The complex responses of social stingless bees (Apidae: Meliponini)
to tropical deforestation
Berry J. Brosi
*
Department of Biology, Stanford University, 385 Serra Mall, Stanford, CA 94305, USA
1. Introduction
Despite ongoing controversy over pollinator declines and a concomitant increase in studies on the effects of anthropogenic environmental change on pollinators (e.g.Ghazoul, 2005; Steffan-Dewenter et al., 2005), we still do not have a comprehensive view of how bee communities respond to land use change. This problem is particularly acute in the tropics, where most tropical forest trees are animal-pollinated (Bawa, 1990), where pollination limitation is most severe (Vamosi et al., 2006) and where land-use change is the largest driver of biodiversity loss (Sala et al., 2000).
The meliponine, or social stingless, bees (Apidae: Meliponini) are a critically important group of tropical bees. They are the most diverse group of eusocial tropical bees (Michener, 2000: p. 779), and may be the most abundant clade of bees on earth (Roubik,
1992). All meliponine bees are eusocial, with perennial hives consisting of a single queen and thousands of workers. Meliponine workers recruit foragers to rich sources of floral forage, thus allowing them to efficiently pollinate tropical plants whose blooming period is brief, including crops such as coffee (Klein et al., 2003a; Ricketts, 2004). This efficient exploitation of floral resources, along with their high densities, means that they are also major players in the cycling of nutrients in tropical forests (Roubik, 1989: p. 354).
This importance carries over to the human enterprise as well, since meliponines contribute to the pollination of >60 tropical crops. They are the primary wild pollinators of coffee (Klein et al., 2003b); and contribute to the effective pollination of avocado, sweet pepper, tomato, cucumber, strawberry, and rambutan (Slaa et al., 2006); as well as coconut, mango, macadamia nuts, chayote, carambola (‘‘star fruit’’), and achiote (Heard, 1999) among many other crops. These meliponine-mediated pollination services likely contribute billions of dollars to tropical economies ever year (Kearns et al., 1998; Ricketts et al., 2004b; Klein et al., 2007). The A R T I C L E I N F O
Article history:
Received 26 November 2008
Received in revised form 22 February 2009 Accepted 25 February 2009 Keywords: Pollinator Land-use change Deforestation Bee communities Biodiversity A B S T R A C T
Despite concern over a putative ‘‘global pollination crisis’’, we still have an incomplete understanding of how bee communities respond to land-use change. I studied the responses of social stingless (or ‘‘meliponine’’) bees (Hymenoptera: Apidae: Meliponini) to surrounding forest cover and floral resources in 35 sites in a largely deforested landscape in Costa Rica over three years, sampling bees with a standardized netting protocol. I recorded a diverse fauna of meliponines, comprised of 20 species and nine genera. I found that meliponine species richness and abundance are strongly related to forest cover, but not floral resource variables (blooming plant species richness and abundance). The effect of forest on meliponine abundance, but not diversity, disappeared when the most common meliponine species, Trigona fulviventris(which comprised 45% of sampled individuals), was excluded from analyses. Meliponine community composition, by contrast, was related most strongly to plant species richness, only weakly to forest cover, and not related to blooming plant abundance. This work differs from past work in the same landscape, which did not find evidence of changes in species richness or abundance of meliponines and forest-related variables (distance to forest or forest fragment size), but did find shifts toward meliponine-dominated communities near forests, especially larger ones. The larger true sample size (i.e. number of sample sites) of the present work likely improved the statistical power to detect these relationships. While meliponines are forest dependent, I recorded some species in the smallest forest fragments in the landscape, and as a group they respond strongly to overall forest cover in the landscape (i.e. including both small and large patches of forest). Both of these observations support arguments for preserving even small fragments of forest in agricultural landscapes. Given the ecological and economic importance of meliponine bees, it is imperative that we better understand their long-term conservation needs in the changing tropical landscapes of the world.
ß2009 Elsevier B.V. All rights reserved.
* Tel.: +1 650 450 3715; fax: +1 650 723 5920. E-mail address:bbrosi@stanford.edu.
Contents lists available atScienceDirect
Forest Ecology and Management
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / f o r e c o
0378-1127/$ – see front matterß2009 Elsevier B.V. All rights reserved. doi:10.1016/j.foreco.2009.02.025
importance of meliponines as pollinators of tropical crops is likely to increase given the ongoing problems of honey bees, including Africanization (Schneider et al., 2004) and parasites and disease (e.g.Oldroyd, 2007).
While the Maya and other indigenous peoples have managed meliponine bees for millennia (Chemas and Rico-Gray, 1991), and some efforts have been made to modernize techniques and promote the husbandry of meliponines, also known as ‘‘melipo-niculture’’ (Cortopassi-Laurino et al., 2006), wild colonies account for the vast majority of crop pollination activities that are conducted by meliponines. Thus, nearly all meliponine pollination activity can be considered a true ecosystem service, rather than a managed human activity.
