and Resistance to Caspofungin among 3,764 Clinical Isolates of
Candida
by Use of CLSI Methods and Interpretive Criteria
Michael A. Pfaller,a,bShawn A. Messer,aDaniel J. Diekema,bRonald N. Jones,aMariana Castanheiraa
‹JMI Laboratories, North Liberty, Iowa, USAa
; University of Iowa, Iowa City, Iowa, USAb
Due to unacceptably high interlaboratory variation in caspofungin MIC values, we evaluated the use of micafungin as a
surro-gate marker to predict the susceptibility of
Candida
spp. to caspofungin using reference methods and species-specific
interpre-tive criteria. The MIC results for 3,764 strains of
Candida
(eight species), including 73 strains with
fks
mutations, were used.
Caspofungin MIC values and species-specific interpretive criteria were compared with those of micafungin to determine the
per-cent categorical agreement (%CA) and very major error (VME), major error (ME), and minor error rates as well as their ability
to detect
fks
mutant strains of
Candida albicans
(11 mutants),
Candida tropicalis
(4 mutants),
Candida krusei
(3 mutants), and
Candida glabrata
(55 mutants). Overall, the %CA was 98.8% (0.2% VMEs and MEs, 0.8% minor errors) using micafungin as the
surrogate marker. Among the 60 isolates of
C. albicans
(9 isolates),
C. tropicalis
(5 isolates),
C. krusei
(2 isolates), and
C.
glabrata
(44 isolates) that were nonsusceptible (either intermediate or resistant) to both caspofungin and micafungin, 54
(90.0%) contained a mutation in
fks1
or
fks2
. An additional 10
C. glabrata
mutants, two
C. albicans
mutants, and one mutant
each of
C. tropicalis
and
C. krusei
were classified as susceptible to both antifungal agents. Using the epidemiological cutoff
val-ues (ECVs) of 0.12
g/ml for caspofungin and 0.03
g/ml for micafungin to differentiate wild-type (WT) from non-WT strains
of
C. glabrata
, 80% of the
C. glabrata
mutants were non-WT for both agents (96% concordance). Micafungin may serve as an
acceptable surrogate marker for the prediction of susceptibility and resistance of
Candida
to caspofungin.
T
he echinocandins caspofungin and micafungin are now well
established as first-line agents for the treatment of candidemia
and other forms of invasive candidiasis (IC) (1–3). The
in vitro
activities of caspofungin and micafungin against
Candida
spp.
have been documented using broth dilution, agar disk diffusion,
and Etest methods (4–8), and most recently, clinically relevant
interpretive breakpoints for broth microdilution (BMD) MIC
testing of
Candida
spp. were established by the Clinical and
Lab-oratory Standards Institute (CLSI) (9). Whereas the CLSI has
de-veloped clinical breakpoints (CBPs) for anidulafungin,
caspofun-gin, and micafungin against the six most common species of
Candida
(9), the European Committee on Antimicrobial
Suscep-tibility Testing (EUCAST) has elected to establish CBPs for
anidu-lafungin and micafungin but not for caspofungin (10).
Further-more, the EUCAST does not currently recommend caspofungin
MIC testing for clinical decision making due to unacceptably high
variation among the caspofungin MIC values obtained from
dif-ferent centers (4,
5,
11,
12). This variation is evident not only using
the EUCAST BMD method (4,
11) but also using the CLSI method
(13). A recent CLSI analysis of the MIC distributions from 16
different laboratories showed variation in the caspofungin
wild-type (WT) modal MIC values of as much as five doubling dilutions
(e.g., 0.015 to 0.5
g/ml) (13). In contrast, the variation in
mica-fungin WT modal MIC values was within
⫾
1 doubling dilution
step for various species (M. A. Pfaller et al., unpublished data).
The reasons for such variation in the caspofungin MIC values
from center to center remain unclear but may involve the
lot-to-lot variation in the potency of caspofungin powders, the use of
dimethyl sulfoxide (DMSO) versus water as a solvent, storage
con-ditions, or the determined MIC endpoints (4,
11–13).
Previously, we demonstrated that
in vitro
cross-resistance
be-tween micafungin and caspofungin does exist among clinical
iso-lates of
Candida
(9,
14) (M. A. Pfaller et al., unpublished data).
The clinical relevance of this cross-resistance has been
docu-mented in studies of the resistance mechanisms (detection of
fks
mutations) (15–17), in animal models of IC (18–21), and in case
series where clinically significant resistance to one or more
echi-nocandins, marked by the acquisition of a resistance mutation in
the
fks
gene, has been reported in immunocompromised patients
(e.g., those in intensive care, stem cell transplant patients, and
solid organ transplant patients) with a high level of prior
echino-candin exposure (11,
22–27). Although many of the resistant
iso-lates and cases of breakthrough IC were due to
Candida glabrata
, a
number of
Candida albicans
,
Candida tropicalis
,
Candida krusei
,
and
Candida lusitaniae
isolates have also been observed to have
reduced susceptibility or resistance to both micafungin and
caspo-fungin (17,
22–24,
26,
28,
29). It should be noted that isolates of
C.
glabrata
with paradoxical reduced caspofungin susceptibility but
increased micafungin susceptibility have been reported (30,
31).
Although the clinical importance of such differential
susceptibil-ities to the echinocandins is not clear, two recent studies found
such differences to be meaningful in murine models of IC,
irre-spective of the presence or absence of
fks
mutations (19,
21).
Despite the fact that CBPs for caspofungin and
Candida
spp.
Received6 September 2013Returned for modification9 October 2013 Accepted18 October 2013
Published ahead of print23 October 2013
Editor:D. W. Warnock
Address correspondence to Michael A. Pfaller, michael-pfaller@jmilabs.com.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
doi:10.1128/JCM.02481-13
on May 16, 2020 by guest
http://jcm.asm.org/
were established by the CLSI (9), the extreme intra- and
interlabo-ratory variations in caspofungin MIC results are of great concern
(4,
11,
13). The fact that micafungin MIC values appear to be
much less variable from laboratory to laboratory (M. A. Pfaller et
al., unpublished data) suggests that this echinocandin may be the
preferred reagent for the
in vitro
testing of echinocandins against
Candida
. Previously, we have shown that fluconazole may serve as
a surrogate marker for evaluating the susceptibility of
Candida
to
ravuconazole (32), voriconazole (33), and posaconazole (34).
