Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
(
l
-Perfluorosebacato-
j
2O
:
O
000)bis[aquabis(1,10-phenanthroline-
j
2N
,
N
000)zinc(II)] perfluorosebacate
bis[triaqua(perfluorosebacato-
jO
)(1,10-phenan-throline-
j
2N
,
N
000)zinc(II)] 3.32-hydrate
Kani I:brahim,aOnur S¸ahin,b Yilmaz Filizaand Orhan Bu¨yu¨kgu¨ngo¨rb*
aDepartment of Chemistry, Anadolu University,
TR-26470 Eskis¸ehir, Turkey, andbDepartment of
Physics, Ondokuz Mayıs University, TR-55139 Samsun, Turkey
Correspondence e-mail: onurs@omu.edu.tr
Key indicators
Single-crystal X-ray study T= 100 K
Mean(C–C) = 0.004 A˚ Disorder in solvent or counterion Rfactor = 0.045
wRfactor = 0.122
Data-to-parameter ratio = 14.7
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 3 July 2006 Accepted 17 July 2006
The asymmetric unit of the title compound, [Zn2{O2C(CF2)8
-CO2}(C12H8N2)4(H2O)2](C10F16O4)[Zn{O2C(CF2)8CO2
}-(C12H8N2)(H2O)3]3.32H2O, contains a neutral mononuclear
[ZnL(phen)(H2O)3] complex (phen is 1,10-phenanthroline
and L is perfluorosebacate), one half each of a centrosym-metric [Zn2L(phen)4(H2O)2]
2+
binuclear complex and an uncoordinated centrosymmetric L ligand, and 1.66 water molecules of solvation. The ZnII atoms in both the mono-nuclear and the bimono-nuclear complexes are six-coordinated in a distorted octahedral geometry.
Comment
Polynuclear d10 metal complexes are very attractive in that they not only exhibit intriguing structures but also show photoluminescent properties. As a d10 metal ion, ZnII in particular is studied for the construction of coordination polymers and networks (Erxleben, 2003). The spherical d10
of supramolecular networks (Ye et al., 2005; McCann et al., 1997; Geragthyet al., 1998; Zheng, Lin & Kong, 2003; Zheng, Lin & Chen, 2003; Devereux et al., 1999). The coordination chemistry of saturated linear ,!-dicarboxylate anions [O2C(CH2)nCO2]
2
(n = 1–8) has not been developed and, indeed, structural information for this class of complex is relatively scarce, since it involves the formation of insoluble polymeric materials, which can be difficult to characterize and almost impossible to crystallize. Devereux et al.(1999, 2000) showed that the introduction of a second competing ligand, such as 1,10-phenanthroline (phen) or 2,2 bipyridine, lowers the dimensionality of the structures, since their chelation to the metal ion leaves fewer sites for dicarboxylic acid coordi-nation. Mixed phen and long-chain,!-dicarboxylate ligand complexes are very rare and no published data have been found in the literature on the X-ray analysis of multinuclear ZnIIcomplexes with perfluorosebacic acid. We report here the crystal structure of the title compound, (I).
The asymmetric unit of (I) contains one half of a centro-symmetric [Zn2L(phen)4(H2O)2]2+ binuclear complex cation
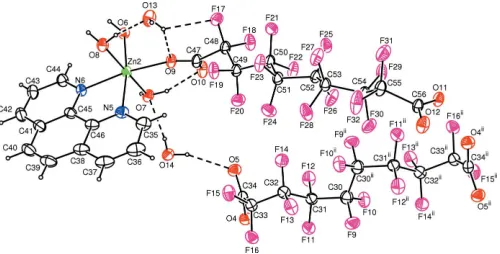
(Fig. 1), a neutral mononuclear [ZnL(phen)(H2O)3] complex
(Fig. 2), one half of an uncoordinated centrosymmetric perfluorosebacate ligand and 1.66 water molecules of solva-tion (Fig. 2), where H2L is HO2C(CF2)8CO2H and phen is
1,10-phenanthroline. Selected bond lengths and angles are listed in Table 1.
