INTRODUCTION
The first sign of hair follicle development is the appearance of epidermal thickenings, placodes, that arise in a regularly spaced pattern. In mouse embryo, placodes destined to form fur hairs appear at embryonic day 14 (E14), while vibrissa placodes arise a few days earlier. According to the prevailing model of hair follicle development, the first inductive signal emanating from the mesenchyme instructs the epidermis to form the placodes. As a response to the first dermal message, the epithelium signals back to the mesenchyme to allow the mesenchymal cells to condensate. The placodal epithelial cells proliferate and invaginate into the dermal condensate as a response to the second dermal signal (Hardy, 1992). Reciprocal signaling between the mesenchymal and epithelial cell layers and the lateral communication between the follicular and interfollicular cells continue throughout hair morphogenesis. As a result, a hair follicle with its several different epidermal cell layers and the mesenchyme-derived dermal papilla develop (Millar, 2002).
The molecular mechanisms that underlie embryonic hair development are currently under intensive study. The most important molecular players in hair development thus far identified are associated with several conserved signaling pathways, including members from Wnt, fibroblast growth factor (FGF), hedgehog (Hh), bone morphogenetic protein (BMP), transforming growth factor  (TGF), ectodysplasin (Eda) and Notch families. Signaling molecules either promote hair follicle fate (e.g. Wnt, Eda and FGF signaling), or inhibit the interfollicular cells to adopt the placodal
fate (BMP signaling) (reviewed by Millar, 2002; Mikkola and Millar, 2006). Shh is required after hair placode initiation to promote the proliferation of follicular epithelial cells (Chiang et al., 1999).
Wnt pathway plays central roles during several stages of hair morphogenesis, including placode formation and mesenchymal condensation, hair follicle spacing and polarity, hair shaft differentiation, and hair cycling (Millar, 2002). Several Wnt ligands are expressed in the epithelial and mesenchymal compartments during embryonic and postnatal development (Reddy et al., 2001), and Wnt/-catenin signaling activity has been localized both in hair follicle epithelium and mesenchyme in transgenic reporter mice (DasGupta and Fuchs, 1999).
The central mediator of the canonical Wnt pathway, -catenin, regulates the transcription of Wnt pathway target genes in the nucleus. When the Wnt pathway becomes active through the Frizzled family of receptors, the cytoplasmic -catenin is able to escape the degradation machinery and accumulate in the nucleus, where it associates with Lef/Tcf transcription factor family members (reviewed by Clevers, 2006). A conditional ablation of  -catenin in mouse skin epithelium prevents hair placode formation in the embryo (Huelsken et al., 2001) and epidermal overexpression of the Wnt inhibitor Dkk1blocks hair placode formation indicating that Wnt/ -catenin signaling is essential for placode formation (Andl et al., 2002). Mice that lack Lef1function lack whisker follicles, as well as the majority of pelage hair follicles; the presence of some follicles is probably due to redundant functions of the Lef1/Tcf transcription factors (van Genderen et al., 1994; Kratochwil et al., 1996).
Forced expression of stabilized -catenin in embryonic ectoderm results in ectopic formation of feather buds in chick embryo (Noramly et al., 1999), while in mouse no embryonic hair defects were noticed in transgenic mice overexpressing a similar degradation-resistant -catenin (Gat et al., 1998). However, these mice exhibit de novo hair follicle formation during postnatal hair development, as well as hair tumors. Thus, current data suggest that in mammalian skin, -catenin is necessary but not sufficient for hair placode induction during embryogenesis.
Sustained epithelial

-catenin activity induces precocious
hair development but disrupts hair follicle down-growth and
hair shaft formation
Katja Närhi1, Elina Järvinen1, Walter Birchmeier2, Makoto M. Taketo3, Marja L. Mikkola1,* and Irma Thesleff1,*,†
During embryonic and postnatal development, Wnt/-catenin signaling is involved in several stages of hair morphogenesis from placode formation to hair shaft differentiation. Using a transgenic approach, we have investigated further the role of -catenin signaling in embryonic hair development. Forced epithelial stabilization of -catenin resulted in precocious and excessive induction of hair follicles even in the absence of Eda/Edar signaling, a pathway essential for primary hair placode formation. In addition, the spacing and size of the placodes was randomized. Surprisingly, the down-growth of follicles was suppressed and hair shaft production was severely impaired. Gene and reporter expression analyses revealed elevated mesenchymal Wnt activity, as well as increased BMP signaling, throughout the skin that was accompanied by upregulation of Sostdc1 (Wise, ectodin) expression. Our data suggest that BMPs are downstream of Wnt/-catenin and that their interplay may be a critical component in establishing correct patterning of hair follicles through the reaction-diffusion mechanism.
KEY WORDS: Hair, Placode, Follicle, -catenin, BMP, Lateral inhibition, Mouse
Development 135, 1019-1028 (2008) doi:10.1242/dev.016550
1Developmental Biology Program, Institute of Biotechnology, Viikki Biocenter, PO Box
56, University of Helsinki, FIN-00014, Helsinki, Finland. 2Max-Delbrück Center for
Molecular Medicine, Robert-Rössle-Strasse 10, 13092 Berlin, Germany. 3Department
of Pharmacology, Graduate School of Medicine, Kyoto University, Yoshida-Konoe-cho, Sakyo-ku, Kyoto 606-8501, Japan.
*These authors contributed equally to this work
†Author for correspondence (e-mail: irma.thesleff@helsinki.fi)
Accepted 7 January 2008
D
E
V
E
LO
P
M
E
N
We have re-examined the consequences of forced -catenin activation on embryonic development of hair and vibrissae by using a transgenic mouse line that carries a conditional construct of stabilized -catenin,and targeted it to skin epithelium by crossing the mice with a K14Cre line. By histological, molecular, in vitro and in vivo skin transplantation experiments, we show that the expression of stabilized -catenin results in accelerated and excessive formation of hair placodes in a random pattern. However, most of the developing mutant follicles eventually failed to produce hair. Sustained -catenin increased Wnt reporter activity both in epithelium and underlying mesenchyme, and induced the expression of several placodal genes. In particular, BMP signaling was stimulated and this was associated with upregulated expression of Bmp2 and Bmp4. Interestingly, Bmp4 expression was induced ectopically in placode epithelium and the BMP target gene Sostdc1 (ectodin, Wise) was highly upregulated in the flanking interfollicular epithelium. We propose that interplay between Wnts and BMPs is essential for the normal patterning of hair follicles. Finally, we also show that forced -catenin expression can compensate for the requirement of Eda signaling during the formation of primary hair placodes.
MATERIALS AND METHODS
Animals and preparation of embryonic tissues
Transgenic mouse lines used in this study have been described earlier:
Catnb⌬ex3K14/+, BAT-gal, immunodeficient HsdCpb:NMRI-Foxn1nu (nude),
B6;129S-Gt(ROSA)26Sor/J (Rosa 26) (Järvinen et al., 2006) and Eda -deficient mice (also referred to as tabbymice, Jackson Laboratories stock #JR0314) (Pispa et al., 1999). The Catnb⌬ex3K14/+mice express one
wild-type and one mutated allele of -catenin in the skin epithelium. Embryos were staged according to morphological criteria, plug day was embryonic day (E) 0. Whole embryos were fixed in 4% paraformaldehyde and processed either for whole-mount in situ hybridization or paraffin sections as previously described (Mustonen et al., 2004). Transversal paraffin sections of 7 m were processed for in situ hybridization or stained with Hematoxylin and Eosin for histological analysis.