This same approach should be applicable to caspofungin, while
the more reliable test reagent, micafungin, may be applied for the
prediction of susceptibility and resistance to these echinocandins.
In the present study, we utilized a large database of
susceptibil-ity test results, all determined by CLSI BMD methods and that
included results for 73
fks
mutant strains, to provide a robust
analysis of cross-resistance between the two agents and
addition-ally to examine the usefulness of micafungin as a surrogate marker
for evaluating caspofungin susceptibility and resistance among
WT
Candida
spp.
MATERIALS AND METHODS
Organisms.We tested a total of 3,764 clinical isolates ofCandidaspp. obtained from⬎100 medical centers worldwide (8,9,34). The collection included 2,010 isolates ofC. albicans, 566 isolates ofC. glabrata, 539 iso-lates ofCandida parapsilosis, 426 isolates ofC. tropicalis, 105 isolates ofC. krusei, 52 isolates ofCandida guilliermondii, 42 isolates ofC. lusitaniae, and 24 isolates ofCandida kefyr. All were incident isolates from individual patients and were obtained from blood or other normally sterile body fluids. Among the included isolates ofC. albicans,C. glabrata,C. tropicalis, andC. kruseiwere 73 isolates (11C. albicans, 4C. tropicalis, 3C. krusei, and 55C. glabrata) with documentedfksresistance mutations. The isolates were identified by use of the Vitek and API yeast identification systems (bioMérieux, Inc., Hazelwood, MO, USA) supplemented with conven-tional methods as needed (35). The isolates were stored as water suspen-sions until use. Prior to testing, each isolate was passaged at least twice on potato dextrose agar (Remel, Lenexa, KS, USA) and CHROMagar
Candida(Becton, Dickinson, Sparks, MD, USA) to ensure purity and viability. The presence or absence of a mutation in the hot spot (HS) regions offks1andfks2(C. glabrataonly) was determined as described previously (36,37).
Antifungal susceptibility testing.All isolates were tested forin vitro
susceptibility to caspofungin and micafungin using CLSI BMD methods (38,39). The MIC results for the agents were read following 24 h of incu-bation. In all instances, the MIC values were determined visually as the lowest concentration of the drug that caused significant growth diminu-tion levels (38,39).
We used the recently revised CBPs to identify the strains of the 6 most common species ofCandida(C. albicans,C. glabrata,C. parapsilosis,C. tropicalis,C. krusei, andC. guilliermondii) that were susceptible (S), inter-mediate (I), or resistant (R) to caspofungin and micafungin (40,41): caspofungin and micafungin MIC values ofⱕ0.25g/ml, 0.5g/ml, and
ⱖ1g/ml were considered to be susceptible, intermediate, and resistant, respectively, forC. albicans,C. tropicalis, andC. krusei, and MIC results of
ⱕ2g/ml, 4g/ml, andⱖ8g/ml were categorized as susceptible, inter-mediate, and resistant, respectively, forC. parapsilosisandC. guilliermon-dii; caspofungin MIC values ofⱕ0.12g/ml, 0.25g/ml, andⱖ0.5g/ml and micafungin MIC values ofⱕ0.06 g/ml, 0.12g/ml, andⱖ0.25
g/ml were considered to be susceptible, intermediate, and resistant, re-spectively, forC. glabrata. In addition to the CBPs for these species, epi-demiological cutoff values (ECVs) were established in order to provide a sensitive means of separating WT from non-WT strains (those that pos-sess an intrinsic or acquired resistance mutation). The ECVs for caspo-fungin and micacaspo-fungin for each species are 0.12g/ml and 0.03g/ml for bothC. albicansandC. glabrata, 1g/ml and 4g/ml forC. parapsilosis,
0.12g/ml and 0.12g/ml forC. tropicalis, 0.25g/ml and 0.12g/ml for
C. krusei, and 2g/ml and 2g/ml forC. guilliermondii, respectively (41). CBPs have not been established for caspofungin and micafungin and less common species, such asC. lusitaniaeandC. kefyr. The caspofungin and micafungin ECVs for these two species are 1g/ml and 0.5g/ml forC. lusitaniaeand 0.03 and 0.12g/ml forC. kefyr, respectively (41).Candida
spp. isolates for which caspofungin or micafungin MICs exceed the ECVs are considered to be non-WT and may harbor acquired mutations in the
fksgenes (17).
Quality control was performed as recommended in CLSI documents M27-A3 (39) and M27-S4 (38) usingC. kruseistrain ATCC 6258 andC. parapsilosisstrain ATCC 22019.
Analysis of results.All MIC results (ing/ml) for micafungin were directly compared with those for caspofungin by regression statistics and by scattergram (data not shown). The error rate bounding method to minimize intermethod interpretive error was also applied using the inter-pretive criteria described above. The acceptable error rate limits used in this comparison were those cited in CLSI document M23-A3 (40,42) and in other studies (43,44).
The definitions of the errors used in this analysis are as follows: a very major error (VME), or a false-susceptible error, was a susceptible result for the surrogate marker micafungin and a resistant result for caspofun-gin; a major error (ME), or a false-resistant error, was a resistant result for micafungin and a susceptible result for caspofungin; and minor errors occurred when the result for one of the agents was susceptible or resistant and that for the other agent was intermediate. In general, for an agent to be considered a reliable surrogate marker, the VME rate should beⱕ1.5% of all results, and the absolute categorical agreement (CA) between methods should beⱖ90% (33,40,42). In addition to the above analysis, we will also examine the detectability offksmutants ofC. albicansandC. glabrata
using the CBPs and ECVs for each echinocandin.
RESULTS AND DISCUSSION
Table 1
depicts the MIC distribution profiles for micafungin and
caspofungin determined for 3,764 strains of
Candida
spp. using
BMD methods validated by the CLSI (39). Overall, 3,694 isolates
(98.2%) were susceptible, 20 isolates (0.5%) were intermediate,
and 50 isolates (1.3%) were categorized as resistant to micafungin.