Each ZnII atom of the binuclear complex cation has a distorted octahedral coordination geometry, defined by four N atoms from two chelating phen ligands and an O atom from the carboxylate group of a bridging perfluorosebacate ligand and the O atom of a water molecule. The observed distortion from octahedral geometry is caused by the small bite angles of the chelating phen ligands (Table 1). Around the central ZnII atom, both chelating phen planes are oriented nearly perpendicular to each other [dihedral angle 85.38 (10)]. The N1—Zn1—O1 angle exhibits less deviation from linearity compared with the othertransangles. The Zn1—O1 distance is slightly longer than the Zn1—O2 distance. All Zn–N bond distances are comparable with those observed in similar compounds (Zhang et al., 2003; Yin et al., 2004; Guo et al., 2004; Viossat et al., 2005). An intramoleculer C—H
interaction and an OW—H O hydrogen bond (Table 2) are observed in the complex cation.
In the neutral mononuclear [ZnL(phen)(H2O)3] complex,
the ZnII atom is six-coordinated in a distorted octahedral geometry by two N atoms from a chelating phen ligand, three O atoms from three water molecules and one O atom from a deprotonated perfluorosebacate ligand. The perfluoro-sebacate ligand is coordinated to the metal atom in mono-dentate fashion. The bite angle of the phen ligand is 77.75 (9) and the cis angles lie in the range 86.88 (9)–96.25 (9). The N6—Zn2—O9transangle exhibits the largest deviation from linearity. Zn—O distances lie in the range 2.0587 (18)– 2.144 (2) A˚ . Intramolecular O—H O hydrogen bonds involving the coordinated water molecules are observed in the mononuclear complex (Table 2).
The free perfluorosebacate anion lies between the two zinc(II) complexes and it is linked to them via strong O— H O hydrogen bonds involvolving the coordinated water molecules (Table 2).
The crystal packing of (I) is stabilized by a complicated network of O—H O and O—H F hydrogen bonds. In addition, a – stacking interaction is observed between adjacent phen ligands. The Cg2 Cg3iii and Cg3 Cg3iv distances are 3.459 (2) and 3.411 (2) A˚ , respectively, where
Cg2 andCg3 are centroids of the central C4–C7/C11/C12 and C39–C41/C45/C46 benzene rings [symmetry codes: (iii) x, y,
1 +z; (iv) 1x, 2y, 2z]. The – interactions are similar to those in the known analogues of Mn, Cu, Zn and Co complexes, with characteristic interplanar distances between the rings of 3.6 A˚ (Geragthy et al., 1999; Zheng & Kong, 2004; Zheng & Ying, 2005).
Experimental
[image:2.610.316.564.70.185.2]Na2CO3 (0.5 ml, 1 mol) was added to a stirred solution of Zn(NO3)26H2O (121.4 mg, 0.408 mmol) in H2O (10 ml). The resul-tant precipitate was separated by filtration, washed several times with distilled water and finally added to a stirred solution of phenan-throline monohydrate (80.78 mg, 0.408 mmol) and perfluorosebacic acid (200 mg, 0.408 mmol) dissolved in EtOH and H2O (1:1 v/v, 20 ml). The mixture was stirred for ca 2 h and the solution was allowed to stand at room temperature for 2 d to obtain colourless crystals of (I) (m.p. 500 K).
Figure 1
The structure of the [Zn2L(phen)4(H2O)2]2+ complex, showing 50%
[image:2.610.316.566.240.367.2]probability displacement ellipsoids and the atomic numbering. Hydrogen bonds are shown as dashed lines. [Symmetry code: (i) 1x, 2y, 1z.]
Figure 2
The structures of the [ZnL(phen)(H2O)3] and C10F16O4 2
Crystal data
[Zn2(C10F16O4)(C12H8N2)4(H2O)2
]-(C10F16O4)[Zn(C10F16O4
)-(C12H8N2)(H2O)3]3.32H2O
Mr= 3499.05 Triclinic,P1 a= 13.505 (2) A˚ b= 15.271 (2) A˚ c= 17.332 (3) A˚
= 70.556 (12)
= 81.673 (13)
= 70.530 (11) V= 3175.6 (9) A˚3
Z= 1
Dx= 1.830 Mg m3
MoKradiation
= 0.92 mm1
T= 100 K Prism, colourless 0.540.350.23 mm
Data collection
Stoe IPDS II diffractometer
!scans
Absorption correction: integration (X-RED32; Stoe & Cie, 2002) Tmin= 0.686,Tmax= 0.809
46049 measured reflections 15080 independent reflections 12163 reflections withI> 2(I) Rint= 0.061
max= 27.9
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.045
wR(F2) = 0.122 S= 0.99 15080 reflections 1028 parameters
H atoms treated by a mixture of independent and constrained refinement
w= 1/[2
(Fo 2
) + (0.0623P)2 + 3.9061P]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.003
max= 1.40 e A˚
3
min=0.78 e A˚
[image:3.610.315.565.70.173.2]3
Table 1
Selected geometric parameters (A˚ ,).
