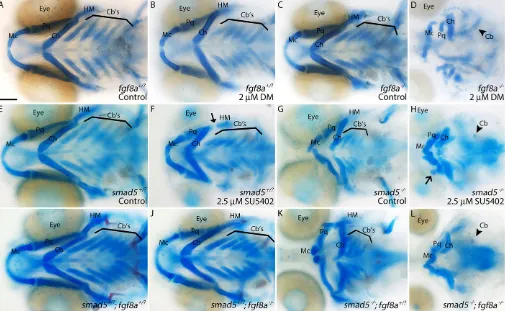
used a suboptimal dose of Dorsomorphin onfgf8amutant embryos. In our hands,fgf8amutants have virtually no viscerocranial defects (Fig. 9C). When treated with 2 µM Dorsomorphin, neither wild-type (WT) nor fgf8a heterozygous (Het) embryos exhibited any viscerocranial defects (Fig. 9B, n=34/34 WT, n=69/69 Het). Dorsomorphin-treated fgf8a mutants have variable viscerocranial defects (Fig. 9D,n=32/32). These defects are highly similar to those in 10 µM Dorsomorphin-treated wild-type embryos and heat-shockeddnBmpr1a-GFPembryos.
We replicated these results in reciprocal experiments using a suboptimal dose of the Fgf inhibitor SU5402 on our hypomorphic smad5mutants. Embryos lackingsmad5have shortened Meckel’s cartilages that fail to meet at the midline and fusions in the posterior arch cartilages, particularly between the ceratohyals and the first ceratobranchials (Fig. 9G) (Swartz et al., 2011). When treated with 2.5 µM SU5402 from 10-18 hpf, both wild-type and smad5 heterozygous embryos exhibited only minor defects to the hyomandibular cartilage (Fig. 9F, arrow; n=15/18 WT, n=29/31 Het). Mutants treated with SU5402 displayed profound viscerocranial defects. Both the Meckel’s and ceratohyal cartilages were reduced and had variable fusions to multiple cartilage elements (Fig. 9H, open arrow;n=17/19). Additionally, the number of ceratobranchials was reduced (Fig. 9H).
To confirm the interaction between the Bmp and Fgf pathways, we createdsmad5; fgf8a double mutants. Embryos containing at
least one wild-type copy ofsmad5have no viscerocranial defects (Fig. 9I,J n=68/68 and n=23/23, respectively). A single mutant allele offgf8a did not alter smad5 mutant phenotypes (Fig. 9K, n=10). However, smad5; fgf8a double mutants had profound viscerocranial defects that phenocopied the inhibitor analyses above (Fig. 9L, n=6). This demonstrates that Bmp and Fgf signaling strongly interact for proper craniofacial development.
DISCUSSION
Collectively, our results provide insight into the regulation of endodermal pouch morphogenesis and subsequent craniofacial development. We show that reception of Bmp signaling by the endoderm is necessary for proper segmentation of the pouches. Through inhibitor and genetic analyses, we demonstrate that Bmp and Fgf signaling synergistically interact in craniofacial development, and loss of Bmp signaling results in reduced Fgf signaling in the endoderm. Overall, we propose that Bmp signaling is required for the endoderm to properly respond to Fgf signaling for endoderm morphogenesis and subsequent craniofacial development.
Bmp signaling regulates endoderm and craniofacial development
The Bmp pathway plays key roles in morphogenesis and development of the endoderm and CNCCs (Alexander et al., 2011; Davis et al., 2004; Miura et al., 2010; Paca et al., 2012; Poulain et al., 2006; Tiso et al., 2002; Yamamoto et al., 2009; Zuniga et al., 2011). Although we have gained a better understanding of the role of Bmp in CNCC development, how Bmp signaling regulates endoderm development remains poorly understood. Previous work, using EGFP driven by a BRE, showed that both the endoderm and CNCCs are Bmp responsive during pharyngeal arch development (Alexander et al., 2011). Our approach utilizing a BRE driving a destabilized form of GFP (Collery and Link, 2011) demonstrated that the endoderm and CNCCs receive Bmp signaling during different time windows, with the endoderm being Bmp responsive prior to the CNCCs. This Bmp response is highly dynamic, occurring in the pre-pouch endoderm and being rapidly downregulated in mature pouches. Blocking Bmp signaling with Dorsomorphin during this endoderm Bmp-response window resulted in severe defects in both endoderm and craniofacial morphology. Our results demonstrate that Bmp signaling is necessary for endoderm and craniofacial development, prior to the role of Bmp signaling in CNCCs.