Given the central place of meliponine bees in provisioning tropical crop pollination services, it is critically important to understand how they respond to ongoing land-use changes. This is particularly true given that studies of bee communities in Central America and Southeast Asia have found that meliponine bees are strongly associated with native forest habitat (Klein et al., 2002; Ricketts, 2004; Brosi et al., 2007; Brosi et al., 2008). On one hand, this association with forest is not surprising given that many (but not all) meliponines are tree-cavity nesters that may rely on tropical forests for nesting habitats (Roubik, 1989). On the other hand, many meliponine species will forage and even nest in human-dominated habitats that have experienced high degrees of deforestation (Klein et al., 2002; Ricketts, 2004; Brosi et al., 2007, 2008).
This tendency of meliponines to forage in human-dominated habitats is a positive attribute when considering crop pollination— and also makes for a direct linkage between forest cover and meliponine-mediated crop pollination. For example,Ricketts et al. (2004a)found that two patches of tropical forest near a large coffee farm in southern Costa Rica supported meliponine bees which contributed approximately $60,000 a year to the value of the coffee harvest, through increases in both the quantity and quality of coffee beans produced.
In order to improve understanding the responses of meliponine bees to land use change, I sampled meliponines over 3 years in a largely deforested landscape in southern Costa Rica, along gradients of both distance to forest and also forest fragment size. I hypothesized that meliponines would be more diverse and abundant in sites with more forest cover surrounding them, and in sites with greater density and diversity of floral resources. I also hypothesized that meliponine species would respond differently to land use change, and that these differences would be reflected in measures of community composition.
2. Methods 2.1. Study region
I conducted this study in the Valle de Coto Brus, Puntarenas province, southern Costa Rica, in the landscape surrounding the Las Cruces Biological Station (88470N, 828570W), near the town of San
Vito. The landscape was converted in the 1960s from mid-elevation tropical forest to a mosaic of pastures, coffee fields, rural dwellings, and subsistence plots of crops like corn, beans, and bananas. Locally collected pollen records, however, show a history of forest clearing and agriculture by indigenous people spanning several thousand years (Clement and Horn, 2001). Remnant tracts of forest comprise about 15% of land cover in the region, with the largest fragment the 230 ha tract at the Las Cruces Biological Station.
2.2. Study sites
The sites and samples—and thus the data presented here—come from one of the larger systematically collected datasets on tropical
bees (Brosi et al., 2007, 2008). With data from both studies and with restrictions I imposed on sites (detailed below), I used 35 sites in the Las Cruces landscape in this analysis. The results presented here are distinct from those inBrosi et al. (2007, 2008) in that analyses combine both datasets (though only including sites that were sampled in a minimum of two full field seasons), along with several new analyses. The sites used inBrosi et al. (2007)fell along a gradient of distance to forest and were sampled in the rainy season (July-September) of 2003 and in the dry season (February-May) of 2005. The sites from Brosi et al. (2008) were along a gradient of forest fragment size, with sample sites situated in pastures at the edges of the forest fragments. These sites were sampled in the rainy season (June-September) of 2004, and the dry season (February–May) of 2005, with the dry season samples being taken concurrently with those fromBrosi et al. (2007). Sites ranged from 500 m to 13 km in geographic distance from one another and from 900 to 1300 m in elevation above sea level.
2.3. Bee and plant sampling
In each site, two field team members netted bees in a 20 m20 m square plot for a 15-minute period, focusing their efforts on flowering plants within the plot. The netting team caught bees in the order that they were seen, and thus would not pursue a relatively rare species at the expense of a common species seen first. The netting trials were focused across all bees in the bee community; in the analyses presented here, we include only the meliponine bees from these samples. We did not net bees in conditions of fog, precipitation, or high winds. For more on the sampling, seeBrosi et al. (2007, 2008). Specimens were pinned, labeled, and identified to species usingRoubik (1992). V. Gonzalez and I. Hinojosa, University of Kansas, evaluated and corrected species determinations. Specimens are housed in the Biology Department, Stanford University.
Blooming plants were counted along 5 parallel 20 m transects in each site, counting and identifying all plants in bloom within 50 cm of either side of the transect line. SeeBrosi et al. (2007, 2008) for more details on plant sampling.
2.4. Data analysis
I analyzed two responses (dependent variables) of meliponine communities to landscape structure and plant resource avail-ability: (1) the species richness and abundance of meliponines (across all species, and also species-specific abundances for those species with >20 individuals recorded); and (2) meliponine community composition (similarity matrices between sites).
I analyzed data using the R statistical programming language (R Core DevelopmentTeam, 2006); additional R packages I used are cited with the details of specific analyses. Sample size was 35 (i.e. the number of sites), except for analyses on forest fragment size, whereN= 19.