N1—Zn1 2.197 (2)
N2—Zn1 2.126 (2)
N3—Zn1 2.206 (2)
N4—Zn1 2.138 (2)
N5—Zn2 2.136 (2)
N6—Zn2 2.168 (2)
O1—Zn1 2.1532 (19)
O2—Zn1 2.040 (2)
O6—Zn2 2.0587 (18)
O7—Zn2 2.144 (2)
O8—Zn2 2.113 (2)
O9—Zn2 2.0980 (18)
O2—Zn1—N2 103.70 (8)
O2—Zn1—N4 92.33 (9)
N2—Zn1—N4 160.24 (9)
O2—Zn1—O1 91.47 (8)
N2—Zn1—O1 97.71 (8)
N4—Zn1—O1 93.28 (8)
O2—Zn1—N1 94.92 (9)
N2—Zn1—N1 77.40 (8)
N4—Zn1—N1 89.95 (8)
O1—Zn1—N1 172.71 (8)
O2—Zn1—N3 167.92 (8)
N2—Zn1—N3 88.03 (8)
N4—Zn1—N3 76.81 (8)
O1—Zn1—N3 84.00 (8)
N1—Zn1—N3 90.39 (8)
O6—Zn2—O9 95.59 (8)
O6—Zn2—O8 87.90 (8)
O9—Zn2—O8 91.13 (8)
O6—Zn2—N5 171.75 (9)
O9—Zn2—N5 90.90 (8)
O8—Zn2—N5 86.88 (9)
O6—Zn2—O7 88.76 (8)
O9—Zn2—O7 90.83 (8)
O8—Zn2—O7 176.28 (7)
N5—Zn2—O7 96.25 (9)
O6—Zn2—N6 95.67 (8)
O9—Zn2—N6 168.64 (8)
O8—Zn2—N6 87.75 (8)
N5—Zn2—N6 77.75 (9)
O7—Zn2—N6 90.95 (8)
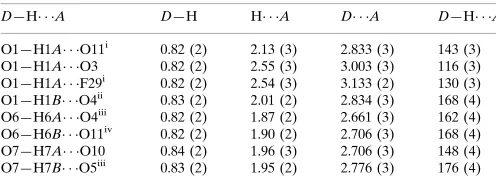
Table 2
Hydrogen-bond geometry (A˚ ,).
Cg1 is the centroid of the N1/C7–C11 ring.
D—H A D—H H A D A D—H A
O1—H1A O11i
0.82 (2) 2.13 (3) 2.833 (3) 143 (3)
O1—H1A O3 0.82 (2) 2.55 (3) 3.003 (3) 116 (3)
O1—H1A F29i
0.82 (2) 2.54 (3) 3.133 (2) 130 (3) O1—H1B O4ii 0.83 (2) 2.01 (2) 2.834 (3) 168 (4) O6—H6A O4iii
0.82 (2) 1.87 (2) 2.661 (3) 162 (4)
iv
D—H A D—H H A D A D—H A
O8—H8A O13 0.83 (2) 1.88 (2) 2.698 (3) 171 (4) O8—H8B O12iv 0.82 (2) 1.93 (2) 2.724 (3) 163 (4) O13—H13A O3v
0.82 (2) 1.99 (2) 2.806 (3) 170 (4) O13—H13B O9 0.82 (2) 2.29 (3) 2.991 (3) 143 (4) O13—H13B F17 0.82 (2) 2.50 (3) 3.151 (3) 137 (4) O14—H14A O7 0.83 (2) 2.12 (3) 2.930 (4) 163 (6) O14—H14B O5 0.82 (2) 2.17 (3) 2.955 (4) 159 (6)
C13—H13 Cg1 0.93 2.84 3.613 (3) 142
Symmetry codes: (i) xþ1;y;z; (ii) xþ2;yþ1;zþ1; (iii)
xþ1;yþ1;zþ2; (iv)x;y;zþ1; (v)xþ1;yþ2;zþ1.