The endoderm must receive Bmp signaling for proper craniofacial development
The complete overlap in timing of the BRE response and Dorsomorphin sensitivity suggests that the endoderm is a direct target of Bmp signaling. Analysis of genetic chimeras confirmed that Bmp signals directly to the endoderm for proper endoderm and craniofacial development. Surprisingly, transplanting wild-type, Bmp-responsive endoderm cells intohs:dnBmpr1a-GFPembryos fully rescued craniofacial development. Consistent with the pre-pouch showing an active Bmp response, these wild-type cells efficiently populate the endodermal pouch, suggesting that, in the chimeras, cells able to respond to Bmp populate the pouches.
[image:8.612.56.290.56.373.2]Consistent with this model, reciprocal transplants did not result in craniofacial defects and thehs:dnBmpr1a-GFPcells were largely excluded from the pouch. By contrast,hs:dnBmpr1a-GFP donor cells transplanted into sox32 mutant hosts did cause craniofacial defects similar to those seen in both hs:dnBmpr1a-GFP and Dorsomorphin-treated embryos. This suggests that as long as a subpopulation of endoderm cells are Bmp responsive then normal Fig. 7. Fgf signaling activity requires Bmp.(A,B) Endodermal pouches
express the Fgf signaling targetetv4at 30 hpf in (A) control but not (B) Dorsomorphin-treated embryos. Lateral views, anterior to the left. (C-H) Expression of the Fgf receptorsfgfr1b,fgfr3andfgfr4at 18 hpf in control and Dorsomorphin-treated embryos. Onlyfgfr4is expressed in the pharyngeal tissues and is reduced in Dorsomorphin-treated embryos (G,H, arrows). (C-F) Dorsal views, (G,H) ventral views, anterior to the left. Scale bars: 50 µm.
DEVEL
O
craniofacial development will proceed, although determining the precise number and location of cells needed requires more detailed analyses. Recently, both Wnt/PCP and Eph/Ephrin signaling have been shown to play important roles in cellular interactions underlying pouch morphogenesis (Choe et al., 2013; Choe and Crump, 2015). It is possible that Bmp signaling might interact with one or both pathways in this process.
Previous work has shown that Bmp signaling is required for dorsal/ventral patterning of the pharyngeal arches (Zuniga et al., 2011). In these analyses, CNCC patterning within the arches, measured bymsxe(msx1a–Zebrafish Information Network),dlx3b andhand2expression, was restored by transplantation of wild-type CNCCs, although the subsequent craniofacial morphology was not examined. Our work shows that Bmp signaling to the endoderm is necessary for endoderm morphogenesis and subsequent skeletal development. Thus, a complex series of Bmp signaling events is necessary for proper craniofacial development.
Bmp signaling interacts with Fgf signaling for proper endoderm development
Bmp signaling has been shown to be important for multiple stages of endoderm development (Davis et al., 2004; Miura et al., 2010; Paca et al., 2012; Poulain et al., 2006; Tiso et al., 2002; Yamamoto et al., 2009). We have found profound defects in endoderm pouch morphogenesis when Bmp signaling is attenuated. In addition, we observed altered expression domains of early endoderm and pouch markers. These later defects were observed after endoderm morphogenesis had failed. These results are consistent with a
model in which Bmp signaling drives endoderm morphogenesis, yet we cannot rule out a primary defect in endoderm patterning or specification. In mouse, Bmp signaling plays a role in morphogenesis of the definitive endoderm and anterior visceral endoderm, although the mechanism is poorly understood (Davis et al., 2004). It will also be of interest to determine if the function of Bmp in pouch morphogenesis is conserved across vertebrates.