Similarly, 3,682 isolates (97.8%) were susceptible, 20 isolates
(0.5%) were intermediate, and 62 isolates (1.7%) were resistant to
caspofungin. The modal MIC for micafungin was 0.015
g/ml
(1,323 results [35.1%]) compared to 0.03
g/ml (1,362 results
[36.2%]) for caspofungin. There was a strong positive correlation
(
r
⫽
0.84) between the micafungin and caspofungin MIC values
(data not shown). Overall, the essential agreement (MIC
⫾
2
dilu-tions) was 97.2%. Decreased potencies of both micafungin and
caspofungin were observed among
C. parapsilosis
(modal MICs, 1
g/ml and 0.5
g/ml, respectively) and
C. guilliermondii
(modal
MICs, 0.25
g/ml and 0.5
g/ml, respectively). The highest rates
of resistance to both agents were observed with
C. glabrata
: 5.8%
were resistant to micafungin and 7.9% were resistant to
caspofun-gin. Among the 33 isolates of
C. glabrata
that were resistant to
micafungin, 30 isolates (90.9%) possessed a mutation in
fks1
or
fks2
, and among 45 isolates that were resistant to caspofungin, 40
(88.9%) possessed a mutation in the
fks
gene (Table 1).
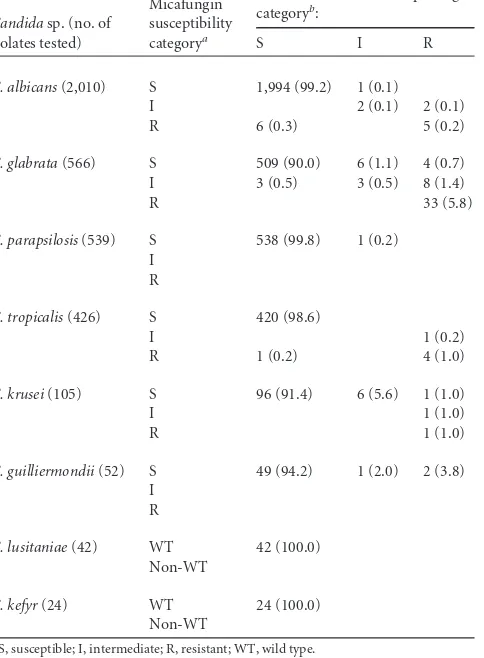
The extent of cross-resistance between micafungin and
caspo-fungin can be seen more clearly in
Table 2. For the 3,694 isolates
that were susceptible to micafungin, 3,672 (99.4%) were also
ceptible to caspofungin. There were seven isolates that were
sus-ceptible to micafungin and resistant to caspofungin; of those, four
isolates were
C. glabrata
, two isolates were
C. guilliermondii
, and
one isolate was
C. krusei
, and two of the four
C. glabrata
isolates
contained an
fks
mutation. Among the 50 isolates that were
on May 16, 2020 by guest
http://jcm.asm.org/
tant to micafungin, 43 isolates (86.0%) were also resistant and
seven isolates (14.0%) were susceptible to caspofungin. Similarly,
17 of the 20 (85.0%) isolates categorized as intermediate to
mica-fungin were either intermediate or resistant to caspomica-fungin. Thus,
99.4% of the micafungin-susceptible and 85.7% of the
micafun-gin-nonsusceptible (NS) (I plus R) isolates were susceptible and
nonsusceptible, respectively, to caspofungin.
When the micafungin test result category (S, I, or R) was used
to predict the caspofungin category, the absolute categorical
agreement (CA) between the test results was 98.8%, with only a
0.2% VME (false-susceptible error) and ME (false-resistant error)
rate and a 0.8% minor error rate (Table 3). Among the eight
spe-cies of
Candida
tested, the CA was
ⱖ92% (range, 92.4% to
100.0%) for all species. Generally, the discrepancies in the
cate-gorical results were minor errors. VMEs were seen more often
with
C. glabrata
,
C. krusei
, and
C. guilliermondii
; however, only the
VME rate involving
C. guilliermondii
(3.8%) exceeded the
allow-able VME rate of
ⱕ
1.5% (Table 3) (40,
42,
43).
Clearly, it is important to detect those isolates of
Candida
that
possess an acquired mutation in the
fks
gene (9,
17), and in that
regard, micafungin performs quite well as a surrogate marker.
Among all 73
fks
mutant strains, 14 (19.2%) were susceptible to
both caspofungin and micafungin, and 54 (74.0%) were
interme-diate or resistant to both, for an overall concordance of 93.2%.