Tissue culture
E12 NMRI and Catnb⌬ex3K14/+vibrissa pads were dissected in Dulbecco’s
PBS (pH 7.4) under a stereomicroscope. Vibrissal follicle explants were grown on nucleopore filters (pore size 0.1 m) at 37°C in a Trowell type culture containing DMEM and F12 medium (1:1) supplemented with 10% fetal calf serum (PAA laboratories, Pasching, Austria), ascorbic acid (0.075 g/l), glutamine and penicillin-streptomycin. The medium was changed every second day. The vibrissa explants were photographed after 3 and 5 days with light microscope (Olympus SZX9).
Skin transplantation
Dorsal skin of E17 Catnb⌬ex3K14/+and control embryos was dissected in PBS
(pH 7.4) Cuts were made in the back skin of anesthetized nude mice and the embryonic explants were transplanted by suturing. The mice were sacrificed after 3 or 5 weeks, and the grafted skin was dissected and photographed by light microscope (Olympus SZX9), fixed in 4% paraformaldehyde for 1 week and processed for embedding in paraffin wax. The sections (7 m) were stained by Hematoxylin and Eosin for histological analysis.
Immunohistochemistry
E14 Catnb⌬ex3K14/+and control embryos were fixed overnight with 4%
paraformaldehyde containing 1% phospho-protection solution (100 mM sodium orthovanadate, 117 mM sodium molybdate, 200 mM imidazole, 484 nM sodium tartrate) and processed to transversal frozen sections. The sections were incubated in methanol with 0.3% H2O2 before
anti-pSmad-1/5/8 antibodies (1:100, Cell Signaling Technology). Immunostaining was performed with the Vectastain Elite ABC Kit (Vector, Burlingame, CA). Anti-rabbit antibodies (Vector) were used as secondary antibodies and horse serum (Vector) was used in blocking solution containing 1% BSA.
In situ hybridization
Whole-mount in situ hybridization was performed by using the InSituPro robot (Intavis AG, Germany). BM Purple AP Substrate Precipitating Solution (Boehringer Mannheim Gmbh, Germany) was used to visualize the digoxigenin-labeled probes. Radioactive in situ hybridization for paraffin sections was carried out according to standard protocols using 35S-UTP
labeling (Amersham). The following probes were used: Sostdc1(Laurikkala et al., 2003), Bmp2(Pispa et al., 1999), Bmp4(Mustonen et al., 2004),
Wnt10b(Wang and Shackleford, 1996), Edarand Shh(Laurikkala et al., 2002), and Dkk1(Andl et al., 2002)
X-gal staining
Cre recombinase and Wnt activity were detected as described earlier (Järvinen et al., 2006). E11-E12.5 and E13.5-E14 embryos were fixed in 2% paraformaldehyde in 0.2% mM glutaraldehyde in PBS for 30 and 60 minutes, respectively, and stained with X-gal staining solution for 24 hours for Cre recombinase and 20 minutes for Wnt activity.
Quantitative RT-PCR
E13.75 and E14.5 dorsal skin, excluding the midline, was dissected from
Catnb⌬ex3K14/+and control mice. The explants were placed into 350 l lysis
buffer of the RNeasy mini kit (Qiagen) containing 1% -mercaptoethanol (Sigma). Total RNA was isolated as recommended by manufacturer and quantified using a nanodrop spectrophotometer. cDNA synthesis using 600 ng of total RNA and qPCR were performed as described earlier (James et al., 2006). Primer sequences of Bmp2, Bmp4, Bmp7, Dkk1, Edar, Shh, Sostdc1 and Ranbp1 are available upon request.
Scanning electron microscopy (SEM)
E14.5 Catnb⌬ex3K14/+ and control embryos were fixed in 2.5%
glutaraldehyde in 0.1 M phosphate buffer (pH 7.3) for 5 hours and processed for viewing with Zeiss DSM 962 scanning electron microscopy (Zeiss, Oberkochen, Germany) as described earlier (Mustonen et al., 2003).
RESULTS
Forced activation of epithelial -catenininduces precocious hair follicle formation
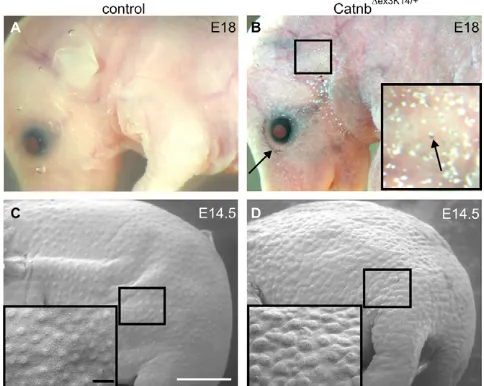
The Cre recombinase activity of K14Cre mice (Huelsken et al., 2001) was used to delete the exon 3 flanked by LoxP sites in  -catenin-flox-exon 3 mice (Catnb⌬ex3K14/+) (Harada et al., 1999). Crossing of these two transgenic mouse lines generated Catnb⌬ex3K14/+mice continuously expressing stabilized -catenin in the basal cells of skin epithelium. As the mutant mice die at birth (Järvinen et al., 2006) we started by analyzing E18 embryos. The examination of the Catnb⌬ex3K14/+mutant embryos under the light microscope uncovered a dramatic skin phenotype suggesting defects in skin and hair morphogenesis. Mutant embryos were covered by white deposits, as well as darkly pigmented areas (Fig. 1A,B), and in contrast to control embryos, were devoid of whiskers (data not shown).
We next examined the mutants at E14.5 when the first wave of hair placodes appears in wild-type embryos. These placodes are visualized as regularly patterned evaginations on the skin surface in control embryos (Fig. 1C). Scanning electron microscopy revealed that the mutant skin was covered by prominent and densely arranged irregular bump-like structures, indicating abnormalities in hair placode formation (Fig. 1D).
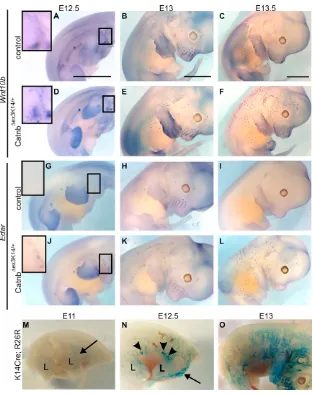
To examine when the first placodes develop in Catnb⌬ex3K14/+ skin, we performed whole-mount in situ hybridization for carefully staged E12.5-E13.5 -catenin mutant and control embryos using several placode markers (Fig. 2; data not shown). Wnt10band Edar are early placode markers that are initially evenly expressed in the wild-type skin epithelium before becoming restricted to the forming placodes (Headon and Overbeek, 1999; Reddy et al., 2001; Laurikkala et al., 2002). Edar encodes the receptor for Eda-A1 isoform of ectodysplasin and mediates signals which are required
D
E
V
E
LO
P
M
E
N
for the formation of the placodes of the first-wave hair follicles (Mikkola and Thesleff, 2003; Mustonen et al., 2004). In control skin, Wnt10band Edar expression was detected in mammary and whisker buds at E12.5 and E13 but not yet in pelage hair placodes (Fig. 2A,B,G,H). At E13.5, both placode markers were localized to hair preplacodes in control embryos (Fig. 2C,I). Wnt10band Edarwere expressed in the developing mammary glands of E12.5 mutants in similar patterns to control embryos (Fig. 2D,E,J,K). In addition, a few placode-like structures expressing both markers were evident on the shoulders of the forelimbs already at E12.5 (Fig. 2D,J). By E13, Wnt10b- and Edar-positive mutant placodes had spread from the shoulders towards the dorsal midline and new placodes had also appeared along and around the mammary milk line (Fig. 2E,K). At E13.5,Wnt10band Edarmarked new placode-like structures in the facial region, forelimbs and dorsal skin where placodes had reached the dorsal midline (Fig. 2B,D). Similar findings were revealed by Shhand Sostdc1-specific probes at E12.5 to E13.5 (data not shown). These results indicate that hair development is induced ~1.5 days earlier in the Catnb⌬ex3K14/+mutants compared with control mice.