Endodermal pouches are crucial in CNCC morphogenesis, patterning and survival, and loss of the pouches results in severe defects to the craniofacial skeleton (Choe et al., 2013; Choe and Crump, 2014; Crump et al., 2004; Piotrowski and Nüsslein-Volhard, 2000; Swartz et al., 2012). As a result of Bmp signaling loss, pouch outpocketing fails. Pouch outpocketing relies on a multistep process, with Fgf signaling acting as a migratory cue during pouch outgrowth (Choe et al., 2013; Choe and Crump, 2014). The pouch defects we observed are reminiscent of phenotypes in embryos with the combined loss offgf3and fgf8a (Crump et al., 2004), consistent with the reduction of the Fgf response in the endoderm that we observe. The Bmp and Fgf signaling pathways are known to interact in multiple tissues during development, including the endoderm (Huang et al., 2009; Pajni-Underwood et al., 2007; Poulain et al., 2006; Yoon et al., 2006; Shin et al., 2007; Wilson and Tucker, 2004). Here, we show that Bmp and Fgf signaling interact during pouch morphogenesis.
[image:9.612.81.528.56.373.2]How does Bmp signaling regulate this Fgf-dependent pouch morphogenesis? Bmp could regulate the expression oftbx1since the Bmp antagonist chordin is a genetic modifier of Tbx1 in craniofacial malformations in mouse (Choi and Klingensmith, Fig. 8. Blocking Bmp reduces the Fgf signaling response in the forming pouch.Still images from confocal time-lapse movies ofsox17:dsred; dusp6:d2GFP
(Fgf-responsive cells) double-transgenic embryos. (A-A″,F-F″) Embryos at 18 hpf, the initiation of the movie (T0). Arrows indicate overlap between transgene expression, whereas arrowheads indicate lack of overlap. (F-J″) Dorsomorphin-treated embryos show a reduced Fgf signaling response in the endoderm and pouches fail to form, in contrast to controls (A-E″). Lateral views, anterior to the left. Scale bar: 50 µm.
DEVEL
O
2009). However, this is unlikely because pouch morphogenesis and Fgf ligand expression are dependent on mesodermally derivedtbx1 (Choe and Crump, 2014). In addition, thefgf3andfgf8aligands are expressed from the ectoderm and mesoderm, respectively (Crump et al., 2004). Given our finding that Bmp signals directly to the endoderm, it is unlikely that altering the expression of these ligands is involved in the endoderm defects we have characterized. Indeed, when we disrupt Bmp signaling, we did not see any change in expression offgf3orfgf8aduring pouch morphogenesis (data not shown). The endoderm also expressestbx1, but this is an unlikely target sincetbx1regulates endoderm proliferation, which appears unaltered in our work (Xu et al., 2005).
Bmp signaling may regulate the Fgf response directly and recent work has shown thatfgfr1aandfgfr2in the endoderm are important in craniofacial development (Larbuisson et al., 2013). However, neither receptor is expressed until at least 24 hpf, after our time window of Bmp dependence. The other Fgf receptors, namely fgfr1b,fgfr3andfgfr4, are potential targets of Bmp signaling and we showed that fgfr4 is expressed in the pharyngeal arches and is downregulated when Bmp signaling is blocked. This suggests that Bmp signaling regulates the Fgf response directly throughfgfr4.
It is also possible that Bmp signaling might regulate fgfr4 expression indirectly. Retinoic acid (RA) signaling from the
mesoderm is necessary for pouch formation (Graham et al., 2005). It is possible that Bmp signaling regulates the expression of RA receptors in the endoderm, which would then regulatefgfr4 expression (Song et al., 2004). Alternatively, Bmp signaling in the endoderm might regulate mesodermal RA expression. It is also possible that Bmp signaling to the endoderm might regulate CNCC gene targets that then regulate endodermal Fgf signaling (Tirosh-Finkel et al., 2010).
[image:10.612.55.560.60.371.2]Previous work in zebrafish has shown that the mesodermally expressed chemokines sdf1a and sdf1b (cxcl12a and cxcl12b – Zebrafish Information Network) regulate endodermal cell movements through the receptor cxcr4 (Mizoguchi et al., 2008). In the mouse digit, Bmp signaling upregulates Sdf1a expression (Lee et al., 2013). In zebrafish eye development, Bmp signaling represses expression of the chemokine receptorcxcr4a(Bielen and Houart, 2012). Thus, loss of Bmp signaling could result in an upregulation of chemokine signaling, which could reduce Fgf signaling, as chemokine signaling can negatively regulate Fgf signaling (Bouzaffour et al., 2009; Presta et al., 1998; Spinetti et al., 2001). In this scenario, chemokine signaling could disrupt endoderm morphogenesis by both acting as a guidance cue and inhibiting Fgf signaling. Future work would be required to address these possibilities.