Furthermore, for the 11 isolates of
C. albicans
with a mutation in
fks1
, nine isolates (82.0%) were either intermediate or resistant to
both micafungin and caspofungin and two isolates were
suscepti-ble to both agents (Tasuscepti-bles 1
and
4). Using the micafungin ECV for
C. albicans
(0.03
g/ml), all 11 isolates would be classified as
non-WT, indicating that they were likely to contain an acquired
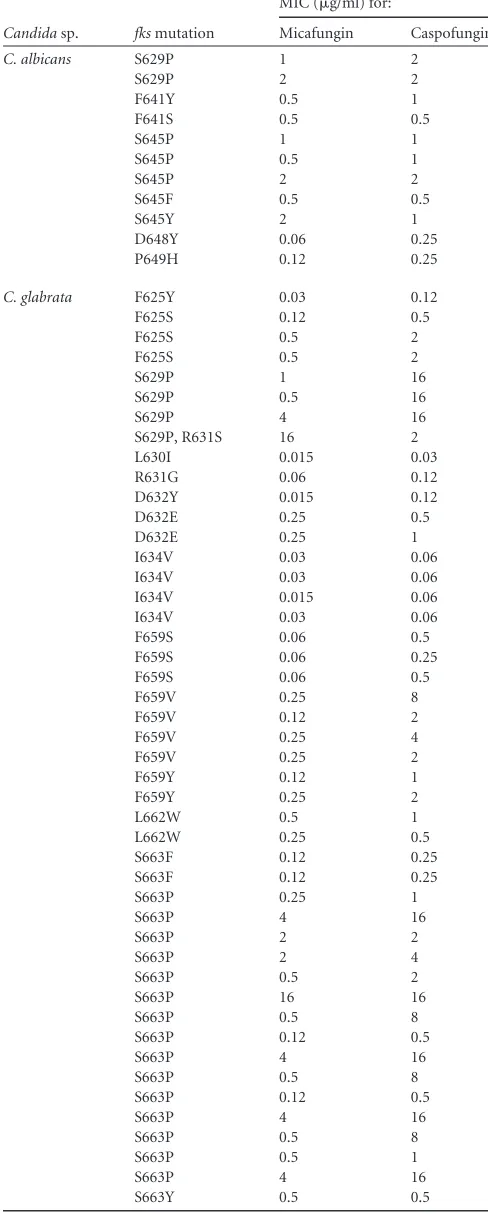
resis-tance mutation. There were a total of 55 isolates of
C. glabrata
that
contained a mutation in
fks1
or
fks2
(Tables 1
and
4). Of these, 10
isolates (18.2%) were susceptible to both micafungin and
caspo-TABLE 1MIC distributions of caspofungin and micafungin versusCandidaspp., including strains withfksmutations using CLSI methods
Candidasp. (no. of
isolates tested) Echinocandins
No. of isolates with an MIC (g/ml) (no. of isolates withfksmutation) of:
0.007 0.015 0.03 0.06 0.12 0.25 0.5 1 2 4 ⱖ8
C. albicans(2,010) Caspofungin 33 539 892 502 22 12 (2) 3 (2) 4 (4) 3 (3) Micafungin 177 1,357 375 80 (1) 5 (1) 1 4 (4) 8 (2) 3 (3)
C. glabrata(566) Caspofungin 34 273 (1) 189 (6) 16 (3) 9 (5) 14 (11) 7 (6) 8 (8) 2 (2) 14 (13) Micafungin 29 437 (3) 39 (7) 14 (5) 14 (10) 11 (9) 10 (10) 2 (1) 3 (3) 5 (5) 2 (2)
C. parapsilosis(539) Caspofungin 1 1 1 17 35 208 219 49 7 1
Micafungin 1 2 25 93 285 133
C. tropicalis(426) Caspofungin 4 137 191 82 3 (1) 4 2 (1) 2 (2) 1 Micafungin 7 126 169 101 15 (1) 2 1 5 (3)
C. krusei(105) Caspofungin 1 44 32 19 (1) 6 2 (1) 1 (1) Micafungin 3 9 75 (1) 15 1 1 (1) 1 (1)
C. guilliermondii(52) Caspofungin 1 2 4 13 22 7 1 2
Micafungin 1 2 3 4 23 18 1
C. lusitaniae(42) Caspofungin 1 1 20 18 2
Micafungin 2 25 13 2
C. kefyr(24) Caspofungin 3 20 1
[image:3.585.42.544.77.334.2]Micafungin 9 15
TABLE 2Use of micafungin to predict susceptibility patterns of caspofungin employing 3,764 clinical isolates ofCandidaspp. from a global surveillance program
Candidasp. (no. of isolates tested)
Micafungin susceptibility categorya
No. (%) of isolates in caspofungin categoryb:
S I R
C. albicans(2,010) S 1,994 (99.2) 1 (0.1)
I 2 (0.1) 2 (0.1) R 6 (0.3) 5 (0.2)
C. glabrata(566) S 509 (90.0) 6 (1.1) 4 (0.7) I 3 (0.5) 3 (0.5) 8 (1.4)
R 33 (5.8)
C. parapsilosis(539) S 538 (99.8) 1 (0.2) I
R
C. tropicalis(426) S 420 (98.6)
I 1 (0.2)
R 1 (0.2) 4 (1.0)
C. krusei(105) S 96 (91.4) 6 (5.6) 1 (1.0)
I 1 (1.0)
R 1 (1.0)
C. guilliermondii(52) S 49 (94.2) 1 (2.0) 2 (3.8) I
R
C. lusitaniae(42) WT 42 (100.0) Non-WT
C. kefyr(24) WT 24 (100.0) Non-WT
a
S, susceptible; I, intermediate; R, resistant; WT, wild type.
bMIC interpretive criteria for each species as shown in reference41.
on May 16, 2020 by guest
http://jcm.asm.org/
[image:3.585.299.541.376.705.2]fungin, 5 isolates (9.1%) were susceptible to micafungin and
ei-ther intermediate or resistant to caspofungin, and 40 (72.7%)
were intermediate or resistant to both agents (Table 4). The
over-all concordance between the two methods (testing of micafungin
versus caspofungin) using the CLSI CBPs to classify
fks
mutant
strains of
C. glabrata
as susceptible or nonsusceptible was 90.9%.
Using the ECVs of 0.03
g/ml for micafungin and 0.12
g/ml for
caspofungin to differentiate WT from non-WT strains of
C.
glabrata
, nine strains (16.4%) were WT and 44 strains (80.0%)
were non-WT for both agents (overall concordance, 96.4%).
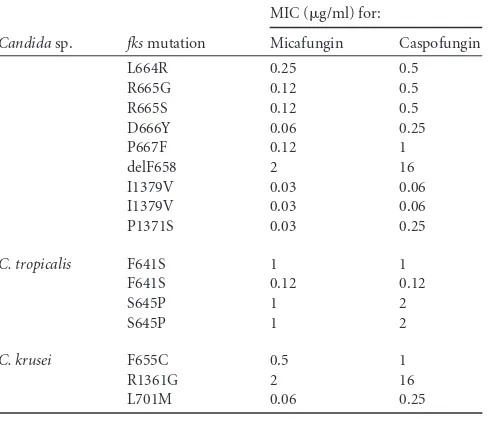
There were four strains of
C. tropicalis
and three of
C. krusei
that harbored an
fks
mutation. Of these, 3 strains of
C. tropicalis
(75.0%) and 2 strains of
C. krusei
(66.7%) were intermediate or
resistant to both agents, whereas one strain of each species was
susceptible to both agents. All of these would be considered
non-WT using the micafungin ECV of 0.12
g/ml for each
spe-cies.