I assessed the effects of (1) landscape structure and (2) local floral resources on meliponine species richness, abundance, and commu-nity composition. The primary measure of landscape structure used in the analyses was ‘‘forest cover’’, or the proportional area of forest surrounding sample sites at a radius of 400 m. I used 400 m because earlier work showed this radial distance to have a consistently strong relationship with meliponine diversity and abundance (Brosi et al., 2007, 2008). In secondary analyses, I also looked at two additional measures of landscape structure: ‘‘distance’’, i.e. whether a site was at the edge of a forest fragment or located in open countryside; ‘‘fragment size’’, the log10of forest fragment area (for those sites at forest edges). Forest cover, distance to forest, and forest fragment area were all highly correlated with one another. I used forest cover in multivariate analyses because it is continuous (unlike
the categorical ‘‘distance’’ measure) and is available for all sites (unlike fragment size, which is only available for a subset). To assess if fragment area or distance to forest behaved differently than forest cover, I conducted separate univariate analyses for these variables. The other major independent variable was the floral resource base in each site, specifically blooming plant abundance and species richness (per 100 m2). Because of uneven sampling in sites, I used a bootstrapped estimate of the mean (Efron, 1981) for both plant richness and abundance. To generate the bootstrapped means, I sampled with replacement from the set of sample dates, repeated this 1,000 times, and took the mean estimate.
To assess the effects of forest cover (proportional area of forest surrounding sample sites) and flowering plant richness and abundance on meliponine diversity and abundance, I used multivariate mixed-effects models. I chose this class of model because it allows for the use of all individual samples while correcting for the temporal pseudoreplication, i.e. different samples from the same site are not independent from one another (Crawley, 2002: pp. 670–671). I used generalized linear models (GLM) with Poisson errors in the mixed-effects model, because the samples are count-based, and because variance increased with the mean for both meliponine richness and abundance (e.g.Olofsson and Shams, 2007). I conducted the mixed-effects models using the LME4 package for R (Bates, 2008), with site as a random variable. I repeated this analysis both with and withoutTrigona fulviventris, the most abundant species in the samples.
To assess species-specific responses, I also separately ran multivariate mixed-effects models with the abundance of each of the nine most common species (those with>20 individuals) as response variables. I also conducted univariate tests on the effects of distance to forest and forest fragment size on meliponine species richness and abundance with mixed-effects models, again with site as a random variable.
To evaluate the impact of forest characteristics (isolation, size, and cover) and floral resources on meliponine community composition, I calculated pairwise meliponine community simi-larity between sites using the Morisita-Horn simisimi-larity index. Because of the very uneven number of samples between sites, I calculated the similarity index as the average of 50 replicates of a bootstrapped collection of 11 sample dates (the minimum number of times each site was sampled), sampling with replacement. I used multivariate matrix permutation tests, with the ADONIS function in the VEGAN package for R (Oksanen et al., 2006) to test the relationship between community similarity and forest cover and floral resources.
To assess spatial and altitudinal autocorrelation between sites, I used Moran’s I for meliponine abundance and diversity, and Mantel tests for pair-wise community similarity, using the aforementioned bootstrapped Morisita-Horn similarity matrix. 3. Results
3.1. Overview
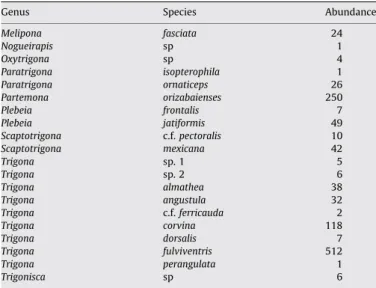
I sampled 1141 meliponine individuals representing 9 genera and 20 species (species list,Table 1). The rank abundance profile of the meliponine community in the Las Cruces landscape is highly uneven. One species,Trigona fulviventris, accounted for 45% of all sampled individuals; the next-most common species,Partamona
orizabaensis, represented 22% of all individuals (rank abundance
curve,Fig. 1).
3.2. Spatial and altitudinal autocorrelation
Meliponine abundance was not spatially autocorrelated as assessed by Moran’s I (p= 0.13), but meliponine richness was
significantly spatially autocorrelated (p= 0.00031). Abundance and richness were both significantly altitudinally autocorrelated (abundance:p= 0.0031; richness:p= 0.0011). Community simi-larity, however, showed no significant relationship with either geographic distance (p= 0.22) or altitudinal differences between sites (p= 0.52), as assessed with Mantel tests. In subsequent analyses, I assumed sites were independent.
3.3. Meliponine diversity and abundance
Multivariate mixed-effects models showed that both melipo-nine abundance and species richness are strongly related to forest cover, but not to the abundance or richness of plants in bloom (Table 2; Fig. 2). When I re-analyzed these models without including Trigona fulviventris, the most common species, these results changed somewhat. Meliponine abundance was no longer significantly related to forest cover, though it remained unrelated to blooming plant richness or abundance. In contrast, meliponine richness was still related to forest cover, though more weakly, and showed marginal relationships with both plant richness and plant abundance (Table 3; Fig. 3). Thus,T. fulviventrisappears to be a Table 1
Species list. Species presented in alphabetical order by genus.
Genus Species Abundance
Melipona fasciata 24 Nogueirapis sp 1 Oxytrigona sp 4 Paratrigona isopterophila 1 Paratrigona ornaticeps 26 Partemona orizabaienses 250 Plebeia frontalis 7 Plebeia jatiformis 49 Scaptotrigona c.f.pectoralis 10 Scaptotrigona mexicana 42 Trigona sp. 1 5 Trigona sp. 2 6 Trigona almathea 38 Trigona angustula 32 Trigona c.f.ferricauda 2 Trigona corvina 118 Trigona dorsalis 7 Trigona fulviventris 512 Trigona perangulata 1 Trigonisca sp 6
driver of the results, particularly with regard to meliponine abundance and forest cover.