One of the uncoordinated water molecules is disordered, with a partial occupancy factor of 0.658 (9). The water H atoms were located in a difference map and refined with an O—H distance restraint of 0.83 (2) A˚ and with Uiso(H) = 1.5Ueq(O). C-bound H atoms were refined using the riding-model approximation, with C—H = 0.93 A˚ andUiso(H) = 1.2Ueq(C). The structure contains a solvent-accessible void of volume 45 A˚3around (0 0.5 0), but there is no evidence of any solvent molecule, as the modelling of the electron density using PLATON(Spek, 2003) showed no electron density in the void space. The highest peak in the final difference map is located 1.20 A˚ from atom F6 and the deepest hole 0.59 A˚ from F1.
Data collection: X-AREA (Stoe & Cie, 2002); cell refinement: X-AREA; data reduction:X-RED32(Stoe & Cie, 2002); program(s) used to solve structure: SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics:ORTEP-3 for Windows(Farrugia, 1997); software used to prepare material for publication:WinGX(Farrugia, 1999).
The authors acknowledge Anadolu University Commission of Scientific Research Projects for financial support (Project No. 031036), and the Faculty of Arts and Sciences, Ondokuz Mayıs University, Turkey, for the use of the Stoe IPDSII diffractometer (purchased under grant F.279 of the University Research Fund).
References
Devereux, M., McCann, M., Cronin, J. F., Ferguson, V. & McKee, V. (1999). Polyhedron,18, 2141–2148.
Devereux, M., McCann, M., Leon, V., Geraghty, M., McKee, V. & Wikaria, J. (2000).Polyhedron,19, 1205–1211.
Erxleben, A. (2003).Coord. Chem. Rev.246, 203–228. Farrugia, L. J. (1997).J. Appl. Cryst.30, 565. Farrugia, L. J. (1999).J. Appl. Cryst.32, 837–838.
Geragthy, M., McCann, M., Casey, M. T., Curran, M., Devereux, M., McKee, V. & McCrea, J. (1998).Inorg. Chim. Acta,277, 257–262.
Geragthy, M., McCann, M., Devereux, M. & McKee, V. (1999).Inorg. Chim. Acta,293, 160–166.
Guo, W., Peng, Z., Li, D. & Zhou, Y. (2004).Polyhedron,23, 1707–1707. McCann, S., McCann, M., Casey, M. T., Devereux, M., McKee, V., McMichael,
P. & McCrea, J. G. (1997).Polyhedron,16, 4247–4252.
Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
Spek, A. L. (2003).J. Appl. Cryst.36, 7–13.
Stoe & Cie (2002).X-RED32andX-AREA. Stoe & Cie, Darmstadt, Germany. Viossat, V., Lemoine, P., Dayan, E. & Vioassat, B. (2005).J. Mol. Struct.741,
45–52.
Ye, B. H., Tong, M. L. & Chen, X. M. (2005).Coord. Chem. Rev.249, 545–565. Yin, M.-C., Yuan, L.-J., Ai, C.-C., Wang, C.-W., Yuan, E.-T. & Sun, J.-T. (2004).
Polyhedron,23, 529–536.
Zhang, L.-Y., Liu, G.-F., Zheng, S.-L., Ye, B.-H., Zhang, X.-M. & Chen, X.-M. (2003).Eur. J. Org. Chem.pp. 2965–2971.
