Case Report
Primary angiosarcoma of the breast: a case report
Soyoung Im1, Byung Joo Chae2, Sung Hun Kim3, Bong-Joo Kang3, Byung Joo Song4, Ahwon Lee5
1Department of Pathology, St. Vincent’s Hospital, The Catholic University of Korea, College of Medicine, Suwon,
Republic of Korea; Departments of 2Surgery, 3Radiology, 4Surgery, 5Hospital Pathology, Seoul St. Mary’s Hospital,
The Catholic University of Korea, College of Medicine, Seoul, Republic of Korea
Received September 6, 2018; Accepted September 27, 2018; Epub February 1, 2019; Published February 15, 2019
Abstract: Background: Primary angiosarcoma of the breast is extremely rare, accounting for less than 0.05% of all primary malignancies of the breast. Here, we report here a case of primary angiosarcoma with full description of ra-diology and histology, including electron microscopic findings. Case presentation: A 39-year-old woman complained of a diffuse hard mass in her right breast. She did not have any history of radiation exposure. Ultrasonography revealed a 7 cm sized mass with an irregular anechoic cystic portion replacing the entire right breast. Modified radical mastectomy was performed. The diagnosis of intermediate grade angiosarcoma was made by microscopic examination, immunohistochemical staining, and electron microscopic examination. The patient underwent four cycles of adriamycin-ifosfamide chemotherapy and received radiation therapy. Multiple bone metastases occurred 9 months after surgery and palliative treatment was given. Follow up was lost at post-operative 22 months. Conclu-sions: We report a rare case of intermediate grade primary angiosarcoma with detailed radiological and histological findings. Despite postoperative chemoradiation therapy, multiple metastases suggest that intermediate grade may have a more aggressive behavior.
Keywords: Breast neoplasms, malignant, sarcoma, angiosarcoma
Background
Angiosarcoma of the breast occurs in two dif-ferent settings, primary or with prior radiation therapy. Primary angiosarcoma of the breast is a malignant vascular neoplasm arising in the breast parenchyma without previous radiation history, in contrast to postradiation angiosar-coma, which is a well-known complication of radiation therapy for breast cancer [1, 2]. Primary angiosarcoma of the breast is extreme-ly rare, accounting for less than 0.05% of all primary malignancies of the breast [3]. It occurs sporadically in young women and usually pres-ents as painless and rapidly growing palpable masses [4, 5]. Here, we report a primary
angio-sarcoma of the breast with the findings of radio -logic, microscopic, and electron microscopic features.
Case presentation
A 39-year-old female patient presented with a diffuse hard mass and fullness in the right breast. She was told by a local hospital that
breast one year ago but refused further evalua-tion or treatment. She did not have any history of radiation exposure. The mammogram showed asymmetrically increased density in the right breast (Figure 1A). Ultrasonography revealed a 7 cm heterogeneous hypoechoic mass with an irregular anechoic cystic portion replacing the entire right breast (Figure 1B). Core needle biopsy revealed a mesenchymal tumor with anastomosing vascular channels and papillary proliferation. Immunohistoche-
mical staining of CD34 and factor VIII confirmed
the endothelial origin, and the histologic fea-tures suggested the possibility of an intermedi-ate grade angiosarcoma. Additional radiologic examination including MRI and PET-CT was per-formed. The axial contrast enhanced T1 weight-ed image displayweight-ed a 7.9×3.6×3.3 cm, irregu-larly shaped, heterogeneously enhancing lesion with relatively smooth margin that occupied the entire breast (Figure 1C). The mass showed
dif-fuse FDG uptake with a maximum SUV of 1.5
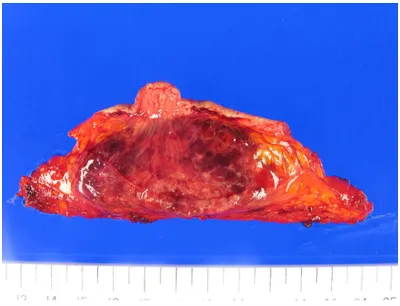
ic mass was identified within the breast paren -chyma. On cut section, the mass had a brick brown to pink color with a soft consistency and hemorrhagic cut surface (Figure 2). Microscopically, the tumor was composed of anastomosing vascular channels with hyper-chromatic endothelial cells. Endothelial tufting, papillary formations, and blood lakes were present. Necrosis was absent, and mitotic index was 1-2/10HPF (Figure 3A-D). Immuno- histochemistry was performed once again on the mastectomy specimen, and the tumor cells
up mammography and ultrasonography at 6 months postoperative showed no evidence of tumor recurrence. However, bone scan at 9 months postoperative discovered newly devel-oped multiple bone metastases in the right rib and left ilium. Palliative chemotherapy and radi-ation therapy was performed. Patient was lost to follow-up 22 months after surgery.