Separate univariate analyses showed strong positive relation-ships with meliponine richness and abundance and both distance to forest (abundance: z= 3.26, p= 0.0011; richness: z= 3.31,
p= 0.00094) and forest fragment size (abundance: z= 2.40,
p= 0.016; richness:z= 3.13,p= 0.0017).
3.4. Species-specific abundance
Because I separately analyzed the abundances of nine species, I used a Bonferroni-corrected significance cut-offp-value of 0.0005
in the species-specific analyses. At this level, I found only three significant associations between landscape or flowering-plant variables and species-specific abundances of meliponines. Two species showed positive relationships with forest cover (T.
fulviventris z= 3.55, p= 0.00039; Plebeia jatiformis z= 8.40,
p= 8.691015). The abundance of Paratrigona orniticeps was positively related to plant species richness (z= 3.65,p= 0.00027).
3.5. Community composition
The results of the multivariate matrix permutation tests were highly dependent on variable order, so I re-ran them for all possible Table 2
Mixed-effects model results for meliponine abundance and species richness.
Abundance Species richness
Estimate Std. error zvalue pvalue Estimate Std. error zvalue pvalue
(Intercept) 0.272 0.411 0.662 0.508 0.498 0.230 2.168 0.030*
Forest cover 1.791 0.674 2.658 0.008** 1.091 0.355 3.071 0.002**
Plant abund 0.000 0.001 0.394 0.693 0.001 0.001 1.118 0.264
Plant spp. rich 0.036 0.038 0.972 0.331 0.020 0.021 0.965 0.335
Significance levels are: .p<0.1; *p<0.05; **p<0.01; ***p<0.001.
Fig. 2.Relationships between forest cover, floral resources, and meliponine species richness and abundance. These graphs are not constructed from the multivariate mixed-effects models, but rather show site-based averages with linear regression fit lines to show basic relationships. Solid regression lines depict significant relationships in the mixed-effects models, with dashed lines for non-significant relationships.
Table 3
Mixed-effects model results for meliponine abundance and species richness, excludingTrigona fulviventris.
Abundance Species richness
Estimate Std. error zvalue pvalue Estimate Std. error zvalue pvalue
(Intercept) 1.044 0.472 2.214 0.027* 1.185 0.305 3.886 0.000***
Forest cover 1.146 0.769 1.491 0.136 0.975 0.473 2.061 0.039*
Plant abund 0.001 0.001 0.835 0.404 0.002 0.001 1.796 0.072.
Plant spp. rich 0.073 0.043 1.694 0.090. 0.050 0.028 1.773 0.076.
order combinations (15 combinations total for all three-way, all two-way, and all one-way combinations). This order specification dependency could be due in part to correlations between variables. Forest cover and plant variables were not significantly correlated with one another, though plant abundance and species richness were significantly correlated. Plant species richness had a consistently significant relationship with meliponine community composition, in all but two of the 11 trials (univariate:F= 3.18,
p0.001). Forest cover was significant in the univariate test, in two of four bivariate tests, and in two of the six trivariate tests (univariate:F= 1.72,p<0.001). Thus, forest is likely playing some role in the relationship, but not as strongly or consistently as plant richness. Plant abundance, on the other hand, was not significant in univariate tests or any bivariate tests, and was only significant in one of the six trivariate tests. Therefore, plant abundance overall does not seem to be an important factor in shaping meliponine community composition (univariate:F= 0.48,p= 0.8). Univariate tests showed no relationship between meliponine community similarity and forest fragment size (F= 0.76, p= 0.6) or distance category (F= 0.87,p= 0.4).
4. Discussion 4.1. Overview
Meliponine species richness and abundance are strongly and positively related to the proportion of forest surrounding sample sites, but are not correlated with blooming plant density or plant species richness. Much of the effect of meliponine abundance (but not species richness) is driven by the most common species,
Trigona fulviventris. In analyses that omitted this species,
abundance was no longer significantly related to forest cover, but species richness remained significantly correlated with forest
cover. I also found species-specific responses: abundance of T.
fulviventris and Plebeia jatiformis was significantly positively
related to forest cover, while abundance ofParatrigona orniticeps
was positively related to plant species richness. None of the six other species analyzed showed any relationship with forest cover or plant resources. Meliponine community composition was most strongly related to plant species richness, weakly related to forest cover, and not related to plant abundance.