[image:4.585.297.541.93.700.2]The most frequently encountered mutations in this collection
occurred at position S663 (18 isolates), followed by F659 (nine
isolates), S645 (seven isolates), and S629 (six isolates), and four
isolates each contained mutations at positions F625, F641, and
I634 (Table 4). Previous reports indicate that isolates of
C.
glabrata
with the S663F mutation respond
in vivo
to high doses of
either micafungin or caspofungin, whereas isolates with the S629P
mutation fail to respond to even the highest dose of either agent
(21). Notably, the pharmacodynamic target for the isolate with the
S663F mutation was below the human area under the
concentra-tion curve (AUC) using standard doses of micafungin but not
caspofungin, suggesting that differences in the echinocandin MIC
values observed with individual
fks
mutations may be associated
with differential antifungal activity
in vivo
(21). These findings are
supported by those of Spreghini et al. (19), who found in a
com-parison between micafungin, caspofungin, and anidulafungin
that micafungin showed the best
in vitro
and
in vivo
activities
against two resistant mutants of
C. glabrata
bearing specific
mu-tations in the
fks2
HS region. Mutations at positions S663 and
F659 in
C. glabrata
have been associated with breakthrough
infec-tions in patients receiving echinocandin therapy (22,
23,
27),
whereas patients infected with
C. glabrata
strains containing the
I1379V and I634V mutations (i.e., susceptible to both micafungin
and caspofungin) tended to respond to therapy with either agent
TABLE 3Absolute categorical agreement and error rates when the micafungin results were used to predict the caspofungin susceptibility of
Candidaspp.
Candidasp. (no. of isolates tested)
% ofa:
CA VME ME
Minor errors
C. albicans(2,010) 99.6 0.0 0.2 0.2
C. glabrata(566) 96.3 0.7 0.0 3.0
C. parapsilosis(539) 99.8 0.0 0.0 0.2
C. tropicalis(426) 99.6 0.0 0.2 0.2
C. krusei(105) 92.4 1.0 0.0 6.6
C. guilliermondii(52) 94.2 3.8 0.0 2.0
C. lusitaniae(42) 100.0 0.0 0.0 0.0
C. kefyr(24) 100.0 0.0 0.0 0.0 AllCandidaspp. (3,764) 98.8 0.2 0.2 0.8 aCA, categorical agreement; VME, very major errors; ME, major errors.
TABLE 4Isolates ofC. albicans,C. glabrata,C. tropicalis, andC. krusei
harboringfksmutations
Candidasp. fksmutation
MIC (g/ml) for:
Micafungin Caspofungin
C. albicans S629P 1 2
S629P 2 2
F641Y 0.5 1
F641S 0.5 0.5
S645P 1 1
S645P 0.5 1
S645P 2 2
S645F 0.5 0.5
S645Y 2 1
D648Y 0.06 0.25 P649H 0.12 0.25
C. glabrata F625Y 0.03 0.12 F625S 0.12 0.5
F625S 0.5 2
F625S 0.5 2
S629P 1 16
S629P 0.5 16
S629P 4 16
S629P, R631S 16 2 L630I 0.015 0.03 R631G 0.06 0.12 D632Y 0.015 0.12 D632E 0.25 0.5
D632E 0.25 1
I634V 0.03 0.06 I634V 0.03 0.06 I634V 0.015 0.06 I634V 0.03 0.06 F659S 0.06 0.5 F659S 0.06 0.25 F659S 0.06 0.5
F659V 0.25 8
F659V 0.12 2
F659V 0.25 4
F659V 0.25 2
F659Y 0.12 1
F659Y 0.25 2
L662W 0.5 1
L662W 0.25 0.5 S663F 0.12 0.25 S663F 0.12 0.25
S663P 0.25 1
S663P 4 16
S663P 2 2
S663P 2 4
S663P 0.5 2
S663P 16 16
S663P 0.5 8
S663P 0.12 0.5
S663P 4 16
S663P 0.5 8
S663P 0.12 0.5
S663P 4 16
S663P 0.5 8
S663P 0.5 1
S663P 4 16
S663Y 0.5 0.5
(Continued on following page)
on May 16, 2020 by guest
http://jcm.asm.org/
[image:4.585.41.290.96.237.2](22). Regarding the
C. albicans
mutants in this study, three of the
11
fks
mutants contained the S645P mutation, a genotype that
cannot be treated with conventional doses of micafungin or
caspofungin (45). Taken together, these results suggest a linkage
between the increased echinocandin MIC results, specific
fks
mu-tations, and the potential for a successful clinical outcome (19,
21,
22,
27).
Previously, we conducted similar analyses using fluconazole
results to predict the susceptibilities of
Candida
spp. to the newer
triazoles as a proof of concept regarding the use of surrogate
mark-ers or class representatives for antifungal susceptibility testing
(32–34). This strategy has been used for decades in antibacterial
susceptibility testing to develop practical alternatives for the
mi-crobiology laboratory when specific diagnostic susceptibility
re-agents produce unreliable results or are limited or unavailable (40,
44). Given the issue of unacceptable variability in caspofungin
MIC results, we concur with the recommendations of the
EUCAST that the caspofungin MIC results for
Candida
spp.
should not be used for clinical decision making at this time (10).
As shown herein, MIC testing with micafungin is highly predictive
of caspofungin categorical results, and it reliably detects clinically
important
fks
mutations.
In addition to providing a strategy for predicting caspofungin
susceptibility and resistance among
Candida
spp., these results
provide strong support for concerns regarding the issue of
cross-resistance between micafungin and caspofungin (5,
15,
16,
18,
22,
26). By using an extensive global collection of clinically important
isolates, including
fks
mutant strains, we validate concerns
origi-nating from single-center case series and rightly focus attention on
C. glabrata
as the species most likely to demonstrate
cross-resis-tance between these two studied echinocandins.
Micafungin functioned well as a surrogate marker for
caspo-fungin susceptibility when applied to this large collection of
clin-ically significant isolates of
Candida
spp. The absolute CA of
98.8%, with only 0.2% VMEs among the 3,764 isolates tested,
easily meets the recognized criteria for a reliable surrogate marker
in antibacterial susceptibility testing (40,
44). The excellent
con-cordance between the micafungin and caspofungin results in
cat-egorizing the
fks
mutants also provides further validation of this
approach. The major limitation of this study, given the
interlabo-ratory variability of caspofungin MICs, is the fact that the data
were obtained from only two laboratories. This may lead to
con-cerns regarding the generalizability of the findings to other
labo-ratories. These concerns are somewhat mitigated by the inclusion
of a large number of
fks
mutants in the study.