[image:3.610.45.293.656.751.2]supporting information
Acta Cryst. (2006). E62, m1909–m1911 [https://doi.org/10.1107/S1600536806027656]
(
µ
-Perfluorosebacato-
κ
2O
:
O
′
)bis[aquabis(1,10-phenanthroline-
κ
2N
,
N
′
)zinc(II)]
perfluorosebacate bis[triaqua(perfluorosebacato-
κ
O
)(1,10-phenanthroline-κ
2N
,
N
′
)zinc(II)] 3.32-hydrate
Kani
İ
brahim, Onur
Ş
ahin, Yilmaz Filiz and Orhan B
ü
y
ü
kg
ü
ng
ö
r
(µ-Perfluorosebacato-κ2O:O′)bis[aquabis(1,10-phenanthroline- κ2N,N′)zinc(II)] perfluorosebacate
bis[triaqua(perfluorosebacato- κO)(1,10-phenanthroline-κ2N,N′)zinc(II)] 3.32-hydrate
Crystal data
[Zn2(C10F16O4)(C12H8N2)4(H2O)2] (C10F16O4)·[Zn(C10F16O4)(C12H8N2) (H2O)3]·3.32H2O
Mr = 3499.05
Triclinic, P1 Hall symbol: -P 1
a = 13.505 (2) Å
b = 15.271 (2) Å
c = 17.332 (3) Å
α = 70.556 (12)°
β = 81.673 (13)°
γ = 70.530 (11)°
V = 3175.6 (9) Å3
Z = 1
F(000) = 1741.2
Dx = 1.830 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 46049 reflections
θ = 1.8–28.0°
µ = 0.92 mm−1
T = 100 K Prism, colourless 0.54 × 0.35 × 0.23 mm
Data collection
Stoe IPDS II diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
Detector resolution: 6.67 pixels mm-1
ω scans
Absorption correction: integration (X-RED32; Stoe & Cie, 2002)
Tmin = 0.686, Tmax = 0.809
46049 measured reflections 15080 independent reflections 12163 reflections with I > 2σ(I)
Rint = 0.061
θmax = 27.9°, θmin = 1.8°
h = −17→17
k = −19→20
l = −22→22
Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.045
wR(F2) = 0.122
S = 0.99
15080 reflections 1028 parameters 12 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(F
o2) + (0.0623P)2 + 3.9061P] where P = (Fo2 + 2Fc2)/3
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq Occ. (<1)
C1 0.7391 (2) 0.5588 (2) 0.26376 (18) 0.0309 (6)
H1 0.7424 0.5346 0.3205 0.037*
C2 0.6712 (2) 0.5352 (2) 0.2256 (2) 0.0350 (7)
H2 0.6286 0.4982 0.2567 0.042*
C3 0.6684 (2) 0.5673 (2) 0.1420 (2) 0.0345 (6)
H3 0.6254 0.5506 0.1157 0.041*
C4 0.7311 (2) 0.6256 (2) 0.09623 (18) 0.0281 (6)
C5 0.7337 (2) 0.6624 (2) 0.00878 (18) 0.0329 (6)
H5 0.6934 0.6465 −0.0206 0.040*
C6 0.7934 (2) 0.7198 (2) −0.03156 (18) 0.0330 (6)
H6 0.7961 0.7403 −0.0885 0.040*
C7 0.8530 (2) 0.7501 (2) 0.01086 (16) 0.0268 (5)
C8 0.9086 (2) 0.8177 (2) −0.02842 (16) 0.0293 (6)
H8 0.9108 0.8426 −0.0852 0.035*
C9 0.9591 (2) 0.8467 (2) 0.01703 (16) 0.0288 (6)
H9 0.9944 0.8925 −0.0083 0.035*
C10 0.9569 (2) 0.8061 (2) 0.10262 (15) 0.0237 (5)
H10 0.9916 0.8256 0.1334 0.028*
C11 0.8539 (2) 0.71416 (19) 0.09647 (15) 0.0223 (5)
C12 0.7941 (2) 0.6488 (2) 0.13982 (16) 0.0233 (5)