4.2. Meliponine species richness and abundance
The results related to species richness and abundance are largely consistent with the few landscape-scale studies that have considered meliponine bees.Brown and Albrecht (2001), working in Brazil, found a strong relationship between the species richness of bees in the genusMeliponaand forest cover, and further showed species-specific responses in that twoMeliponaspecies were not associated with the degree of forest cover, while another two species showed a very strong association with forest. In Indonesia, Klein et al. (2002)demonstrated a negative relationship between land-use intensity and the diversity and abundance of social bees, which there included meliponines as well as two speciesApis. In southern Costa Rica, about 100 km NW of my study region,Ricketts (2004)showed significantly greater visitation to coffee flowers by meliponine bees, as well as enhanced bee species richness, near two large forest fragments, but not near a thin riparian strip of trees. Finally, earlier work in the same landscape did not directly show effects of distance to forest (Brosi et al., 2007) or forest fragment size (Brosi et al., 2008) on meliponine species richness and abundance. Both studies, however, did show a positive relationship between forest size and proximity and the proportion of the total bee community represented by meliponines. Compar-ing the results presented here with those fromBrosi et al. (2007, Fig. 3.Relationships between forest cover, floral resources, and meliponine species richness and abundance, excludingTrigona fulviventris. These graphs are not constructed from the multivariate mixed-effects models, but rather show site-based averages, excludingTrigona fulviventris(the most common species in the samples) using linear regression fit lines to show basic relationships. Solid regression lines depict significant relationships in the mixed-effects models, with dashed lines for non-significant relationships.
2008), it is likely that in the present work, the larger true sample size (increased number of sites), as well as the larger number of samples considered within some individual sites, contributed to greater statistical power to detect trends within the meliponine community.
I did not find a relationship between meliponine species richness and abundance and the availability of floral resources within a site. While all meliponines in this region are dependent on nectar and pollen for essentially 100% of their dietary require-ments, the relationship between the species richness and abundance of plants and bees is not straightforward, for several reasons. One factor is that different plant species have distinct pollen and nectar rewards, both in terms of quantity and quality, as well as access to those rewards, through both morphological (floral structure) and chemical (nectar and pollen chemistry) means (e.g. Chittka et al., 1999). It was beyond the scope of this study to quantify these rewards, and thus by necessity I used somewhat coarse measures of the availability of floral resources. Second, meliponines and many other pollinators, by virtue of their ability to fly, have large areas over which they can forage, and quantifying the floral resource base over the entire study area, let alone over a larger area in each study site, was beyond the scope of the study. Finally, there can be lag effects in the responses of bee communities to changes in floral resources. Due to the time constraints of provisioning larval cells, larval and pupal develop-ment, and (in some species) periods of dormancy, bee abundance can take several weeks to a year to respond to increases in the floral resource base (e.g.Tepedino and Stanton, 1981).
Meliponines as a group are associated with the quantity of forest cover, as evidenced by both the results presented here as well as those reported by others, from a range of disparate and distant locations (Brazil, Brown and Albrecht, 2001; Indonesia, Klein et al., 2003a,b; and Costa Rica,Ricketts, 2004; Brosi et al., 2007, 2008). This pattern is likely driven by two primary life-history requirements: nesting and feeding needs. While melipo-nines have diverse nesting habits and substrates, many species prefer to nest in tree cavities, as is befitting of their evolution in tropical forest habitats (Roubik, 1989). In addition, while many meliponine species will forage both within tropical forests and also in deforested areas, some of the rich floral resources within forests (such as mass-blooming tropical trees) likely help support meliponine species richness and abundance. Measuring floral resources within tropical forests is logistically daunting and was beyond the scope of this study.
Another factor that could have contributed to the association between meliponines and forest is human exploitation and destruction of stingless bee nests. In many parts of the tropics, including southern Costa Rica, stingless bee honey is valued for its medicinal properties and carries a high price (Souza et al., 2006); nests are often destroyed in the process of harvesting this honey. Additionally, meliponine nest defense mechanisms (hair pulling; flying in the eyes, ears, and mouth; etc.) can present a nuisance to people (Roubik, 1989: p. 196), and nests near human habitation are often purposefully destroyed for this reason.
4.3. Community composition
The community composition of meliponines was consistently related only to the species richness of blooming plants in the sampled pastures, and consistently not related to plant abundance. There was a more complex response of forest cover, which showed inconsistent evidence of being an important factor in shaping meliponine communities. In about half of the combinations of variable order, it was significant, whereas it was not in the other half. Univariate tests on the effects of forest fragment size and proximity to forest on meliponine community composition did not
show any significant effects, as was expected given the correlation of those values with forest cover. Thus, there is only very weak evidence to support my hypothesis that meliponine species would be grouped into sets of species that are more or less resilient to deforestation.
The result that meliponine community composition is driven primarily by blooming plant diversity is somewhat surprising, given the complex relationship between bee and plant commu-nities discussed previously. One potential mechanism that could cause plant richness to structure bee communities is the interference competition and dominance hierarchy that melipo-nines display, which is thought to contribute to resource partitioning (e.g.Hubbell and Johnson, 1978). Such a mechanism may lead, for example, to an increased likelihood of some ‘‘submissive’’ bee species appearing in species-rich plant commu-nities, where they can exploit floral resources that are not being used by more dominant species.
Potts et al. (2004)showed that nectar resource diversity, which is strongly related to plant species richness, is a major factor structuring pollinator community composition. They also found a significant relationship, however, between nectar resource diver-sity and bee diverdiver-sity. Perhaps quantifying the species-specific floral rewards in the flowering plant community would strengthen the association between plants and bees, both in terms of diversity and abundance and also in terms of community composition.