To monitor the Cre recombinase activity in the skin, we crossed Rosa26 reporter mice, which expressed -galactosidase as a reporter, to K14Cre mice. E11 skin showed weak Cre activity in limbs, shoulder region and around the mammary milk line (Fig. 2M). At E12.5, the dorsolateral skin was positive for -galactosidase expression but the most intense Cre activity was detected in the mammary buds and in the shoulder region where we detected the first mutant placodes (Fig. 2N). By E13, reporter expression had further spread towards the dorsal midline (Fig. 2O). These observations indicate that the first appearance of placodes in the mutant embryos correlates with the onset of Cre activity.
Catnb⌬ex3K14/+mice have aberrant hair follicle
morphogenesis
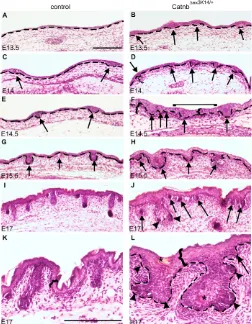
We examined the morphological features of hair follicle development in histological sections of E13.5 to E17 Catnb⌬exK14/+ and control back skin (Fig. 3). E13.5 control mice lacked epithelial thickenings, but by E14 primary hair placodes had appeared, and at E17 they had developed to stage 4 hair follicles containing a stem with several epithelial layers and a round bulb region with a dermal papilla surrounded by epithelial cells (Fig. 3A,C,E,G,I,K). The back skin of E13.5 Catnb⌬exK14/+mutants contained epithelial thickenings resembling hair placodes (Fig. 3B). At E14, the placode density was markedly higher in mutant skin than in control skin (Fig. 3D) and the density of the mutant follicles increased during following days. E14.5 mutant follicles resembled enlarged wild-type germ-stage follicles and new placodes arose between them in close juxtaposition (Fig. 3F). Follicles were also detected in the dorsal midline (Fig. 3F). By E15.5, the irregular shapes and sizes of the mutant follicles (Fig. 3H) were more prominent and new placodes had appeared in the interfollicular epithelium. The aberrant morphology of mutant follicles became more obvious at E17 as the down-growth of the follicles had apparently ceased and many follicles had a thick and short stem with large bulb-like epithelium with no evidence of a proper dermal papilla (Fig. 3J,L). New placodes were budding from the outer root sheath and bulb of the mutant follicles. Furthermore, at E17, deposits of keratin-like material were present within the epithelium of the mutant follicles and in epidermis (Fig. 3L). At E19, the abnormalities of the mutant skin were even more pronounced, and all follicles were severely stunted (data not shown).
The layer of condensed dermal mesenchyme in Catnb⌬exK14/+skin was markedly thick during E14-E17 and surrounded the continuously arising epithelial placodes and aberrant follicles (Fig. 3D,F). Moreover, the Catnb⌬exK14/+epidermis was clearly thicker than that in the control skin from E14 onwards (Fig. 3F,K,L). Taken together, the observations indicate that forced activation of -catenin resulted in markedly disturbed hair follicle morphogenesis, a failure in their proper down-growth, overproduction of rudimentary follicles and abnormal differentiation of epithelial cells.
Sustained Wnt/-catenin signaling perturbs hair
shaft production
Owing to the perinatal death of the Catnb⌬exK14/+mice, we examined the advancing development of the mutant hair follicles and subsequent hair filament formation by transplanting pieces of back skin of E17 Catnb⌬exK14/+and control embryos on the dorsal fascia of immunodeficient nude mice. After 3 weeks, the control grafts had dense tufts of black and white hairs, whereas the mutant grafts contained only few hairs (data not shown). After 5 weeks, the exterior features of the control grafts were unchanged (Fig. 4A), while the hairs in the mutant grafts had elongated but had not increased in number (Fig. 4B). The control grafts contained all four hair types – the long (>9 mm) guard hairs, as well as awl, auchene and zig-zag hairs – in approximately normal proportions (Fig. 4C). Majority of the mutant hairs resembled awl hairs, but we also observed a few zigzag and auchene hairs (Fig. 4E). Microscopic examination of the medulla in control and mutant awl hairs did not reveal major differences (Fig. 4D,F).
[image:3.612.54.296.64.257.2]Histological analysis revealed that after 3 weeks, the control skin grafts displayed typical features of normal skin with keratinized epidermis and catagen hair follicles (Fig. 4G). Mutant grafts contained mainly undeveloped short hair follicles and only few hair shaft-forming follicles detected (Fig. 4H). After 5 weeks, an even layer of anagen hair follicles were observed in control grafts (Fig. Fig. 1. Catnb⌬ex3K14/+mice show a dramatic skin phenotype.
(A,B) Macroscopic examination at E18 revealed severely disturbed skin morphology in Catnb⌬ex3K14/+mice. Mutant skin was covered by pigmented dots (arrows) and white granules. Eyes were open and external ears were poorly developed. (C,D) Scanning electron microscopic analysis of the skin of a Catnb⌬ex3K14/+mutant embryo (D) and its control littermate (C) at E14.5 revealed the random and dense pattern of epithelial evaginations on the surface of the mutant skin when compared with the regular array of hair placodes in the control embryo. Insets in B-D are higher magnifications of the boxed regions of head (B) and back skin (C,D). Scale bars: 1 mm in C,D; 0.2 mm in inset in C.
D
E
V
E
LO
P
M
E
N
4I). In the mutant grafts, the epidermis and adjacent dermis appeared grossly normal, while hair follicles were rudimentary with numerous invaginations (Fig. 4J,M). Many of these had a large number of sebocytes often localized at the tips of epithelial invaginations (Fig. 4K). The sebocytes also formed large clusters (Fig. 4L) that were often connected to the hair-producing follicles (data not shown). In addition, large epithelial cysts filled with keratin-like material were readily observed (Fig. 4L,M). Thus, forced -catenin expression caused aberrant embryonic hair morphogenesis that progressed into a severe epidermal phenotype with impaired follicle down-growth and hair shaft formation, as well as stimulated keratin expression and sebocyte differentiation.
Constitutively active Wnt/-catenin signaling
results in irregular hair placode patterning
Hair placode patterning in Catnb⌬ex3K14/+ skin was examined at E14.5 by whole-mount in situ hybridization analyses using Wnt10b, Shhand Sostdc1as placode markers. Sostdc1, also known as ectodin or Wise, is a secreted BMP inhibitor and Wnt modulator that is expressed in the developing ectodermal organs but is absent from the actual signaling centers, including hair placodes and tooth enamel knots (Laurikkala et al., 2003; Itasaki et al., 2003; Mou et al., 2006). Sostdc1proved to be a suitable marker because of its characteristic expression around the placodes. The expression of
Sostdc1was noticeably intense in the mutant skin (Fig. 5A-D,J) and the irregular morphology of the mutant skin surface with the protruding evaginations was readily visualized in the Sostdc1 whole-mount staining (Fig. 5B).