Discussion and conclusions
Primary breast sarcomas excluding phyllodes tumors are rare and account for less than 0.1% of all breast malignancy [4]. Major histologic subtypes of breast sarcoma are angiosarcoma,
malignant fibrous histiocytoma and fibrosarco -ma [6]. Angiosarco-mas of the breast are divid-ed in two categories according to their etiology; de novo development (primary) or post-radia-tion therapy related (secondary). Primary angio-sarcoma has a median age of 42 years, while secondary angiosarcoma occurs in older women with a median age of 72 years [7]. The MRI of angiosarcoma shows a heteroge-neous mass with low signal intensity on T1-wighted images and hyperintensity on T2-wighted images [5, 8]. Enhancement of the mass depends on the tumor grade. Low grade angiosarcomas show progressive
[image:2.612.89.376.72.264.2]enhance-Figure 1. Radiologic findings. A. The mammogram shows asymmetrically increased density of the right breast. B. The sonogram of the right breast shows a huge, heterogeneous hypoechoic lesion replacing nearly the entire breast. C. The axial contrast enhanced T1 weighted image shows a large, heterogeneously enhancing lesion that occupies the entire breast. D. FDG PET scanning shows diffuse uptake in the right breast.
Figure 2. Gross findings. A 8.0×4.5 cm partially cir -cumscribed mass with a brick brown to pink color and hemorrhagic cut surface is noted within the breast parenchyma.
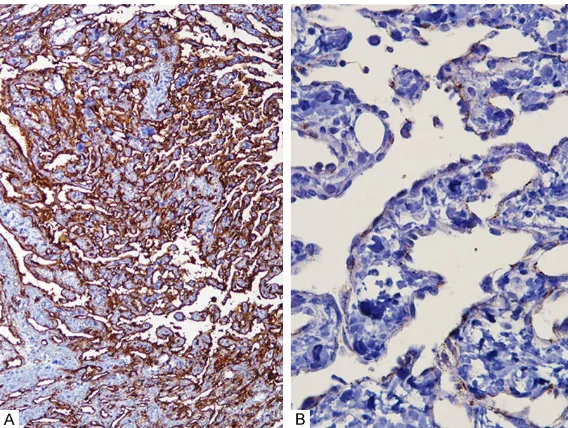
were positive for vimentin,
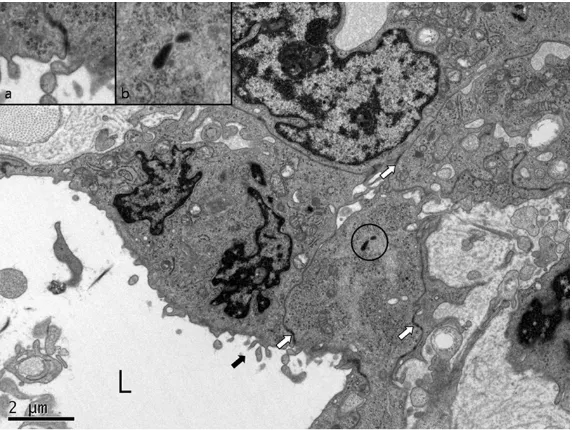
CD34 and factor VIII-related antigen, confirming the diag -nosis of an intermediate grade angiosarcoma (Figure 4A and 4B). Electron microscopy was performed, and the vascular lumens were lined by plump cells with cytoplasmic cesses. The cytoplasmic pro-cesses were joined by inter-cellular junctions. Weibel-Pal- ade bodies were noted within the endothelial cytoplasm (Fig- ure 5). Sixteen lymph nodes were retrieved from axillary dissection, and lymph node metastasis was absent. The patient underwent four cy- cles of adriamycin-ifosfamide chemotherapy and received radiation therapy with a total
[image:2.612.89.289.353.506.2]ment. On the other hand, high grade angiosar-comas show rapid enhancement and washout, and large draining vessels may be visualized [5]. The present case showed a heterogeneous-ly enhancing mass with smooth margin on con-trast enhanced T1 weighted image and diffuse
FDG uptake with a maximum SUV of 1.5 on
PET-CT.