4.4. Meliponine foraging behavior and land use change
Meliponine foraging strategies are extremely diverse and are a major driver of the distribution of workers of a given species on the landscape. In the context of this study, this is a particularly important point because some the strategy of some meliponine species is geared toward the efficient exploitation of ‘‘bonanza’’-type resources, by having a few scouts locate resources, followed by massive recruitment. Foraging workers of such species are not distributed evenly across the landscape, which means that my sampling strategy would not have detected such species propor-tionally to their abundance. By contrast, other meliponine species forage in a primarily or exclusively solitary manner, and others are facultative recruiters, which forage in a solitary mode but will sometimes recruit nestmates to high-density floral resources (e.g. Hubbell and Johnson, 1978; Roubik et al., 1986; Slaa et al., 2003). Foraging strategy, however, is closely tied to the distribution of flowering plant resources (Johnson and Hubbell, 1975), which is in turn strongly associated with land cover. Forested and deforested habitats contrast strongly in the spatiotemporal distributions of flowering plant resources in many places in the tropics. In particular, tropical forest trees often bloom in intense, short bursts, leading to an extremely patchy distribution of floral resources in both space and time (Bawa, 1990). By contrast, many deforested habitats, such as the rustic pastures I sampled meliponines in, have low densities of flowering herbs and shrubs, many of which are in bloom for much of the year (Brosi et al., 2007, 2008).
Thus, it is likely that meliponine species that employ a solitary or facultative-recruiting foraging style will fare better in defor-ested habitats, and forage more in those habitats, than will species that use intensive forager recruitment. For example, Trigona
fulviventris, by far the most commonly sampled bee in this study, is
a facultative recruiter that conducts a great deal of solitary foraging (Johnson and Hubbell, 1975; Hubbell and Johnson, 1978; Slaa et al., 2003). WhileT. fulviventriscan apparently efficiently utilize the low-density floral resources of the deforested habitats in the study region, its distribution is also strongly linked to forest habitat. Its need for relatively large tree cavities for nesting sites (Hubbell and Johnson, 1977) likely drives this pattern.
Considering foraging patterns in meliponines, the sampling strategy that I used is appropriate for the deforested habitats I sampled in due to their relatively consistent, low-density floral resources. At the same time, this sampling strategy would not serve well for understanding the meliponine community in tropical forest habitats with a much patchier distribution of floral resources; such a goal was beyond the scope of this study.
Foraging strategy is also a very important aspect of meliponine biology in the context of crop pollination services. Crops with mass-blooming phenology, particularly those with a short flower-ing duration, should theoretically be particularly well pollinated by meliponines with strong recruitment behavior. Coffee (Coffea
arabicaL.), a cornerstone of tropical export agriculture, fits this
exact profile with a typical blooming period of three days (e.g. Drinnan and Menzel, 1995). In a study of coffee pollination near San Isidro del General, Costa Rica (about 100 km north-northwest of my study site), Ricketts (2004) found that ten species of meliponines, along with feral honey bees (Apis mellifera) were the most common visitors to coffee flowers. While foraging-strategy data are not available for all species, of the four most common meliponines in theRicketts (2004)study, one is a solitary forager
(Plebeia frontalis, the second-most common floral visitor) and
another is a facultative group forager (T. fulviventris, the fourth most common floral visitor). Thus, while foraging recruitment can play a role in meliponine-mediated crop pollination services, it is not the only factor in determining the density of flower visitors. It is possible that land-use change, and the concomitant change in the distribution of floral resources, reduced the abundance of mass-recruiting meliponines in the San Isidro landscape.
4.5. Management recommendations
Based on my results and those of others, several recommenda-tions for landscape management to support meliponine bees and their crop pollination services can be made:
Preserve even small patches of forest near pollination-dependent crops to support meliponine nesting. I recorded meliponines in fragments as small as0.25 ha.
Consider conserving forest even when not adjacent to pollina-tion-dependent crops, for their option value if land use were to change. This is particularly important given ongoing problems with honey bee declines.
Work to re-connect isolated forest patches. While I did not find an effect of forest isolation on meliponine communities, the maximum isolation distance between forest fragments in this landscape was short, <2 km in all cases (Brosi, 2009). More extreme isolation distances may have strong impacts on meliponines, since their colonies do not swarm. Instead, workers take thousands of trips between old and new nests to transport nest material and provisions (Michener, 2000), which likely makes them particularly susceptible to habitat isolation at scales greater than normal foraging distances.
Work to reduce human destruction of meliponine nests, for honey harvests or otherwise.
Reduce use of agrochemicals; though this study did not focus on the effects of agrochemicals on meliponine bees, bees as a group are particularly susceptible to pesticides (Gross, 2008).