In conclusion, we have demonstrated the existence of
cross-resistance between micafungin and caspofungin, with the greatest
emphasis on
C. glabrata
. Furthermore, we have shown that in the
face of the unreliable caspofungin MIC results documented
else-where, a prediction of caspofungin susceptibility in a medical
cen-ter currently performing antifungal susceptibility testing of
mica-fungin can be accomplished by using the micamica-fungin result as a
surrogate marker for caspofungin susceptibility and resistance.
Arguably, the most important role of
in vitro
susceptibility testing
is to predict the resistance of the infecting organism to the agent
under consideration for use in a patient (46). The occurrence of
false-resistant and false-susceptible errors with this application of
the class representative concept to the echinocandin antifungal
was low and was considered very acceptable for use as a surrogate
marker. The excellent CA documented in this study was further
supported by the high level of concordance in identifying strains
of
Candida
with clinically important
fks
resistance mutations.
Fur-ther efforts to clarify and correct the issues of caspofungin testing
using the CLSI and EUCAST BMD methods should be a priority
for future research.
ACKNOWLEDGMENTS
The global antifungal surveillance programs that served as the source of data used in the development of the manuscript were supported in part by Pfizer, Inc., and Astellas.
We acknowledge the excellent technical assistance of S. Benning in the preparation of the manuscript.
JMI Laboratories, Inc., received research and educational grants in 2011 to 2013 from AIReS, the American Proficiency Institute (API), Ana-cor, Astellas, AstraZeneca, Bayer, bioMérieux, Cempra, Cerexa, Contra-Fect, Cubist, Dipexium, Furiex, GlaxoSmithKline, Johnson & Johnson (J&J), LegoChem Biosciences, Inc., Meiji Seika Kaisha, Merck, Nabriva, Novartis, Pfizer, PPD Therapeutics, Premier Research Group, Rempex, Rib-X Pharmaceuticals, Seachaid, Shionogi, The Medicines Company, Theravance, and Thermo Fisher Scientific. Some JMI employees are ad-visors and/or consultants for Astellas, Cubist, Pfizer, Cempra, Cerexa-Forest, J&J, and Theravance. These authors have no conflicts of interest with regard to speakers’ bureaus and stock options. D. Diekema has re-ceived research funding from Cerexa, bioMérieux, Pfizer, T2Biosystems, and PurThread, Inc. D. Diekema has no conflicts of interest with regard to speakers’ bureaus or consultant and/or advisor work.
REFERENCES
1.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortho-lary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Flörl C, Petrikkos G, Rich-ardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group.2012. ESCMID guideline for the diagno-sis and management ofCandidadiseases 2012: non-neutropenic adult patients. Clin. Microbiol. Infect.18(Suppl 7):19 –37.http://dx.doi.org/10 .1111/1469-0691.12039.
2.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of America.2009. Clinical practice guidelines for the management of
candi-TABLE 4(Continued)
Candidasp. fksmutation
MIC (g/ml) for:
Micafungin Caspofungin
L664R 0.25 0.5 R665G 0.12 0.5 R665S 0.12 0.5 D666Y 0.06 0.25
P667F 0.12 1
delF658 2 16
I1379V 0.03 0.06 I1379V 0.03 0.06 P1371S 0.03 0.25
C. tropicalis F641S 1 1 F641S 0.12 0.12
S645P 1 2
S645P 1 2
C. krusei F655C 0.5 1
R1361G 2 16
L701M 0.06 0.25
on May 16, 2020 by guest
http://jcm.asm.org/
[image:5.585.41.287.77.289.2]diasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis.48:503–535.http://dx.doi.org/10.1086/596757.
3.Ullmann AJ, Akova M, Herbrecht R, Viscoli C, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Cornely OA, Donnelly JP, Garbino J, Groll AH, Hope WW, Jensen HE, Kullberg BJ, Lass-Flörl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Cuenca-Estrella M, ESCMID Fungal In-fection Study Group. 2012. ESCMID guideline for the diagnosis and management ofCandidadiseases 2012: adults with haematological malig-nancies and after haematopoietic stem cell transplantation (HCT). Clin. Microbiol. Infect. 18(Suppl 7):53– 67. http://dx.doi.org/10.1111/1469 -0691.12041.
4.Arendrup MC, Garcia-Effron G, Lass-Flörl C, Lopez AG, Rodriguez-Tudela JL, Cuenca-Estrella M, Perlin DS.2010. Echinocandin suscepti-bility testing ofCandidaspecies: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and isosensitest media. Antimicrob. Agents Chemother.54:426 – 439.http: //dx.doi.org/10.1128/AAC.01256-09.
5.Arendrup MC, Pfaller MA, Danish Fungaemia Study Group. 2012. Caspofungin Etest susceptibility testing ofCandidaspecies: risk of mis-classification of susceptible isolates ofC. glabrataandC. kruseiwhen adopting the revised CLSI caspofungin breakpoints. Antimicrob. Agents Chemother.56:3965–3968.http://dx.doi.org/10.1128/AAC.00355-12. 6.Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S,
Jones RN, Turnidge J, Diekema DJ.2010. Wild-type MIC distributions and epidemiological cutoff values for the echinocandins andCandidaspp. J. Clin. Microbiol.48:52–56.http://dx.doi.org/10.1128/JCM.01590-09. 7.Arendrup MC, Park S, Brown S, Pfaller M, Perlin DS.2011. Evaluation
of CLSI M44-A2 disk diffusion and associated breakpoint testing of caspo-fungin and micacaspo-fungin using a well-characterized panel of wild-type and
fkshot spot mutantCandidaisolates. Antimicrob. Agents Chemother.
55:1891–1895.http://dx.doi.org/10.1128/AAC.01373-10.
8.Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M.2013. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifun-gal resistance. J. Clin. Microbiol.51:2571–2581.http://dx.doi.org/10.1128 /JCM.00308-13.
9.Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, Motyl M, Perlin DS, CLSI Subcommittee for Antifungal Testing.
2011. Clinical breakpoints for the echinocandins andCandidarevisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist. Updat. 14:164 –176.
http://dx.doi.org/10.1016/j.drup.2011.01.004.
10. Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope W, EUCAST-AFST.2012. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum in-hibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect.18:E246 –E247.http://dx.doi .org/10.1111/j.1469-0691.2012.03880.x.
11. Arendrup MC, Garcia-Effron G, Buzina W, Mortensen KL, Reiter N, Lundin C, Jensen HE, Lass-Flörl C, Perlin DS, Bruun B.2009. Break-throughAspergillus fumigatusandCandida albicansdouble infection dur-ing caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob. Agents Chemother. 53:1185–1193.
http://dx.doi.org/10.1128/AAC.01292-08.
12. Arendrup MC, Rodriguez-Tudela JL, Park S, Garcia-Effron G, Del-mas G, Cuenca-Estrella M, Gomez-Lopez A, Perlin DS.2011. Echi-nocandin susceptibility testing ofCandidaspp. using EUCAST EDef 7.1 and CLSI M27-A3 standard procedures: analysis of the influence of bovine serum albumin supplementation, storage time, and drug lots. Antimicrob. Agents Chemother.55:1580 –1587.http://dx.doi.org/10 .1128/AAC.01364-10.
13. Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante EB, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Flörl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SRBS, St-Germain G, Szeszs MW, Turnidge J.9 September 2013. Interlaboratory variability of caspofungin MICs forCandidaspp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob. Agents Chemother.http://dx.doi.org/10.1128/AAC.01519-13.
14. Pfaller MA, Diekema DJ, Ostrosky-Zeichner L, Rex JH, Alexander BD,
Andes D, Brown SD, Chaturvedi V, Ghannoum MA, Knapp CC, Sheehan DJ, Walsh TJ.2008. Correlation of MIC with outcome for
Candidaspecies tested against caspofungin, anidulafungin, and micafun-gin: analysis and proposal for interpretive MIC breakpoints. J. Clin. Mi-crobiol.46:2620 –2629.http://dx.doi.org/10.1128/JCM.00566-08. 15. Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS.2009. Effect of
Candida glabrata FKS1andFKS2mutations on echinocandin sensitivity and kinetics of 1,3-beta-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother.53:3690 –3699.
http://dx.doi.org/10.1128/AAC.00443-09.
16. Garcia-Effron G, Chua DJ, Tomada JR, DiPersio J, Perlin DS, Ghan-noum M, Bonilla H.2010. NovelFKSmutations associated with echino-candin resistance inCandidaspecies. Antimicrob. Agents Chemother.54:
2225–2227.http://dx.doi.org/10.1128/AAC.00998-09.
17. Perlin DS. 2011. Echinocandin-resistantCandida: molecular methods and phenotypes. Curr. Fungal Infect. Rep.5:113–119.http://dx.doi.org/10 .1007/s12281-011-0054-x.
18. Lepak A, Castanheira M, Diekema D, Pfaller M, Andes D. 2012. Optimizing echinocandin dosing and susceptibility breakpoint determi-nation viain vivopharmacodynamic evaluation againstCandida glabrata
with and withoutfksmutations. Antimicrob. Agents Chemother.56:
5875–5882.http://dx.doi.org/10.1128/AAC.01102-12.
19. Spreghini E, Orlando F, Sanguinetti M, Posteraro B, Giannini D, Manso E, Barchiesi F.2012. Comparative effects of micafungin, caspo-fungin, and anidulafungin against a difficult-to-treat fungal opportunistic pathogen,Candida glabrata. Antimicrob. Agents Chemother.56:1215– 1222.http://dx.doi.org/10.1128/AAC.05872-11.
20. Howard SJ, Livermore J, Sharp A, Goodwin J, Gregson L, Alastruey-Izquierdo A, Perlin DS, Warn PA, Hope WW.2011. Pharmacodynamics of echinocandins againstCandida glabrata: requirement for dosage esca-lation to achieve maximal antifungal activity in neutropenic hosts. Anti-microb. Agents Chemother. 55:4880 – 4887. http://dx.doi.org/10.1128 /AAC.00621-11.
21. Arendrup MC, Perlin DS, Jensen RH, Howard SJ, Goodwin J, Hope W.
2012. Differentialin vivoactivity of anidulafungin, caspofungin and mi-cafungin againstC. glabrataisolates with and withoutFKSresistance mu-tations. Antimicrob. Agents Chemother.56:2435–2442.http://dx.doi.org /10.1128/AAC.06369-11.
22. Alexander BD, Johnson MD, Pfeiffer CD, Jiménez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA.2013. Increasing echinocandin resistance inCandida glabrata: clinical failure correlates with presence ofFKSmutations and elevated minimum inhib-itory concentrations. Clin. Infect. Dis.56:1724 –1732.http://dx.doi.org/10 .1093/cid/cit136.
23. Dannaoui E, Desnos-Ollivier M, Garcia-Hermoso D, Grenouillet F, Cassaing S, Baixench MT, Bretagne S, Dromer F, Lortholary O, French Mycoses Study Group.2012.Candidaspp. with acquired echinocandin resistance, France, 2004 –2010. Emerg. Infect. Dis.18:86 –90.http://dx.doi .org/10.3201/eid1801.110556.
24. Durán-Valle MT, Gago S, Gómez-López A, Cuenca-Estrella M, Díez-Canseco LJ, Gómez-Garcés JL, Zaragoza O.2013. Recurrent episodes of candidemia due toCandida glabratawith a mutation in the hot spot 1 of theFKS2gene developed after prolonged therapy with caspofungin. An-timicrob. Agents Chemother. 56:3417–3419.http://dx.doi.org/10.1128 /AAC.06100-11.
25. Lortholary O, Desnos-Ollivier M, Sitbon K, Fontanet A, Bretagne S, Dromer F, French Mycosis Study Group.2011. Recent exposure to caspo-fungin or fluconazole influences the epidemiology of candidemia: a prospec-tive multicenter study involving 2,441 patients. Antimicrob. Agents Che-mother.55:532–538.http://dx.doi.org/10.1128/AAC.01128-10.