Catnb⌬ex3K14/+ mice displayed a dramatic placode patterning defect: irregularly spaced placodes had spread throughout the skin and covered regions that still lacked placodes in control embryos such as dorsal paw skin, the immediate surroundings of the eye, the ear lobe, tail skin and the dorsal midline (Fig. 5B,D). Mutant placodes were also observed in regions that remain largely hairless in wild-type adults, such as ventral paw skin (Fig. 5B⬘). Similar findings were recently reported in E14.5 embryos of mice lacking the Wnt antagonist adenomatous polyposis coli (APC) protein in developing ectoderm (Kuraguchi et al., 2006).
[image:4.612.51.364.57.452.2]Excessive placode formation occurred in the mutants in whisker pads and a close look of Wnt10band Sostdc1expression patterns at E13 indicated tight associations of adjacent follicles (Fig. 5D; see Fig. S1A-D in the supplementary material). The progressive addition of new placodes and their aberrant patterning in the mutants was also followed in cultured explants of E12 whisker pads (see Fig. S1E-H in the supplementary material). New placodes seemed to bud from the existing follicles much in the same way as earlier demonstrated for teeth in the same Catnb⌬ex3K14/+mutants (Järvinen et al., 2006). Histological analysis showed irregularly shaped and
Fig. 2. Forced expression of epithelial  -catenin leads to precocious hair follicle induction.(A-L) Detection of placodes in control (A-C,G-I) and Catnb⌬ex3K14/+skin (D-F,J-L) by in situ hybridization at E12.5 (A,D,G,J), E13 (B,E,H,K) and E13.5 (C,F,I,L) using Wnt10b(A-F) and Edar(G-L) as markers. In the Catnb⌬ex3K14/+mutants, first hair placode-like structures have already appeared at E12.5 (insets in D and J). Insets (A,D,G,J) are higher magnifications of the boxed areas in the shoulder region of E12.5 skin. (M-O) Whole-mount X-gal staining for E11, E12.5 and E13 Rosa26;K14Cre mice showing the Cre recombinase activity. Arrowheads indicate mammary placodes. L, limb. Arrows indicate Cre activity in shoulder region. Scale bars: 2 mm.
D
E
V
E
LO
P
M
E
N
enlarged epithelial invaginations arising in the whisker region (see Fig. S1I-J in the supplementary material), further confirming the results from the histological analysis of mutant pelage hair follicles (Fig. 3).
We next examined in more detail the patterning of back skin placodes and analyzed their size and arrangement using high-magnification pictures of the whole mounts of E14.5 Catnb⌬ex3K14/+ and control skin (Fig. 5E-J). Wnt10band Shhshowed similar spot-like expression patterns revealing the severely disturbed patterning of mutant placodes (Fig. 5E,F,H,I). The distances between adjacent placodes varied greatly compared with those between control follicles, and the neighboring mutant placodes had even formed fusions of several placodes. In addition, the shape of the mutant Fig. 3. The prenatal morphogenesis of Catnb⌬ex3K14/+mutant hair follicles is disturbed.(A-L) Histological sections of embryonic skin showed that the first morphological signs of hair placodes were evident in the controls at E14 (C). They reached germ stage by E15.5 (G) and stage 4 at E17 (I,K). Placodes of second-wave hair follicles had also appeared at E17 (I). In Catnb⌬ex3K14/+mutant skin, epithelial thickenings were already present at E13.5 (B). New placodes and buds of enlarged size and irregular shape were induced continuously during advancing development (D,F,H,J). Mutant placodes were already observed in dorsal midline at E14.5 (bracket, F). In addition, epithelial budding was initiated from the outer root sheath of follicles (arrowheads in J and L). The layer of condensed mesenchyme was thicker in the mutants both under the placode and under interfollicular epithelium (C-E, curly bracket in F). In addition, the epidermis was thicker in the mutants when compared with control skin (curly brackets in F,K,L). Broken lines show the epithelial-mesenchymal border (A-H,L). Asterisks indicate keratin deposits within the mutant epithelium (L). Arrows indicate placodes and buds. Scale bars: 200 m.
Fig. 4. Hair shaft production is severely impaired in Catnb⌬ex3K14/+ skin.(A,B) Advancing hair development was examined by transplanting back skin from E17 embryos to nude mice. After 5 weeks, dense hair was present in control graft, whereas the Catnb⌬ex3K14/+mutant graft had produced only a few hairs. (C-F) Light microscopic examination revealed no major differences in hair structure between control and mutant grafts. Arrows indicate zig-zags and arrowheads indicate awl hairs (C,E). High-magnification pictures (D,F) show internal structure of awl hairs. (G-N) Histological analysis of control (G,I) and mutant (H-N) grafts after 3 (G,H,K,L) or 5 weeks (I,J,M,N). Control skin showed regular row of catagen (G) and anagen-stage hair follicles (I), but in the mutant graft only few well-developed follicles were observed (arrows in H and J). In the mutant skin grafts, the down-growth of hair follicles was dramatically impaired and a variety of abnormalities were obvious (K-N): extra sebocytes in large clusters (arrows in K and L), epithelial budding from the outer root sheath of aberrant follicles (arrows in M) and large epithelial cysts filled with keratin-like material (arrowheads in J,L,N). Scale bars: 2 mm in A; 200 m in C,E,G,I; 100 m in D,F,L-N;
50m in K.
D
E
V
E
LO
P
M
E
N
placodes was irregular compared with the round control placodes (Fig. 5E-J). The placode numbers counted from different body regions indicated ~1.6-fold increase in the mutant mice at E14.5 (Fig. 5K). Interestingly, the widths of the majority of mutant
placodes were only half of those in the control skin. However, the size of mutant placodes varied markedly, and both tiny and very large placodes were common (Fig. 5L).
Placode markers show upregulation in the Catnb⌬ex3K14/+mice
To study the downstream effects of forced -catenin activation at the molecular level, we first monitored Wnt activity in E14 control and Catnb⌬exK14/+skin by crossing the Catnb⌬exK14/+mice with BAT-gal reporter mice (Maretto et al., 2003). Because Wnt reporter activity is strong throughout the mid-gestation skin, in particular in the dermis (Atit et al., 2006) (data not shown), the tissues were understained to reveal the regions displaying the most prominent Wnt activity. High Wnt activity was restricted to placode regions both in the control and mutant skin, and was present in placode epithelium, as well as in underlying mesenchyme (Fig. 6A-D). In the Catnb⌬exK14/+mutants, mesenchymal Wnt activity was more intense and extended further from the placode when compared with control skin.
We next localized the expression of some candidate genes associated with Wnt signaling by radioactive in situ hybridization of E14.5 control and Catnb⌬exK14/+skin sections. Wnt10b, Shhand Edarwere confined to placode epithelium in both mutant and control skin (Fig. 6E,F; data not shown). In line with the whole-mount analysis, the intensity of Sostdc1expression was markedly increased in the mutant skin and extended further from placodes compared with that seen in the control skin (Fig. 6G,H). The Wnt inhibitor dickkopf 1 (Dkk1), a direct Wnt target (Niida et al., 2004; Chamorro et al., 2005), was restricted to mesenchyme underneath the placodes in control skin (Fig. 6I), whereas in the mutants, it was expressed at higher intensity throughout the dermis almost as a continuous line (Fig. 6J).
Using quantitative RT-PCR analysis of E13.75 Catnb⌬exK14/+and control line skin (Fig. 6K), we detected approximately twofold upregulation of Dkk1 andSostdc1in the mutant when compared with the controls. In addition, Shh was elevated in the mutant skin, which was expected as Shhis known to act downstream of the Wnt pathway during embryonic hair development (Huelsken et al., 2001; Andl et al., 2002) and overexpression of -catenin in adult skin leads to upregulation of Shh(Gat et al., 1998; Lo Celso et al., 2003; Silva-Vargas et al., 2005). Interestingly, the expression level of Edarwas not altered (Fig. 6K).