4.6. Future research
Future research should include the effects of species traits (such as flight range, nesting substrate, foraging and recruitment strategies, position in foraging dominance hierarchies) on meli-ponine responses to habitat loss and change. Specifically, there is little known about how foraging strategy may condition the effects
of land use change on different species of meliponines. For example, do mass-recruiting meliponines fare worse in deforested habitats? Will crop fields near very large tracts of tropical forest receive higher levels of crop pollination services, due to a greater abundance of meliponine species that exhibit mass-recruitment? In addition to questions on meliponine species traits, we also know little about the particular attributes of tropical forest that support their foraging and nesting. In particular, there is little information on how meliponines respond to re-growth forest, or how to design ecological restoration programs to support meliponine bees and the crop pollination services they provide. 5. Conclusion
Meliponine bees are important both ecologically and economic-ally, and more work is needed to both understand their complex responses to habitat change, and to conserve their populations, over the long term. Given the increasing concern over the status of pollinators worldwide, conserving this critically important group of bees in the most biodiverse habitats on Earth should be prioritized.
Acknowledgements
I thank the many Costa Rican families who allowed me to work on their land. G. Daily contributed crucial support, advice, and funding at all stages of this work. V. Gonzalez and I. Hinojosa, University of Kansas, confirmed my species determinations. L. Billadello, E. Brosi, J. DeNoyer, K. Frangioso, B. Graham, J. Ilama, F. Oviedo, and T. Shih provided superb field assistance. The staff of the Las Cruces Biological Station and the Organization for Tropical Studies (especially R. Quiro´s, E. Ramirez, and Z. Zahawi) provided cheerful field research support. G. Dura´n of Las Cruces provided key spatial data. J. Ranganathan provided GIS advice, data, and assistance. I am grateful for funding from the Anne M. and Robert T. Bass Stanford Graduate Fellowship in Science and Engineering, a Teresa Heinz Scholarship for Environmental Research, and the Moore Family Foundation; the Stanford University Field Studies and Human Biology Research Experiences for Undergraduates (HB-REX) Programs; and grants to the Center for Conservation Biology at Stanford University from the Koret, McDonnell, Sherwood, and Winslow Foundations and Peter and Helen Bing.
References
Bates, D. 2008. LME4: linear mixed-effects models using S4 classes. <http://lme4.r-forge.r-project.org/Version 0.99875-9>(accessed November 2008.
Bawa, K., 1990. Plant-pollinator interactions in tropical rain-forests. Ann. Rev. Ecol. Systemat. 21, 399–422.
Brosi, B.J., 2009. The effects of forest fragmentation on euglossine bee communities (Hymenoptera: Apidae: Euglossini). Biol. Conserv. 142, 414–423.
Brosi, B.J., Daily, G., Ehrlich, P., 2007. Bee community shifts with landscape context in a tropical countryside. Ecol. Appl. 17, 418–430.
Brosi, B.J., Daily, G.C., Shih, T.M., Oviedo, F., Duran, G., 2008. The effects of forest fragmentation on bee communities in tropical countryside. J. Appl. Ecol. 45, 773–783.
Brown, J.C., Albrecht, C., 2001. The effect of tropical deforestation on stingless bees of the genus Melipona (Insecta: Hymenoptera: Apidae: Meliponini) in central Rondonia, Brazil. J. Biogeogr. 28, 623–634.
Chemas, A., Rico-Gray, V., 1991. Apiculture and management of associated vegeta-tion by the Maya of Tixcacaltuyub, Yucatan, Mexico. Agroforestry Syst. 13, 13– 25.
Chittka, L., Thomson, J.D., Waser, N.M., 1999. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften 86, 361–377.
Clement, R., Horn, S., 2001. Pre-Columbian land-use history in Costa Rica: a 3000-year record of forest clearance, agriculture and fires from Laguna Zoncho. Holocene 11, 419–426.
Cortopassi-Laurino, M., Imperatriz-Fonseca, V.L., Roubik, D.W., Dollin, A., Heard, T., Aguilar, I., Venturieri, G.C., Eardley, C., Nogueira-Neto, P., 2006. Global melipo-niculture: challenges and opportunities. Apidologie 37, 275–292.
Crawley, M.J., 2002. Statistical computing: an introduction to data analysis using S-Plus. Wiley, Chichester, West Sussex, England; New York.
Drinnan, J.E., Menzel, C.M., 1995. Temperature affects vegetative growth and flowering of coffee (Coffea-arabica L.). J. Horticult. Sci. 70, 25–34.
Efron, B., 1981. Nonparametric estimates of standard error—the jackknife, the bootstrap and other methods. Biometrika 68, 589–599.
Ghazoul, J., 2005. Buzziness as usual? Questioning the global pollination crisis. Trends Ecol. Evol. 20, 367–373.
Gross, M., 2008. Pesticides linked to bee deaths. Curr. Biol. 18, R684.
Heard, T.A., 1999. The role of stingless bees in crop pollination. Ann. Rev. Entomol. 44, 183–206.
Hubbell, S.P., Johnson, L.K., 1977. Competition and nest spacing in a tropical stingless bee community. Ecology 58, 949–963.
Hubbell, S.P., Johnson, L.K., 1978. Comparative foraging behavior of 6 stingless bee species exploiting a standardized resource. Ecology 59, 1123–1136. Johnson, L.K., Hubbell, S.P., 1975. Contrasting foraging strategies and coexistence of
2 bee species on a single resource. Ecology 56, 1398–1406.