Fig. 9. Bmp and Fgf signaling synergistically interact to achieve proper craniofacial development.Whole-mount images of 5 dpf viscerocranium. (A-C) No gross craniofacial defects are present in wild-type or heterozygous controls (A), wild-type embryos treated with suboptimal doses of Dorsomorphin (B) or untreatedfgf8amutants (C). (D) Treatingfgf8amutants with suboptimal doses of Dorsomorphin causes severe craniofacial defects. (E,F) Similarly, wild-type or
smad5heterozygous embryos do not display any craniofacial defects (E), whereas these embryos treated with suboptimal doses of SU5402 (Pan-Fgf inhibitor, 10-18 hpf ) display minor hyomandibular defects (F, arrow). (G) Untreatedsmad5mutants have characterized craniofacial defects (Swartz et al., 2011). (H) Suboptimal SU5402-treatedsmad5mutants display severe craniofacial defects, including loss of ceratobranchial cartilages (arrowhead). (I-L) Severe craniofacial defects mirroring those of SU5402-treatedsmad5mutants (see H) are present insmad5; fgf8acompound mutants (L) and not any other allelic combination (I-K). Ventral views, anterior to the left. Cartilages: Mc, Meckel’s; Ch, ceratohyal; Cb’s, ceratobranchial; Pq, palatoquadrate; HM, hyomandibular. Scale bar: 100 µm.
DEVEL
O
In conclusion, we have shown that Bmp signals directly to the endoderm for proper morphogenesis of the pouches and subsequent craniofacial development. Our work demonstrates that Bmp signaling regulates pouch development through interactions with the Fgf pathway. This provides a foundation for future examinations of endoderm development and its role in craniofacial development, as well as the role of Bmp signaling in these processes.
MATERIALS AND METHODS Zebrafish (Danio rerio) care and use
Zebrafish embryos were raised and cared for using established protocols (Westerfield, 1993) with IACUC approval at the University of Texas at Austin. Adult fish were maintained at 28.5°C with a 14/10 h light/dark cycle. Embryos were morphologically staged according to Kimmel et al. (1995). All mutant and transgenic alleles have been described previously (Table S1). Heat shocks were performed at 39°C for 1 h starting at 10 hpf. Embryos were treated with either 2 or 10 µM Dorsomorphin (Tocris) (Yu et al., 2008) or 2.5 µM SU5402 (Tocris) (Reifers et al., 2000) dissolved in 100% DMSO during the windows indicated in Fig. 2.
Tissue and embryo labeling
Embryos were stained with Alcian Blue for cartilage and Alizarin Red for bone (Walker and Kimmel, 2007). Whole-mount TUNEL staining of embryos was modified from Yabu et al. (2001) using the TUNEL assay (Roche). Dechorinated embryos were fixed overnight in 4% paraformaldehyde in PBS at 4°C. Samples were washed twice in 1 ml PBS with 0.25% Tween 20 (PBT) and then dehydrated in methanol. Samples were rehydrated in PBT and treated with 1 ml proteinase K (10 µg/ml in PBT) at room temperature for 1 min. Samples were washed four times with PBT for 5 min each and incubated in 50 µl of 1:10 Enzyme +Fluorescein Label solution (Roche) in the dark at 37°C for 2 h. The reaction was stopped by washing the samples twice in 1 ml PBT. Antibody labeling was performed as reported (McCarthy et al., 2013). Primary antibodies were diluted 1/200: anti-active Caspase 3 (G748A, Promega); Living Colors DsRed polyclonal antibody (632496, Clontech); monoclonal anti-phospho-Histone H3 antibody (H6409, Sigma-Aldrich). Secondary antibodies were diluted 1/500: Alexa Fluor 488, 568 anti-rabbit IgG (A10042, Invitrogen).