BMP signaling is stimulated in the Catnb⌬ex3K14/+ skin
As Sostdc1was absent from the placodes but was expressed at augmented levels around the placodes in the mutant epithelium, we suspected that it was stimulated by secondary signals from the placode and/or underlying mesenchyme rather than being directly regulated by-catenin. As Sostdc1is known to be induced by BMPs and is a likely direct target of Bmp4 in the developing skin (Laurikkala et al., 2003; Mou et al., 2006), we decided to examine whether BMP signaling was affected in Catnb⌬ex3K14/+ mutant skin. First, we localized phosphorylated Smad1/5/8 by immunohistochemistry in sections of E14 Catnb⌬ex3K14/+and control skin (Fig. 7A-D). The intensity of pSmad1/5/8 staining was substantially increased in the Catnb⌬ex3K14/+skin when compared with the control littermates both in the epithelium and underlying mesenchyme.
[image:6.612.46.299.61.436.2]We next compared the expression levels of BMP ligands in mutant and control skin. Bmp2, Bmp4 and Bmp7 have all been associated with placode development; Bmp2 and Bmp4 have been Fig. 5. Catnb⌬ex3K14/+hair placodes form throughout the skin in a
random and dense pattern.(A-J) Detection of placodes by expression of Sostdc1(A-D,G,J), Wnt10b(E,H) and Shh(F,I) in E14.5 control (A,C,E-G) and Catnb⌬ex3K14/+ (B,D,H-J) skin showed dramatic spreading of mutant hair placodes throughout the skin, even in regions that, in control embryos, still lacked placodes: dorsal paw skin (arrow in B); the immediate surroundings of eye, ear lobe and vibrissa pads (arrows in D); and dorsal midline and tail skin (arrows in B⬙). Mutant placodes were also observed in ventral paw skin (B⬘), which in adult wild-type mice remain largely hairless. (E-J) High magnifications of dorsal skin revealed the regularly spaced control placodes (E-G), whereas the distances varied greatly between adjacent mutant placodes (H-J). Arrows indicate fusions of mutant placodes (H-J). (K) Placode numbers calculated from E14.5 Sostdc1wholemounts indicated a 1.6-fold increase in Catnb⌬ex3K14/+mice when compared with control. Data are represented as mean±s.d. (L) The distribution of placodes according to diameter as measured from E14.5Sostdc1 wholemounts. Mutant placodes showed larger variation in size and were, on average, smaller than control placodes. The placode was determined as the unstained area surrounded by the positive Sostdc1 expression. Number of placodes measured: mutant,n=218; control, n=137. Scale bars: 2 mm in A,A⬙,C; 0.5 mm in A⬘; 200 m in E.
D
E
V
E
LO
P
M
E
N
implicated as inhibitors of placode formation in hairs and feathers (Jung et al., 1998; Noramly and Morgan, 1998; Botchkarev et al., 1999; Mustonen et al., 2004) and Bmp7 as a positive regulator at stages preceding placode initiation in feathers (Harris et al., 2004). Quantitative PCR analysis of E14.5 skin indicated that Bmp2and Bmp4 expression was increased ~1.3-fold in the Catnb⌬ex3K14/+ mutants when compared with the controls, whereas Bmp7 expression level was not altered (Fig. 7M).
Whole-mount in situ hybridization of Bmp2and Bmp4 expression in E14 embryonic skin showed that both genes were expressed in the placodes and that the level of expression was increased in the Catnb⌬ex3K14/+mutants (Fig. 7E-H). In vibratome sections of the control embryos, Bmp2was restricted to the placode epithelium, whereas Bmp4was confined to underlying mesenchyme (Fig. 7I,J), as previously reported (Lyons et al., 1990; Bitgood and McMahon, 1995). In the Catnb⌬ex3K14/+mutant skin, Bmp2continued to be
expressed in the placode epithelium but at higher intensity (Fig. 7K). Interestingly, the pattern of Bmp4 expression was changed: although it was still expressed in the mesenchyme under the placodes, there was also intense ectopic expression in the epithelium of most placodes, in particular in the most protruding placodes (Fig. 7L; see Fig. S2 in the supplementary material). Taken together, these observations indicate that overexpression of -catenin in the epithelium resulted in increased BMP pathway activation and ectopic expression of Bmp4in placode epithelium.
Catnb⌬ex3K14/+placode phenotype is unaffected in
the absence of Eda/Edar signaling
Like the Wnt/-catenin pathway, the Eda/Edar pathway also stimulates placode formation (Mustonen et al., 2003; Mustonen et al., 2004). In the absence of Eda, the primary hair placode formation is blocked but the following ones develop normally (Headon and Overbeek, 1999; Laurikkala et al., 2002). Because the epistatic relationships of the two signal pathways have remained unclear (Huelsken et al., 2001; Andl et al., 2002; Laurikkala et al., 2002), we decided to explore this issue by crossing the Catnb⌬ex3K14/+mice with Eda–/–(tabby) mice (Falconer, 1952). The resulting embryos were analyzed at E13 to E15.5, and the formation of the first hair placodes was monitored by localizing Sostdc1 expression (Fig. 7N-Q; data not shown). Intriguingly, the dramatic placodal phenotype of the E14.5 Catnb⌬ex3K14/+ mutant embryos was apparently unaffected when Edafunction was deleted (Fig. 7P,Q, compare with Fig. 5B), and precocious formation of hair follicles was evident at E13 in Catnb⌬ex3K14/+;Eda–/–embryos (Fig. 7N,O). Hence, increased Wnt/-catenin signaling compensated for the lack of Eda signaling in primary hair placodes.
DISCUSSION
Forced -catenin activation results in accelerated and continuous hair placode induction
independent of Eda
Previous studies with genetically modified mice have indicated that canonical Wnt/-catenin signaling is necessary for the formation of all hair follicles (Huelsken et al., 2001; Andl et al., 2002). Here, we show that forced activation of epithelial -catenin during embryogenesis is also sufficient for hair follicle induction. To our knowledge, conditional Catnb⌬ex3K14/+mouse is the first mouse model displaying precocious appearance of hair placodes. Because placode initiation correlated both temporally and spatially with the onset of Cre activity in the ectoderm, it is possible that forced -catenin activity could induce placodes even earlier than E12.5. The fact that transgenic overexpression of a stabilized  -catenin under K14 promoter (⌬N87cat) in wild-type background did not cause any embryonic defects (Gat et al., 1998) is probably due to selection for mice with low transgene expression, as high ectodermal -catenin activity is perinatally lethal. In contrast to Catnb⌬ex3K14/+skin grafts, ⌬N87cat mice had a dense fur. This dissimilarity is most probably due to difference in timing and/or strength of -catenin activity between the two mouse models. In postnatal skin, the ability of -catenin to induce de novo follicle morphogenesis is well documented (Gat et al., 1998; Lo Celso et al., 2004; Silva-Vargas et al., 2005; Lowry et al., 2005), but it has remained unclear whether similar molecular mechanisms govern induction of hair formation in embryonic and adult skin (Estrach et al., 2006).