26. Pfeiffer CD, Garcia-Effron G, Zaas AK, Perfect JR, Perlin DS, Alexander BD.2010. Breakthrough invasive candidiasis in patients on micafungin. J. Clin. Microbiol.48:2373–2380.http://dx.doi.org/10.1128/JCM.02390-09. 27. Shields RK, Nguyen MH, Press EG, Updike CA, Clancy CJ. 2013. Caspofungin MICs correlate with treatment outcomes among patients withCandida glabratainvasive candidiasis and prior echinocandin expo-sure. Antimicrob. Agents Chemother.57:3528 –3535.http://dx.doi.org/10 .1128/AAC.00136-13.
28. Desnos-Ollivier M, Moquet O, Chouaki T, Guérin AM, Dromer F.
2011. Development of echinocandin resistance inClavispora lusitaniae
during caspofungin treatment. J. Clin. Microbiol.49:2304 –2306.http: //dx.doi.org/10.1128/JCM.00325-11.
29. Jensen RH, Johansen HK, Arendrup MC.2013. Stepwise development of
on May 16, 2020 by guest
http://jcm.asm.org/
a homozygous S80P substitution in Fks1p, conferring echinocandin resis-tance inCandida tropicalis. Antimicrob. Agents Chemother.57:614 – 617.
http://dx.doi.org/10.1128/AAC.01193-12.
30. Healey KR, Katiyar SK, Castanheira M, Pfaller MA, Edlind TD.2011.
Candida glabratamutants demonstrating paradoxical caspofungin reduced susceptibility but micafungin increased susceptibility. Antimicrob. Agents Chemother.55:3947–3949.http://dx.doi.org/10.1128/AAC.00044-11. 31. Healey KR, Katiyar SK, Raj S, Edlind TD.2012. CRS-MIS inCandida
glabrata: sphingolipids modulate echinocandin-Fks interaction. Mol. Micro-biol.86:303–313.http://dx.doi.org/10.1111/j.1365-2958.2012.08194.x. 32. Pfaller MA, Messer SA, Boyken L, Rice C, Tendolkar S, Hollis RJ,
Diekema DJ.2004. Cross-resistance between fluconazole and ravucona-zole and the use of fluconaravucona-zole as a surrogate marker to predict suscepti-bility and resistance to ravuconazole among 12,796 clinical isolates of
Candidaspp. J. Clin. Microbiol.42:3137–3141.http://dx.doi.org/10.1128 /JCM.42.7.3137-3141.2004.
33. Pfaller MA, Messer SA, Boyken L, Rice C, Tendolkar S, Hollis RJ, Diekema DJ.2007. Use of fluconazole as a surrogate marker to prvedict susceptibility and resistance to voriconazole among 13,338 clinical isolates ofCandidaspp. tested by Clinical and Laboratory Standards Institute-recommended broth microdilution methods. J. Clin. Microbiol.45:70 – 75.http://dx.doi.org/10.1128/JCM.01551-06.
34. Pfaller MA, Messer SA, Boyken L, Tendolkar S, Hollis RJ, Diekema DJ.
2008. Selection of a surrogate agent (fluconazole or voriconazole) for ini-tial susceptibility testing of posaconazole againstCandidaspp.: results-from a global antifungal surveillance program. J. Clin. Microbiol.46:551– 559.http://dx.doi.org/10.1128/JCM.01952-07.
35. Howell SA, Hazen KC.2011.Candida,Cryptococcus, and other yeasts of medical importance, p 1793–1821.InVersalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW (ed), Manual of clinical micro-biology, 10th ed. ASM Press, Washington, DC.
36. Castanheira M, Woosley LN, Diekema DJ, Messer SA, Jones RN, Pfaller MA.2010. Low prevalence offks1hotspot 1 mutations in a worldwide collection ofCandidaspp. Antimicrob. Agents Chemother.54:2655– 2659.http://dx.doi.org/10.1128/AAC.01711-09.
37. Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN.2012. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Can-dida glabrata. J. Clin. Microbiol.50:1199 –1203.http://dx.doi.org/10.1128 /JCM.06112-11.
38. Clinical and Laboratory Standards Institute.2012. Reference method for broth dilution antifungal susceptibility testing of yeasts; 4th informational supplement. CLSI M27-S4. Clinical and Laboratory Standards Institute, Wayne, PA.
39. Clinical and Laboratory Standards Institute.2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved stan-dard—3rd ed. CLSI document M27-A3. Clinical and Laboratory Stan-dards Institute, Wayne, PA.
40. Clinical and Laboratory Standards Institute.2013. Performance stan-dards for antimicrobial susceptibility testing; 23rd informational supple-ment. CLSI M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA.
41. Pfaller MA, Diekema DJ.2012. Progress in antifungal susceptibility test-ing ofCandidaspp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J. Clin. Microbiol.50:2846 – 2856.http://dx.doi.org/10.1128/JCM.00937-12.
42. Clinical and Laboratory Standards Institute.2008. Development ofin vitrosusceptibility testing criteria and quality control parameters; ap-proved standard—3rd ed. CLSI document M23-A3. Clinical and Labora-tory Standards Institute, Wayne, PA.
43. Ferraro MJ, Jorgensen JH.1995. Instrument-based antibacterial suscep-tibility testing, p 1379 –1384.InMurray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH (ed), Manual of clinical microbiology, 6th ed. ASM Press, Washington, DC.
44. Jones RN, Pfaller MA, SENTRY Antimicrobial Surveillance Program Participants Group (USA).2001. Can antimicrobial susceptibility testing results for ciprofloxacin or levofloxacin predict susceptibility to a newer fluoroquinolone, gatifloxacin? Report from the SENTRY Antimicrobial Surveillance Program (1997–99). Diagn. Microbiol. Infect. Dis.39:237– 243.http://dx.doi.org/10.1016/S0732-8893(01)00229-2.
45. Slater JL, Howard SJ, Sharp A, Goodwin J, Gregson LM, Alastruey-Izquierdo A, Arendrup MC, Warn PA, Perlin DS, Hope WW.2011. Disseminated candidiasis caused byCandida albicanswith amino acid substitutions in Fks1 at position Ser645 cannot be successfully treated with micafungin. Antimicrob. Agents Chemother.55:3075–3083.http://dx.doi .org/10.1128/AAC.01686-10.
46. Turnidge J, Paterson DL.2007. Setting and revising antibacterial suscep-tibility breakpoints. Clin. Microbiol. Rev.20:391– 408.http://dx.doi.org
/10.1128/CMR.00047-06.