RNA injection andin situhybridization
We injected one-cell stage embryos with ∼3 nl sox32 mRNA made using the mMESSAGE mMACHINE Kit (Ambion). Stock solutions (1000 µg/ml) were diluted 1:5 with water and Alexa 568 dextran (Invitrogen). Genetic chimeras were generated at 4 hpf as previously described (Eberhart et al., 2008). RNAin situhybridization was performed as previously described (Miller et al., 2000). Table S2 lists all probes used.
Image capture and processing
Confocalz-stacks and movies were collected on a Zeiss LSM 710. Surface renderings were created using Imaris (Bitplane). Bright-field images of cartilage stains and DIC images of RNA in situ hybridizations were collected on a Zeiss Axioimager. Images were processed in Photoshop Elements 11 (Adobe).
Flow cytometry analysis
Embryos (ten per sample) were dissociated in 1 mg/ml collagenase D (Roche) in Hank’s Balanced Salt Solution (HBSS; Thermo Scientific). Dissociated cells were filtered through a 40 µm mesh filter. 50,000 events per sample were assayed on an LSRII Fortessa FCM (BD Biosciences) and the data were analyzed using FlowJo software (Tree Star). Statistical analyses were performed using a one-way ANOVA with a Tukey’s multiple comparisons test in Prism 5.02 (Graphpad).
Acknowledgements
We thank Dr Steve Vokes for allowing his graduate student, Jacqueline Norrie, to perform FACS analysis for this manuscript; and Dr Brian Link for providing theBRE: d2GFPtransgenic zebrafish line.
Competing interests
The authors declare no competing or financial interests.
Author contributions
C.B.L. and J.K.E. conceived, wrote and edited the manuscript. C.B.L. performed all of the analysis of Bmp signaling in endoderm development. M.E.S. assisted with the creation of genetic chimeras and edited the manuscript. N.M. created and developed the Fgfrin situhybridizations and edited the manuscript.J.L.N. determined total endoderm cell number via FACS and edited the manuscript.
Funding
Funding to support this research was provided by the National Institutes of Health/ National Institute of Dental and Craniofacial Research (NIH/NIDCR) [R01DE020884 to J.K.E.] and NIH/National Institute on Alcohol Abuse and Alcoholism (NIAAA) [U24AA014881 to J.K.E.; F32AA021320 and K99AA023560 to C.B.L.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at
http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.129379/-/DC1
References
Alexander, J., Rothenberg, M., Henry, G. L. and Stainier, D. Y. R.(1999). casanovaplays an early and essential role in endoderm formation in zebrafish. Dev. Biol.215, 343-357.
Alexander, C., Zuniga, E., Blitz, I. L., Wada, N., Le Pabic, P., Javidan, Y., Zhang, T., Cho, K. W., Crump, J. G. and Schilling, T. F.(2011). Combinatorial roles for BMPs and Endothelin 1 in patterning the dorsal-ventral axis of the craniofacial skeleton.Development138, 5135-5146.
Balczerski, B., Matsutani, M., Castillo, P., Osborne, N., Stainier, D. Y. R. and Crump, J. G.(2012). Analysis of sphingosine-1-phosphate signaling mutants reveals endodermal requirements for the growth but not dorsoventral patterning of jaw skeletal precursors.Dev. Biol.362, 230-241.
Bielen, H. and Houart, C.(2012). BMP signaling protects telencephalic fate by repressing eye identity and its Cxcr4-dependent morphogenesis.Dev. Cell23, 812-822.
Bouzaffour, M., Dufourcq, P., Lecaudey, V., Haas, P. and Vriz, S.(2009). Fgf and Sdf-1 pathways interact during zebrafish fin regeneration.PLoS ONE4, e5824.
Chai, Y. and Maxson, R. E. Jr. (2006). Recent advances in craniofacial morphogenesis.Dev. Dyn.235, 2353-2375.
Choe, C. P. and Crump, J. G. (2014). Tbx1 controls the morphogenesis of pharyngeal pouch epithelia through mesodermal Wnt11r and Fgf8a. Development141, 3583-3593.
Choe, C. P. and Crump, J. G.(2015). Eph-Pak2a signaling regulates branching of the pharyngeal endoderm by inhibiting late-stage epithelial dynamics. Development142, 1089-1094.
Choe, C. P., Collazo, A., Trinh, L. A., Pan, L., Moens, C. B. and Crump, J. G.