Our findings place -catenin upstream of all known activators of placode formation and show that forced stabilization of epithelial  -catenin can bypass the requirement for the still unknown first dermal Fig. 6. Forced -catenin expression causes increased epithelial
and mesenchymal Wnt activity and upregulation of placode marker genes.(A-D) Vibratome sections of X-gal stained E14 BAT-gal and BAT-BAT-gal; Catnb⌬ex3K14/+mice indicated increased Wnt activity in mutant skin both in placode epithelium and underlying
mesenchyme (B,D) compared with control (A,C). (E-J) By radioactive in situ hybridization, Wnt10b was localized to placode epithelium. (E,F)Sostdc1was restricted to epithelium flanking the placodes but was absent from the placode, and its expression was more intense in the mutant (G,H). Dkk1was expressed in mesenchyme underlying the placodes, and was more intense and widespread in the mutant skin (I,J). Arrows indicate hair placodes. Broken lines indicate epithelial-mesenchymal border. Scale bars: 100 m. (K) Quantitative RT-PCR reveals doubling of Dkk1, Sostdc1and Shhexpression in E13.75 Catnb⌬ex3K14/+ skin compared with control. Edar expression levels were unaltered in the mutant skin. Data are represented as mean±s.d.
D
E
V
E
LO
P
M
E
N
[image:7.612.52.300.62.352.2]signal(s) that is normally under strict temporal control. Whether the phenotype of Catnb⌬ex3K14/+embryos mimics epithelial -catenin activity responding to mesenchymal Wnts being part of the first inductive signal, or whether epithelial -catenin activity is a secondary response to non-Wnt dermal cues that induce the expression of placodal Wnts (such as Wnt10a and Wnt10b) is currently unknown.
Eda signaling is essential for the formation of primary hair placodes and promotes the expansion of hair placodes (Mikkola and Millar, 2006). However, the consequences of forced stabilization of epithelial -catenin are notably different from the effects of superfluous Eda, which cannot induce ectopic nor precocious hair follicle development in vivo or ex vivo (Mustonen et al., 2004). Surprisingly, forced epithelial -catenin activity could compensate for the absence of Eda. Although moderately (approximately fourfold) augmented expression of Edar has been shown to lead to ligand-independent signaling and to rescue absence of Eda in vivo (Mou et al., 2006), it is unlikely that this mechanism could explain our observations because the amount of Edar transcripts was unaffected in Catnb⌬ex3K14/+embryos. Our finding would tend to place canonical Wnt activity downstream of Eda/Edar signaling. However, our recent microarray screen on embryonic skin treated with a short exposure of recombinant Eda failed to reveal any obvious agonists of the Wnt pathway as putative direct Eda target genes (I. Fliniaux and I.T., unpublished) although other previously identified Eda targets (Pummila et al., 2007) were identified in the screen. In line with these data, Eda did not induce the expression of
-catenin, Wnt10bor Lef1in E14 skin explants when analyzed by RT-qPCR (I. Fliniaux and I.T., unpublished). Although we cannot exclude the possibility that Eda indirectly promotes Wnt signaling, we favor the following interpretation: during placode induction, Wnt/-catenin is upstream of Eda, but during placode formation Eda and Wnt pathways might act in parallel perhaps to stabilize nascent placodes. Overstimulation of one pathway could then compensate for the lack of the other. In principle, Eda and Wnt pathways could regulate common target genes, or they could control similar cellular functions via different genes.
Disturbed hair follicle morphogenesis results in poor hair filament production in Catnb⌬exK14/+skin The morphogenesis of the mutant developing follicles proceeded fairly normally from placode to germ stage but soon the abnormal size and irregular shape of the follicle became evident. The mutant skin was also characterized by unusually prominent condensation of the mesenchyme. This correlated with ectopic mesenchymal activation of both Wnt and BMP signaling, suggesting that either or both of the pathways may be involved. Strikingly, it appeared that most of the follicles were unable to encase the condensed mesenchyme to form a proper dermal papilla.
[image:8.612.55.296.61.417.2]The enlarged mutant follicles and the thickened epidermis were plausibly the result of a proliferative effect on epithelial cells induced and maintained by stabilized -catenin, as earlier studies have shown that chronic -catenin activity causes a prominent increase in BrdU incorporation in follicular epithelial cells leading Fig. 7. Forced activation of epithelial -catenin increases BMP signaling and compensates for the absence of Eda.
(A-D) Immunohistological detection of phosphorylated Smad1/5/8 in E14 control (A,C) and Catnb⌬ex3K14/+(B,D) skin, indicating stimulation of BMP pathway activity in the mutant skin. (E-H) Whole-mount in situ hybridization analysis of E14 embryos showed stimulated expression of Bmp2(E,F) and Bmp4 (G,H) in Catnb⌬ex3K14/+skin when compared with control. High magnifications of mutant wholemounts revealed prominent epithelial evaginations with Bmp2and Bmp4 expression in the tips (inserts in F and H). (I-L) Vibratome sections of wholemounts (E-H) indicated more intense expression of Bmp2and Bmp4in Catnb⌬ex3K14/+skin compared with controls (I-L). Ectopic expression of Bmp4had been induced in mutant placode epithelium (L).
(M) Quantitative RT-PCR showed 1.3-fold upregulation of Bmp2and Bmp4in E14.5 Catnb⌬ex3K14/+skin compared with controls. No difference was seen in Bmp7expression. Data are represented as mean±s.d. (N-Q) Expression of Sostdc1in E13 and E14.5 Eda-deficient mice (Eda–/–) and Eda–/–; Catnb⌬ex3K14/+mice. Sostdc1was absent from E13 and E14.5 Eda–/–skin (N,P) owing to lack of primary hair placodes. When Eda–/–mutants were crossed to Catnb⌬ex3K14/+mice, placode formation was rescued and the placode phenotypes at E13 and E14.5 were indistinguishable from the Catnb⌬ex3K14/+mutant phenotype (O,Q). Broken lines indicate epithelial-mesenchymal borders. Scale bars: 200 m in A; 100 m in C and inset in F,I; 2 mm in E,N. (R) A model of the interactions between Wnts and BMPs in hair placode formation, suggesting roles for BMPs as reaction-diffusion inhibitors of placode patterning. Placodal cells display Wnt activity. Wnts induce the expression of their feedback inhibitors Dkk1 and Dkk4, as well as of Bmp2 and Bmp4, which in turn downregulate Wnt activation at least through inhibition of Lef1. In interplacode region BMPs induce the expression of Sostdc1which may act by suppressing Wnt activity in interplacodal cells. Black lines indicate transcriptional regulation, and red lines show protein-protein interaction. Green area, placode; yellow area, condensed mesenchyme; white area, interfollicular epithelium.
D
E
V
E
LO
P
M
E
N
to thickening of the follicles (Van Mater et al., 2003; Lo Celso et al., 2004). At later developmental stages, we observed formation of large epithelial cysts filled with keratin-like material. Previously, prominent hair follicle-associated hyperkeratosis was observed in a mouse model with inducible activation of -catenin in adult skin (Van Mater et al., 2003). Grafted Catnb⌬ex3K14/+skin also contained excessive amount of sebocytes. Earlier studies have indicated that -catenin may either promote or suppress sebocyte differentiation depending on the level and duration of signaling (Lo Celso et al., 2004).
Embryonic analyses and skin graft studies revealed that hair shaft production was greatly impaired in the Catnb⌬exK14/+skin, and the vast majority of hair follicles were very short and rudimentary, and never reached the length of the wild-type anagen follicle. This is a rather surprising finding, as epithelial Wnt/-catenin activity is thought to promote the active growth phase of the hair cycle (Van Mater et al., 2003; Lowry et al., 2005). In contrast to the adult follicle, it appears that chronic -catenin activity suppresses the down-growth of the embryonic follicle (but not the adult one) (Van Mater et al., 2003; Lowry et al., 2005), suggesting that downregulation of Wnt signaling after placode formation is essential to successful hair morphogenesis. In conclusion, our results imply that the first embryonic anagen may be fundamentally different from the following ones.