Kearns, C.A., Inouye, D.W., Waser, N.M., 1998. Endangered mutualisms: the con-servation of plant–pollinator interactions. Ann. Rev. Ecol. Systemat. 29, 83–112. Klein, A., Steffan-Dewenter, I., Buchori, D., Tscharntke, T., 2002. Effects of land-use intensity in tropical agroforestry systems on coffee flower-visiting and trap-nesting bees and wasps. Conserv. Biol. 16, 1003–1014.
Klein, A., Steffan-Dewenter, I., Tscharntke, T., 2003a. Bee pollination and fruit set of Coffea arabica and C-canephora (Rubiaceae). Am. J. Bot. 90, 153–157. Klein, A., Steffan-Dewenter, I., Tscharntke, T., 2003b. Fruit set of highland coffee
increases with the diversity of pollinating bees. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 270, 955–961.
Klein, A.M., Vaissiere, B.E., Cane, J.H., Steffan-Dewenter, I., Cunningham, S.A., Kre-men, C., Tscharntke, T., 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. Biol. Sci. Ser. B 274, 303–313.
Michener, C.D., 2000. The bees of the world. Johns Hopkins University Press, Baltimore, Md.
Oksanen, J., Kindt, R., Legendre, P., O’Hara, R., 2006. Vegan: Community Ecology Package.http://cc.oulu.fi/jarioksa/, p. Ordination methods and other useful functions for community and vegetation ecologists.
Oldroyd, B.P., 2007. What’s killing American honey bees? PLoS Biol. 5, 1195–1199. Olofsson, J., Shams, H., 2007. Determinants of plant species richness in an alpine
meadow. J. Ecol. 95, 916–925.
Potts, S.G., Vulliamy, B., Roberts, S., O’Toole, C., Dafni, A., Ne’eman, G., Willmer, P.G., 2004. Nectar resource diversity organises flower–visitor community structure. Entomol. Exp. Appl. 113, 103–107.
Ricketts, T.H., 2004. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conserv. Biol. 18, 1262–1271.
Ricketts, T., Daily, G., Ehrlich, P., Michener, C., 2004a. Economic value of tropical forest to coffee production. Proc. Natl. Acad. Sci. U.S.A. 101, 12579–12582. Ricketts, T.H., Daily, G.C., Ehrlich, P.R., Michener, C.D., 2004b. Economic value of
tropical forest to coffee production. Proc. Natl. Acad. Sci. U.S.A. 101, 12579– 12582.
Roubik, D.W., 1989. Ecology and Natural History of Tropical Bees. Cambridge University Press, Cambridge, New York.
Roubik, D.W., 1992. Stingless bees. A guide to Panamanian and Mesoamerican species and their nests (Hymenoptera:Apidae:Meliponinae. In: Quintero, D., Aiello, A. (Eds.), Insects of Panama and Mesoamerica—Selected Studies. Oxford University Press, Oxford, UK, pp. 495–524.
Roubik, D.W., Moreno, J.E., Vergara, C., Wittmann, D., 1986. Sporadic food competi-tion with the African honey bee: projected impact on Neotropical social bees. J. Trop. Ecol. 2, 97–111.
Sala, O.E., Chapin, F.S., Armesto, J.J., Berlow, E., Bloomfield, J., Dirzo, R., Huber-Sanwald, E., Huenneke, L.F., Jackson, R.B., Kinzig, A., Leemans, R., Lodge, D.M., Mooney, H.A., Oesterheld, M., Poff, N.L., Sykes, M.T., Walker, B.H., Walker, M., Wall, D.H., 2000. Biodiversity—global biodiversity scenarios for the year 2100. Science 287, 1770–1774.
Schneider, S.S., Hoffman, G.D., Smith, D.R., 2004. The African honey bee: factors contributing to a successful biological invasion. Annu. Rev. Entomol. 49, 351– 376.
Slaa, E.J., Wassenberg, J., Biesmeijer, J.C., 2003. The use of field-based social information in eusocial foragers: local enhancement among nestmates and heterospecifics in stingless bees. Ecol. Entomol. 28, 369–379.
Slaa, E.J., Sanchez Chaves, L.A., Malagodi-Braga, K.S., Hofstede, F.E., 2006. Stingless bees in applied pollination: practice and perspectives. Apidologie 37, 293– 315.
Souza, B., Roubik, D., Barth, O., Heard, T., Enriquez, E., Carvalho, C., Villas-Boas, J., Marchini, L., Locatelli, J., Persano-Oddo, L., Almeida-Muradian, L., Bogdanov, S., Vit, P., 2006. Composition of stingless bee honey: setting quality standards. Interciencia 31, 867–875.
Steffan-Dewenter, I., Potts, S.G., Packer, L., 2005. Pollinator diversity and crop pollination services are at risk. Trends Ecol. Evol. 20, 651–652.
R Development Core Team, R.D.C., 2006. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Tepedino, V.J., Stanton, N.L., 1981. Diversity and competition in bee-plant
commu-nities on short-grass prairie. Oikos 36, 35–44.
Vamosi, J.C., Knight, T.M., Steets, J.A., Mazer, S.J., Burd, M., Ashman, T.L., 2006. Pollination decays in biodiversity hotspots. Proc. Natl. Acad. Sci. U.S.A. 103, 956–961.