C13 1.0977 (2) 0.5472 (2) 0.14667 (16) 0.0250 (5)
H13 1.0790 0.6079 0.1070 0.030*
C14 1.1800 (2) 0.4722 (2) 0.12811 (17) 0.0281 (6)
H14 1.2161 0.4832 0.0776 0.034*
C15 1.2066 (2) 0.3818 (2) 0.18560 (17) 0.0286 (6)
H15 1.2612 0.3308 0.1744 0.034*
C16 1.1510 (2) 0.3668 (2) 0.26175 (17) 0.0261 (5)
C17 1.1750 (2) 0.2752 (2) 0.32563 (19) 0.0320 (6)
H17 1.2258 0.2210 0.3159 0.038*
C18 1.1248 (2) 0.2666 (2) 0.39949 (19) 0.0328 (6)
H18 1.1412 0.2064 0.4398 0.039*
C19 1.0465 (2) 0.3484 (2) 0.41731 (17) 0.0277 (6)
C20 0.9955 (2) 0.3452 (2) 0.49433 (17) 0.0305 (6)
H20 1.0129 0.2878 0.5377 0.037*
H21 0.8859 0.4239 0.5563 0.035*
C22 0.8968 (2) 0.5130 (2) 0.44000 (16) 0.0267 (5)
H22 0.8453 0.5678 0.4479 0.032*
C23 1.0185 (2) 0.4384 (2) 0.35512 (15) 0.0229 (5)
C24 1.0714 (2) 0.44742 (19) 0.27573 (15) 0.0220 (5)
C25 0.8182 (2) 0.8209 (2) 0.31066 (16) 0.0270 (5)
C26 0.7263 (3) 0.8860 (2) 0.3509 (2) 0.0345 (6)
C27 0.6491 (3) 0.8380 (3) 0.4073 (2) 0.0370 (7)
C28 0.5456 (2) 0.9016 (2) 0.44048 (19) 0.0337 (6)
C29 0.5514 (3) 0.9780 (3) 0.4765 (2) 0.0387 (7)
C30 0.5568 (2) 0.4696 (2) 0.51195 (17) 0.0305 (6)
C31 0.5955 (2) 0.4708 (2) 0.59231 (16) 0.0254 (5)
C32 0.5808 (2) 0.5698 (2) 0.60479 (16) 0.0258 (5)
C33 0.6521 (2) 0.5661 (2) 0.66886 (16) 0.0256 (5)
C34 0.6568 (2) 0.4848 (2) 0.75284 (15) 0.0238 (5)
C35 0.4542 (2) 0.8358 (2) 0.85193 (16) 0.0325 (6)
H35 0.4131 0.8150 0.8274 0.039*
C36 0.5260 (3) 0.8819 (3) 0.80270 (19) 0.0387 (7)
H36 0.5333 0.8896 0.7469 0.046*
C37 0.5846 (2) 0.9150 (2) 0.83712 (19) 0.0366 (7)
H37 0.6310 0.9470 0.8048 0.044*
C38 0.5746 (2) 0.9008 (2) 0.92224 (19) 0.0305 (6)
C39 0.6338 (2) 0.9321 (2) 0.9637 (2) 0.0355 (7)
H39 0.6784 0.9674 0.9336 0.043*
C40 0.6259 (2) 0.9112 (2) 1.0459 (2) 0.0345 (7)
H40 0.6661 0.9315 1.0716 0.041*
C41 0.5570 (2) 0.8584 (2) 1.09435 (18) 0.0283 (6)
C42 0.5475 (2) 0.8330 (2) 1.18006 (19) 0.0323 (6)
H42 0.5888 0.8486 1.2088 0.039*
C43 0.4766 (2) 0.7849 (2) 1.22132 (18) 0.0311 (6)
H43 0.4697 0.7676 1.2781 0.037*
C44 0.4151 (2) 0.7621 (2) 1.17733 (16) 0.0271 (5)
H44 0.3666 0.7304 1.2059 0.032*
C45 0.4937 (2) 0.83029 (19) 1.05507 (17) 0.0241 (5)
C46 0.5028 (2) 0.8511 (2) 0.96745 (17) 0.0254 (5)
C47 0.2385 (2) 0.6976 (2) 0.90502 (15) 0.0256 (5)
C48 0.1678 (2) 0.7259 (2) 0.83132 (15) 0.0245 (5)
C49 0.2220 (2) 0.7636 (2) 0.74747 (15) 0.0233 (5)
C50 0.1646 (2) 0.7722 (2) 0.67231 (14) 0.0222 (5)
C51 0.1990 (2) 0.8366 (2) 0.58908 (15) 0.0236 (5)
C52 0.1640 (2) 0.8221 (2) 0.51389 (14) 0.0224 (5)
C53 0.1680 (2) 0.9024 (2) 0.43125 (15) 0.0229 (5)
C54 0.1643 (2) 0.86979 (19) 0.35577 (14) 0.0212 (5)