[image:3.612.89.376.72.288.2][4]. Recently, Nascimento et al. studied 49 cases of primary angiosarcoma of the breast and reported that there were no correlation between histological grade and patient out-come [1]. In this present case, the tumor was an intermediate grade but multiple metastases occurred in spite of postoperative chemoradia-tion therapy, suggesting that intermediate
Figure 3. Microscopic findings. A. Anastomosing vasculature of the tumor. B. Focal areas of blood lakes. C. Papillary proliferation of the tumor cells. D. The lining cells of the anastomosing vessels are slightly plump and hyper-chromatic with mild nuclear pleomorphism.
Figure 4. Immunohistochemistry. The lining cells of the anastomosing vascu-lar channel are positive for CD34 (A) and Factor VIII (B).
Histologically, angiosarcoma displays heterogenous appea- rances including well-formed anastomosing vascular chan-nels, endothelial tufting and papillary formations, solid and spindle cell foci, presence of hyperchromatic endothelial cells, and necrosis. Histologic grading is performed with Rosen’s 3-tiered grading sys-tem based on morphology [9, 10]. The presenting case revealed anastomosing vascu-lar channels with hyperchro-matic endothelial cells invad-ing the breast parenchyma, endothelial tufting, papillary formations, and blood lakes. However, necrosis was absent and mitoses were rare with an index of 1-2/10HPF, which was compatible for an inter-mediate grade angiosarcoma. Although the histologic grade was intermediate, multiple bone metastasis occurred 9
months after surgery. Despite
palliative chemoradiation the- rapy, the disease progressed during the 22 months of follow up and had a poor outcome. Rosen et al. reported that dis-ease-free survival 5 years after treatment are 76%, 70%, and 15% for low-, intermedi-ate-, and high-grade angiosar-comas, respectively, and that intermediate-grade angiosar-comas behave more like low-grade angiosarcomas [10]. However, Adem et al. reported that overall survival was asso-ciated with tumor size, but did
not significantly differ between
[image:3.612.90.374.356.570.2]grade may have a more aggressive behavior. However, angiosarcoma of the breast is rare, and the clinical data in literature are limited with mostly descriptive studies of a small sam-ple size or retrospective studies based on review of databases such as Surveillance, Epidemiology, and End Result Program (SEER)
or Florida Cancer Data System (FCDS) [3, 4,
11-13]. Further studies on a larger scale are needed to validate histologic grade as a prog-nostic factor.
Primary angiosarcioma tends to spread hema-togenously and shows a high propensity for metastasis including bone, lung, and liver. Complete surgical resection of the tumor with negative margins is the mainstay of treatment for breast sarcomas [6, 14]. Lymph node metastasis is very rare in breast sarcomas and radical dissection of axillary lymph nodes should only be performed in histologically prov-en isolated nodal disease or to relieve symp-toms [6, 15]. Systemic adjuvant chemotherapy may be offered, since angiosarcoma is a poten-tially chemosensitive disease with a response rate of 48% to anthracycline- and gemcitabine-based chemotherapy in the metastatic setting [7]. The role of radiation therapy is controver-sial. Adjuvant radiation therapy for primary breast angiosarcomas has not been proven in randomized trials [6, 11].
Disclosure of conflict of interest None.