Catnb⌬exK14/+mice show random patterning of hair
follicles and increased BMP pathway activity Our analyses on embryonic Catnb⌬exK14/+back skin showed that the number of hair placodes was increased. Moreover, their sizes varied greatly and their spacing was random. The spacing of hair placodes is thought to be a result of signaling by diffusible molecules that either promote or repress follicular fate. These competing factors are initially uniformly expressed but become localized to nascent placodes (or the underlying mesenchyme) (Millar, 2002; Jiang et al., 2004; Mikkola and Millar, 2006). Whereas Wnts, FGFs and Eda are thought to be placode activators, Dkks and BMPs (in particular Bmp4) are generally regarded as placode inhibitors that mediate lateral inhibition in neighboring cells. The best characterized mathematical model to explain the periodic patterning of hair follicles is the reaction-diffusion mechanism that can account for pattern formation through self-organization (Turing, 1952; Jung et al., 1998; Jiang et al., 2004). It has recently been proposed that the interplay between Wnts and Dkks, in particular Dkk4, is the key element of the reaction-diffusion process (Andl et al., 2002; Sick et al., 2006). However, as the phenotypes of Dkk4(or combined Dkk1 and Dkk4) loss-of-function mutants have not been reported, the functional relevance of Wnt-Dkk interactions for follicle patterning has remained elusive.
The Catnb⌬exK14/+skin displayed increased mesenchymal Wnt activity, as well as highly elevated levels of BMP signaling activity. We propose that the interplay between Wnts and BMPs may be involved in establishing the correct hair follicle spacing in wild-type skin (Fig. 7R). This conclusion is in line with the phenotype of K14-Noggin mutant mice, and is consistent with idea that BMPs work as reaction-diffusion inhibitors for hair follicle patterning (Plikus et al., 2004). Our findings suggest that epithelial Wnt activity causes upregulation of mesenchymal Wnt signaling, possibly through placodal Wnts such as Wnt10a and Wnt10b. They induce, directly or indirectly, the expression Bmp2and Bmp4. BMPs in turn inhibit placode fate in neighboring cells at least through restricting responsiveness to Wnts by downregulating Lef1(Jamora et al., 2003) and by suppressing expression of Edar(Mou et al., 2006).
Simultaneously, placodal cells are protected by BMP antagonists that are expressed in the placode and in the underlying dermal condensate (Botchkarev et al., 1999; Mou et al., 2006; Pummila et al., 2007). Moreover, BMPs induce the expression of Sostdc1in the epithelium flanking the placode (Laurikkala et al., 2003; Mou et al., 2006). Sostdc1 is known to be able to modulate both Bmp and Wnt pathways (Itasaki et al., 2003; Laurikkala et al., 2003; Kassai et al., 2005; Beaudoin et al., 2005) but its primary mode of action during initiation of hair development is currently unknown. However, during the onset of postnatal anagen it has been proposed to act as a Wnt repressor (Beaudoin et al., 2005) and if the same holds true for placode formation, expression of Sostdc1around the placode would further strengthen the circular Wnt-inhibited zone around the nascent hair bud (Fig. 7R).
It is evident that the Catnb⌬exK14/+mutants are insensitive to Wnt inhibitors upstream of -catenin. We suggest that the increased placode density and randomness of their patterning results (at least partly) from the impaired inhibitory mechanisms mediated by both Wnt induced feedback inhibitors (Dkks) and inhibitors induced secondarily by BMPs (Sostdc1). However, the fact that interplacodal epithelium did form in Catnb⌬exK14/+skin and the majority of the placodes were small in size suggests that the essential -catenin partner Lef1 and/or other Tcfs were still under inhibitory mechanisms leading to spatially restricted expression of placode markers.
In conclusion, we have shown that forced activation of -catenin during embryogenesis led to excessive formation of hair follicles, as recently shown for teeth and taste papillae (Järvinen et al., 2006; Liu et al., 2007). Surprisingly, chronic -catenin activation disturbed proper morphogenesis of the hair follicle, as well as hair shaft production. Our data suggest that BMPs are downstream of Wnt/ -catenin during placode formation and that sustained -catenin signaling interferes with their normal crosstalk. It is apparent that multiple pairs of activators and inhibitors of different pathways operate in parallel to establish correct patterning of hair follicles (Jiang et al., 2004; Sick et al., 2006; Pummila et al., 2007).
We thank Merja Mäkinen, Riikka Santalahti and Raija Savolainen for excellent technical assistance. This work was supported by the Academy of Finland, the Sigrius Jusélius Foundation, and the Helsinki Graduate School in Biotechnology and Molecular Biology.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/135/6/1019/DC1
References
Andl, T., Reddy, S. T., Gaddapara, T. and Millar, S. E.(2002). WNT signals are required for the initiation of hair follicle development. Dev. Cell2, 643-653. Atit, R., Sgaier, S. K., Mohamed, O. A., Taketo, M. M., Dufort, D., Joyner, A.
L., Niswander, L. and Conlon, R. A.(2006). Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev. Biol. 296, 164-176.
Beaudoin, G. M. J., III, Sisk, J. M., Coulombe, P. A. and Thompson, C. C. (2005). Hairless triggers reactivation of hair growth by promoting Wnt signaling.
Proc. Natl. Acad. Sci. USA102, 14653-14658.
Bitgood, M. J. and McMahon, A. P.(1995). Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo.
Dev. Biol. 172, 126-138.
Botchkarev, V. A., Botchkareva, N. V., Roth, W., Nakamura, M., Chen, L.-H., Herzog, W., Lindner, G., McMahon, J. A., Peters, C., Lauster, R. et al. (1999). Noggin is a mesenchymally derived stimlator of hair-follicle induction.
Nat. Cell Biol. 1, 158-164.
Chamorro, M. N., Schwartz, D. R., Vonica, A., Brivanlou, A. H., Cho, K. R. and Varmus, H. E.(2005). FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. EMBO J. 24, 73-84.
Chiang, C., Swan, R. Z., Grachtchouk, M., Bolinger, M., Litingtung, Y.,
Robertson, E. K., Cooper, M. C., Gaffield, W., Westphal, H., Beachy, P. A. et
D
E
V
E
LO
P
M
E
N
al.(1999). Essential role for Sonic hedgehog during hair follicle morphogenesis.
Dev. Biol. 205, 1-9.
Clevers, H.(2006). Wnt/beta-catenin signaling in development and disease. Cell 127, 469-480.
DasGupta, R. and Fuchs, E.(1999). Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation.
Development126, 4557-4568.
Estrach, S., Ambler, C. A., Lo Celso, C., Hozumi, K. and Watt, F. M.(2006). Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation adult epidermis. Development133, 4427-4438.
Falconer, D. S.(1952). A totally sex-linked gene in the house mouse. Nature169, 664-665.
Gat, U., DasGupta, R., Degenstein, L. and Fuchs, E.(1998). De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated -catenin in skin.
Cell95, 605-614.
Harada, N., Tamai, Y., Ishikawa, T., Sauer, B., Takaku, K., Oshima, M. and Taketo, M. M.(1999). Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 18, 5931-5942.
Hardy, M. H.(1992). The secret life of the hair follicle. Trends Genet. 8, 55-61. Harris, M. P., Linkhart, B. L. and Fallon, J. F.(2004). Bmp7 mediates early
signaling events during induction of chick epidermal organs. Dev. Dyn. 231, 22-32.
Headon, D. J. and Overbeek, P. A.(1999). Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat. Genet. 22, 370-374.