(2013). Wnt-dependent epithelial transitions drive pharyngeal pouch formation. Dev. Cell24, 296-309.
Choi, M. and Klingensmith, J.(2009). Chordin is a modifier of tbx1 for the craniofacial malformations of 22q11 deletion syndrome phenotypes in mouse. PLoS Genet.5, e1000395.
Chung, W.-S. Stainier, D. Y. R.(2008). Intra-endodermal interactions are required for pancreatic beta cell induction.Dev. Cell14, 582-593.
Cobourne, M. T. and Sharpe, P. T.(2003). Tooth and jaw: molecular mechanisms of patterning in the first branchial arch.Arch. Oral Biol.48, 1-14.
Collery, R. F. and Link, B. A.(2011). Dynamic smad-mediated BMP signaling revealed through transgenic zebrafish.Dev. Dyn.240, 712-722.
Couly, G., Creuzet, S., Bennaceur, S., Vincent, C. and Le Dourain, N.(2002). Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head.Development 129, 1061-1073.
Crump, J. G., Maves, L., Lawson, N. D., Weinstein, B. M. and Kimmel, C. B.
(2004). An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning.Development131, 5703-5716.
David, N. B., Saint-Etienne, L., Tsang, M., Schilling, T. F. and Rosa, F. M.(2002). Requirement for endoderm and FGF3 in ventral head skeleton formation. Development129, 4457-4468.
Davis, S., Miura, S., Hill, C., Mishina, Y. and Klingensmith, J.(2004). BMP receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning.Dev. Biol.270, 47-63.
Eberhart, J. K., He, X., Swartz, M. E., Yan, Y.-L., Song, H., Boling, T. C., Kunerth, A. K., Walker, M. B., Kimmel, C. B. and Postlethwait, J. H.(2008). MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis.Nat. Genet.40, 290-298.
Fischer, S., Draper, B. W. and Neumann, C. J.(2003). The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation.Development130, 3515-3524.
DEVEL
O
Graham, A., Okabe, M. and Quinlan, R.(2005). The role of the endoderm in the development and evolution of the pharyngeal arches.J. Anat.207, 479-487.
Gross, J. B. and Hanken, J.(2008). Review of fate-mapping studies of osteogenic cranial neural crest in vertebrates.Dev. Biol.317, 389-400.
Haworth, K. E., Wilson, J. M., Grevellec, A., Cobourne, M. T., Healy, C., Helms, J. A., Sharpe, P. T. and Tucker, A. S.(2007). Sonic hedgehog in the pharyngeal endoderm controls arch pattern via regulation of Fgf8 in head ectoderm.Dev. Biol. 303, 244-258.
Huang, J., Dattilo, L. K., Rajagopal, R., Liu, Y., Kaartinen, V., Mishina, Y., Deng, C.-X., Umans, L., Zwijsen, A., Roberts, A. B. et al.(2009). FGF-regulated BMP signaling is required for eyelid closure and to specify conjunctival epithelial cell fate.Development136, 1741-1750.
Johnson, C. W., Hernandez-Lagunas, L., Feng, W., Melvin, V. S., Williams, T. and Artinger, K. B.(2011). Vgll2a is required for neural crest cell survival during zebrafish craniofacial development.Dev. Biol.357, 269-281.
Kikuchi, Y., Agathon, A., Alexander, J., Thisse, C., Waldron, S., Yelon, D., Thisse, B. and Stainier, D. Y. R.(2001).casanovaencodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish.Genes Dev.15, 1493-1505.
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. and Schilling, T. F.
(1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310.
Knight, R. D. and Schilling, T. F.(2006). Cranial neural crest and development of the head skeleton.Adv. Exp. Med. Biol.589, 120-133.
Kopinke, D., Sasine, J., Swift, J., Stephens, W. Z. and Piotrowski, T.(2006). Retinoic acid is required for endodermal pouch morphogenesis and not for pharyngeal endoderm specification.Dev. Dyn.235, 2695-2709.
Kucenas, S., Takada, N., Park, H.-C., Woodruff, E., Broadie, K. and Appel, B.
(2008). CNS-derived glia ensheath peripheral nerves and mediate motor root development.Nat. Neurosci.11, 143-151.