Address correspondence to: Dr. Ahwon Lee, Department of Hospital Pathology, The Catholic University of Korea, Seoul St. Mary’s Hospital, 222 Banpodaero, Seochogu, Seoul 06591, Republic of Korea. Tel: 82-2-2258-1621; Fax: 82-2-2258-1628; E-mail: klee@catholic.ac.kr
References
[1] Nascimento AF, Raut CP and Fletcher CD. Pri -mary angiosarcoma of the breast: clinicopath-ologic analysis of 49 cases, suggesting that grade is not prognostic. Am J Surg Pathol 2008; 32: 1896-1904.
[2] Scow JS, Reynolds CA, Degnim AC, Petersen IA, Jakub JW and Boughey JC. Primary and sec-ondary angiosarcoma of the breast: the mayo clinic experience. J Surg Oncol 2010; 101: 401-407.
[3] Hodgson NC, Bowen-Wells C, Moffat F, France-schi D and Avisar E. Angiosarcomas of the breast: a review of 70 cases. Am J Clin Oncol 2007; 30: 570-573.
[4] Adem C, Reynolds C, Ingle JN and Nascimento AG. Primary breast sarcoma: clinicopathologic series from the mayo clinic and review of the literature. Br J Cancer 2004; 91: 237-241. [5] Glazebrook KN, Magut MJ and Reynolds C. An
[image:4.612.90.376.71.286.2]-giosarcoma of the breast. AJR Am J Roentgenol 2008; 190: 533-538.
Figure 5. Electron microscopic findings. Vascular lumen (L) with lining cells. The vascular lining cells have cytoplasmic processes (arrow) and intercellu-lar junctions (open arrow & inset a). Weibel-Palade bodies (circle & inset b) are noted within the endothelial cytoplasm.
In conclusion, we report a pri-mary angiosarcoma replacing the entire right breast with detailed radiological and
his-tological findings. The patient
was treated by surgery and postoperative chemoradiation therapy but developed metas-tasis 9 months after surgery. Acknowledgements
This study has been approved by the Institutional Review Board at The Catholic Univer- sity of Korea (KC17ZESI0630), and was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (NRF-
[6] Lim SZ, Ong KW, Tan BK, Selvarajan S and Tan PH. Sarcoma of the breast: an update on a rare entity. J Clin Pathol 2016; 69: 373-381. [7] Sher T, Hennessy BT, Valero V, Broglio K, Wood
-ward WA, Trent J, Hunt KK, Hortobagyi GN and Gonzalez-Angulo AM. Primary angiosarcomas of the breast. Cancer 2007; 110: 173-178. [8] Yang WT, Hennessy BT, Dryden MJ, Valero V,
Hunt KK and Krishnamurthy S. Mammary an-giosarcomas: imaging findings in 24 patients. Radiology 2007; 242: 725-734.
[9] Donnell RM, Rosen PP, Lieberman PH, Kaufman RJ, Kay S, Braun DW Jr and Kinne DW. Angiosarcoma and other vascular tumors of the breast. Am J Surg Pathol 1981; 5: 629-642.
[10] Rosen PP, Kimmel M and Ernsberger D. Mam -mary angiosarcoma. The prognostic signifi -cance of tumor differentiation. Cancer 1988; 62: 2145-2151.
[11] Masai K, Kinoshita T, Jimbo K, Asaga S and Hojo T. Clinicopathological features of breast angiosarcoma. Breast Cancer 2016; 23: 718-723.
[12] Yin M, Wang W, Drabick JJ and Harold HA. Prognosis and treatment of non-metastatic primary and secondary breast angiosarcoma: a comparative study. BMC Cancer 2017; 17: 295.
[13] Vorburger SA, Xing Y, Hunt KK, Lakin GE, Ben -jamin RS, Feig BW, Pisters PW, Ballo MT, Chen L, Trent J 3rd, Burgess M, Patel S, Pollock RE and Cormier JN. Angiosarcoma of the breast. Cancer 2005; 104: 2682-2688.
[14] Pandey M, Mathew A, Abraham EK and Rajan B. Primary sarcoma of the breast. J Surg Oncol 2004; 87: 121-125.