Huelsken, J., Vogel, R., Erdmann, B., Cotsarelis, G. and Birchmeier, W.(2001). Beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell105, 533-545.
Itasaki, N., Jones, C. M., Mercurio, S., Rowe, A., Domingos, P. M., Smith, J. C. and Krumlauf, R.(2003). Wise, a context-dependent activator and inhibitor of Wnt signaling. Development130, 4295-4305.
James, M. J., Järvinen, E., Wang, X. P. and Thesleff, I.(2006). Different roles of Runx2 during early neural crest-derived bone and tooth development. J. Bone Miner. Res. 21, 1034-1044.
Jamora, C., DasGupta, R., Kocieniewski, P. and Fuchs, E.(2003). Links between signal transduction, transcription and adhesion in epithelial bud development. Nature422, 317-322.
Järvinen, E., Salazar-Ciudad, I., Birchmeier, W., Taketo, M. M., Jernvall, J. and Thesleff, I.(2006). Continuous tooth regeneration in mouse is induced by activated epithelial Wnt/-catenin signaling. Proc. Natl. Acad. Sci. USA103, 18627-18632.
Jiang, T. X., Widelitz, R. B., Shen, W. M., Will, P., Wu, D. Y., Lin, C. M., Jung, H. S. and Chuong, C. M.(2004). Integument pattern formation involves genetic and epigenetic controls: feather arrays simulated by digital hormone models. Int. J. Dev. Biol. 48, 117-135.
Jung, H. S., Francis-West, P. H., Widelitz, R. B., Jiang, T. X., Ting-Berreth, S., Tickle, C., Wolpert, L. and Chuong, C. M.(1998). Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev. Biol. 196, 11-23.
Kassai, Y., Munne, P., Hotta, Y., Penttila, E., Kavanagh, K., Ohbayashi, N., Takada, S., Thesleff, I., Jernvall, J. and Itoh, N.(2005). Regulation of mammalian tooth cusp patterning by ectodin. Science309, 2067-2070. Kratochwil, K., Dull, M., Farinas, I., Galceran, J. and Grosschedl, R.(1996).
Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 10, 1382-1394.
Kuraguchi, M., Wang, X. P., Bronson, R. T., Rothenberg, R., Ohene-Baah, N. Y., Lund, J. J., Kucherlapati, M., Maas, R. L. and Kucherlapati, R.(2006). Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 2, e146.
Laurikkala, J., Pispa, J., Jung, H. S., Nieminen, P., Mikkola, M., Wang, X., Saarialho-Kere, U., Galceran, J., Grosschedl, R. and Thesleff, I.(2002). Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development129, 2541-2553.
Laurikkala, J., Kassai, Y., Pakkasjärvi, L., Thesleff, I. and Itoh, N.(2003). Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev. Biol. 264, 91-105.
Liu, F., Thirumangalathu, S., Gallant, N. M., Yang, S. H., Stoick-Cooper, C. L., Reddy, S. T., Andl, T., Taketo, M. M., Dlugosz, A. A., Moon, R. T. et al. (2007). Wnt-beta-catenin signaling initiates taste papilla development. Nat. Genet. 39, 106-112.
Lo Celso, C., Prowse, D. M. and Watt, F. M.(2004). Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair
follicles but continuous activation is required to maintain hair follicle tumours.
Development131, 1787-1799.
Lowry, W. E., Blanpain, C., Nowak, J. A., Guasch, G., Lewis, L. and Fuchs, E. (2005). Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 19, 1596-1611.
Lyons, K. M., Pelton, R. W. and Hogan, B. L.(1990). Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A). Development109, 833-844.
Maretto, S., Cordenonsi, M., Dupont, S., Braghetta, P., Broccoli, V., Hassan, A. B., Volpin, D., Bressan, G. M. and Piccolo, S.(2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA100, 3299-3304.
Mikkola, M. L. and Thesleff, I.(2003). Ectodysplasin signaling in development.
Cytokine Growth Factor Rev. 14, 211-224.
Mikkola, M. L. and Millar, S. E.(2006). The mammary bud as a skin appendage: unique and shared aspects of development. J. Mammary Gland Biol. Neoplasia 11, 187-203.
Millar, S. E.(2002). Molecular mechanisms regulating hair follicle development. J. Invest. Dermatol. 118, 216-225.
Mou, C., Jackson, B., Schneider, P., Overbeek, P. A. and Headon, D. J.(2006). Generation of the primary hair follicle pattern. Proc. Natl. Acad. Sci. USA103, 9075-9080.
Mustonen, T., Pispa, J., Mikkola, M. L., Pummila, M., Kangas, A. T., Pakkasjärvi, L., Jaatinen, R. and Thesleff, I.(2003). Stimulation of ectodermal organ development by Ectodysplasin-A1. Dev. Biol. 259, 123-136. Mustonen, T., Ilmonen, M., Pummila, M., Kangas, A. T., Laurikkala, J.,
Jaatinen, R., Pispa, J., Gaide, O., Schneider, P., Thesleff, I. et al.(2004). Ectodysplasin A1 promotes placodal cell fate during early morphogenesis of ectodermal appendages. Development131, 4907-4919.
Niida, A., Hiroko, T., Kasai, M., Furukawa, Y., Nakamura, Y., Suzuki, Y., Sugano, S. and Akiyama, T.(2004). Dkk1, a negative regulator of Wnt signaling, is target of the beta-catenin/TCF pathway. Oncogene23, 8520-8526.
Noramly, S. and Morgan, B. A.(1998). BMPs mediate lateral inhibition at successive stages in feather tract development. Development125, 3775-3787. Noramly, S., Freeman, A. and Morgan, B. A.(1999). Beta-catenin signaling can
initiate feather bud development. Development126, 3509-3521.
Pispa, J., Jung, H. S., Jernvall, J., Kettunen, P., Mustonen, T., Tabata, M. J., Kere, J. and Thesleff, I.(1999). Cusp patterning defect in Tabby mouse teeth and its partial rescue by FGF. Dev. Biol. 216, 521-534.
Plikus, M., Wang, W. P., Liu, J., Wang, X., Jiang, T.-X. and Chuong, C.-M. (2004). Integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway.
Am. J. Pathol. 164, 1099-1114.
Pummila, M., Fliniaux, I., Jaatinen, R., James, M. J., Laurikkala, J., Schneider, P., Thesleff, I. and Mikkola, M. L.(2007). Ectodysplasin has a dual role in ectodermal organogenesis: inhibition of Bmp activity and induction of Shh expression. Development 134, 117-125.
Reddy, S., Andl, T., Bagasra, A., Lu, M. M., Epstein, D. J., Morrisey, E. E. and Millar, S. E.(2001). Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 107, 69-82.
Sick, S., Reinker, S., Timmer, J. and Schlake, T.(2006). WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science314, 1447-1450.
Silva-Vargas, V., Lo Celso, C., Giangreco, A., Ofstad, T., Prowse, D. M., Braun, K. M. and Watt, F. M.(2005). Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev. Cell9, 21-31.
Turing, A. M.(1952). The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 237, 37-72.
van Genderen, C., Okamura, R. M., Fariñas, I., Quo, R. G., Parslow, T. G., Bruhn, L. and Grosschedl, R.(1994). Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 8, 2691-2703.
Van Mater, D., Kolligs, F. T. and Dlugosz, A. A.(2003). Transient activation of beta-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 17, 1219-1224. Wang, J. and Shackleford, G. M.(1996). Murine Wnt10a and Wnt10b: cloning
and expression in developing limbs, face and skin of embryos and in adults.
Oncogene13, 1537-1544.