Larbuisson, A., Dalcq, J., Martial, J. A. and Muller, M.(2013). Fgf receptors Fgfr1a and Fgfr2 control the function of pharyngeal endoderm in late cranial cartilage development.Differentiation86, 192-206.
Lee, J., Marrero, L., Yu, L., Dawson, L. A., Muneoka, K. and Han, M.(2013). SDF-1α/CXCR4 signaling mediates digit tip regeneration promoted by BMP-2.Dev. Biol.382, 98-109.
McCarthy, N., Wetherill, L., Lovely, C. B., Swartz, M. E., Foroud, T. M. and Eberhart, J. K. (2013). Pdgfra protects against ethanol-induced craniofacial defects in a zebrafish model of FASD.Development140, 3254-3265.
McGurk, P. D., Lovely, C. B. and Eberhart, J. K.(2014). Analyzing craniofacial morphogenesis in zebrafish using 4D confocal microscopy. J. Vis. Exp. 83, e51190.
Medeiros, D. M. and Crump, J. G. (2012). New perspectives on pharyngeal dorsoventral patterning in development and evolution of the vertebrate jaw.Dev. Biol.371, 121-135.
Miller, C. T., Schilling, T. F., Lee, K., Parker, J. and Kimmel, C. B.(2000).sucker encodes a zebrafish Endothelin-1 required for ventral pharyngeal arch development.Development127, 3815-3828.
Miura, S., Singh, A. P. and Mishina, Y.(2010). Bmpr1a is required for proper migration of the AVE through regulation of Dkk1 expression in the pre-streak mouse embryo.Dev. Biol.341, 246-254.
Mizoguchi, T., Verkade, H., Heath, J. K., Kuroiwa, A. and Kikuchi, Y.(2008). Sdf1/Cxcr4 signaling controls the dorsal migration of endodermal cells during zebrafish gastrulation.Development135, 2521-2529.
Molina, G. A., Watkins, S. C. and Tsang, M.(2007). Generation of FGF reporter transgenic zebrafish and their utility in chemical screens.BMC Dev. Biol.7, 62.
Moore-Scott, B. A. and Manley, N. R.(2005). Differential expression of Sonic hedgehog along the anterior-posterior axis regulates patterning of pharyngeal pouch endoderm and pharyngeal endoderm-derived organs.Dev. Biol. 278, 323-335.
Niederreither, K., Vermot, J., Le Roux, I., Schuhbaur, B., Chambon, P. and Dollé, P.(2003). The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system.Development130, 2525-2534.
Paca, A., Séguin, C. A., Clements, M., Ryczko, M., Rossant, J., Rodriguez, T. A. and Kunath, T.(2012). BMP signaling induces visceral endoderm differentiation of XEN cells and parietal endoderm.Dev. Biol.361, 90-102.
Pajni-Underwood, S., Wilson, C. P., Elder, C., Mishina, Y. and Lewandoski, M.
(2007). BMP signals control limb bud interdigital programmed cell death by regulating FGF signaling.Development134, 2359-2368.
Piotrowski, T. and Nüsslein-Volhard, C.(2000). The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio).Dev. Biol.225, 339-356.
Poulain, M., Fürthauer, M., Thisse, B., Thisse, C. and Lepage, T.(2006). Zebrafish endoderm formation is regulated by combinatorial Nodal, FGF and BMP signalling.Development133, 2189-2200.
Presta, M., Belleri, M., Vecchi, A., Hesselgesser, J., Mantovani, A. and Horuk, R.
(1998). Noncompetitive, chemokine-mediated inhibition of basic fibroblast growth factor-induced endothelial cell proliferation.J. Biol. Chem.273, 7911-7919.
Pyati, U. J., Webb, A. E. and Kimelman, D.(2005). Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development.Development132, 2333-2343.
Reifers, F., Bohli, H., Walsh, E. C., Crossley, P. H., Stainier, D. Y. and Brand, M.
(1998). Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development125, 2381-2395.
Reifers, F., Walsh, E. C., Léger, S., Stainier, D. Y. and Brand, M.(2000). Induction and differentiation of the zebrafish heart requires fibroblast growth factor 8 (fgf8/ acerebellar).Development127, 225-235.
Shin, D., Shin, C. H., Tucker, J., Ober, E. A., Rentzsch, F., Poss, K. D., Hammerschmidt, M., Mullins, M. C. and Stainier, D. Y. R.(2007). Bmp and Fgf signaling are essential for liver specification in zebrafish.Development 134, 2041-2050.
Song, Y., Hui, J. N., Fu, K. K. and Richman, J. M.(2004). Control of retinoic acid synthesis and FGF expression in the nasal pit is required to pattern the craniofacial skeleton.Dev. Biol.276, 313-329.
Spinetti, G., Camarda, G., Bernardini, G., Romano Di Peppe, S., Capogrossi, M. C. and Napolitano, M.(2001). The chemokine CXCL13 (BCA-1) inhibits FGF-2 effects on endothelial cells.Biochem. Biophys. Res. Commun.289, 19-24.
Swartz, M. E., Sheehan-Rooney, K., Dixon, M. J. and Eberhart, J. K.(2011). Examination of a palatogenic gene program in zebrafish. Dev. Dyn. 240, 2204-2220.
Swartz, M. E., Nguyen, V., McCarthy, N. Q. and Eberhart, J. K.(2012). Hh signaling regulates patterning and morphogenesis of the pharyngeal arch-derived skeleton.Dev. Biol.369, 65-75.
Tirosh-Finkel, L., Zeisel, A., Brodt-Ivenshitz, M., Shamai, A., Yao, Z., Seger, R., Domany, E. and Tzahor, E.(2010). BMP-mediated inhibition of FGF signaling promotes cardiomyocyte differentiation of anterior heart field progenitors. Development137, 2989-3000.
Tiso, N., Filippi, A., Pauls, S., Bortolussi, M. and Argenton, F.(2002). BMP signalling regulates anteroposterior endoderm patterning in zebrafish.Mech. Dev. 118, 29-37.
Wada, N., Javidan, Y., Nelson, S., Carney, T. J., Kelsh, R. N. and Schilling, T. F.
(2005). Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull.Development 132, 3977-3988.
Walker, M. B. and Kimmel, C. B.(2007). A two-color acid-free cartilage and bone stain for zebrafish larvae.Biotech. Histochem.82, 23-28.
Westerfield, M.(1993).The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio). Eugene, OR: University of Oregon Press.
Wilson, J. and Tucker, A. S.(2004). Fgf and Bmp signals repress the expression of Bapx1 in the mandibular mesenchyme and control the position of the developing jaw joint.Dev. Biol.266, 138-150.
Xu, H., Cerrato, F. and Baldini, A.(2005). Timed mutation and cell-fate mapping reveal reiterated roles of Tbx1 during embryogenesis, and a crucial function during segmentation of the pharyngeal system via regulation of endoderm expansion. Development132, 4387-4395.
Yabu, T., Todoriki, S. and Yamashita, M.(2001). Stress-induced apoptosis by heat shock, UV andγ-ray irradiation in zebrafish embryos detected by increased caspase activity and whole-mount TUNEL staining.Fish. Sci.67, 333-340.
Yamamoto, M., Beppu, H., Takaoka, K., Meno, C., Li, E., Miyazono, K. and Hamada, H. (2009). Antagonism between Smad1 and Smad2 signaling determines the site of distal visceral endoderm formation in the mouse embryo. J. Cell Biol.184, 323-334.
Yelick, P. C. and Schilling, T. F.(2002). Molecular dissection of craniofacial development using zebrafish.Crit. Rev. Oral Biol. Med.13, 308-322.
Yoon, B. S., Pogue, R., Ovchinnikov, D. A., Yoshii, I., Mishina, Y., Behringer, R. R. and Lyons, K. M.(2006). BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways.Development133, 4667-4678.
Yu, P. B., Hong, C. C., Sachidanandan, C., Babitt, J. L., Deng, D. Y., Hoyng, S. A., Lin, H. Y., Bloch, K. D. and Peterson, R. T.(2008). Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism.Nat. Chem. Biol.4, 33-41.
Zuniga, E., Rippen, M., Alexander, C., Schilling, T. F. and Crump, J. G.(2011). Gremlin 2 regulates distinct roles of BMP and Endothelin 1 signaling in dorsoventral patterning of the facial skeleton.Development138, 5147-5156.