C55 0.1273 (2) 0.9527 (2) 0.27605 (15) 0.0248 (5)
C56 0.1512 (2) 0.91734 (19) 0.19886 (14) 0.0214 (5)
N1 0.79913 (17) 0.61437 (17) 0.22268 (14) 0.0240 (4)
N2 0.90697 (17) 0.74087 (16) 0.14127 (12) 0.0210 (4)
N4 0.94561 (18) 0.51914 (17) 0.36712 (13) 0.0224 (4)
N5 0.44311 (19) 0.82103 (18) 0.93215 (13) 0.0264 (5)
N6 0.42296 (18) 0.78383 (16) 1.09631 (13) 0.0226 (4)
O1 1.05782 (15) 0.67892 (14) 0.29094 (11) 0.0227 (4)
H1A 1.057 (3) 0.7357 (16) 0.282 (2) 0.034*
H1B 1.1156 (19) 0.644 (2) 0.278 (2) 0.034*
O2 0.82544 (16) 0.73301 (15) 0.33026 (12) 0.0295 (4)
O3 0.87687 (19) 0.86375 (17) 0.26480 (14) 0.0401 (5)
O4 0.73773 (16) 0.41427 (15) 0.76271 (12) 0.0291 (4)
O5 0.57842 (16) 0.50037 (16) 0.79911 (12) 0.0307 (4)
O6 0.21278 (15) 0.73669 (15) 1.10209 (11) 0.0230 (4)
H6A 0.235 (3) 0.6836 (18) 1.1370 (18) 0.035*
H6B 0.183 (3) 0.775 (2) 1.128 (2) 0.035*
O7 0.40724 (16) 0.61865 (15) 1.03947 (11) 0.0259 (4)
H7A 0.379 (3) 0.593 (3) 1.017 (2) 0.039*
H7B 0.415 (3) 0.583 (2) 1.0872 (13) 0.039*
O8 0.24015 (16) 0.91993 (15) 0.99704 (11) 0.0265 (4)
H8A 0.196 (2) 0.940 (3) 0.9622 (19) 0.040*
H8B 0.213 (3) 0.938 (3) 1.0368 (17) 0.040*
O9 0.25083 (16) 0.77031 (14) 0.91641 (10) 0.0258 (4)
O10 0.27299 (19) 0.61056 (16) 0.94211 (12) 0.0352 (5)
O11 0.12639 (16) 0.84333 (14) 0.20584 (10) 0.0242 (4)
O12 0.19102 (18) 0.96729 (15) 0.13913 (11) 0.0296 (4)
O13 0.11155 (19) 0.97708 (16) 0.87225 (12) 0.0326 (5)
H13A 0.117 (3) 1.019 (2) 0.8287 (17) 0.049*
H13B 0.139 (3) 0.9245 (19) 0.864 (3) 0.049*
O14 0.5717 (3) 0.6144 (3) 0.9091 (2) 0.0346 (11) 0.658 (9)
H14A 0.529 (4) 0.603 (5) 0.948 (3) 0.052* 0.658 (9)
H14B 0.557 (5) 0.591 (5) 0.877 (3) 0.052* 0.658 (9)
F1 0.6653 (2) 0.95839 (17) 0.28837 (14) 0.0633 (7)
F2 0.76424 (18) 0.92810 (18) 0.39051 (16) 0.0580 (7)
F3 0.61555 (14) 0.78863 (15) 0.37142 (12) 0.0390 (4)
F4 0.70507 (18) 0.77189 (14) 0.47344 (11) 0.0430 (5)
F5 0.47889 (17) 0.95016 (17) 0.37703 (13) 0.0484 (5)
F6 0.50129 (18) 0.83885 (16) 0.49450 (13) 0.0482 (5)
F7 0.5721 (2) 1.05102 (16) 0.41391 (16) 0.0596 (7)
F8 0.63076 (17) 0.9364 (2) 0.52831 (16) 0.0631 (7)
F9 0.62119 (14) 0.50011 (15) 0.44884 (10) 0.0377 (4)
F10 0.56749 (15) 0.37534 (13) 0.52340 (11) 0.0349 (4)
F11 0.69820 (14) 0.42130 (14) 0.59256 (11) 0.0345 (4)
F12 0.54794 (15) 0.41963 (13) 0.65630 (10) 0.0341 (4)
F13 0.60186 (15) 0.63050 (13) 0.53243 (10) 0.0341 (4)
F14 0.47957 (14) 0.60587 (13) 0.62763 (10) 0.0327 (4)
F15 0.61709 (15) 0.65495 (13) 0.68013 (11) 0.0347 (4)
F16 0.74979 (13) 0.55726 (13) 0.63230 (10) 0.0312 (4)
F17 0.07888 (13) 0.79814 (14) 0.83879 (10) 0.0325 (4)
F18 0.13908 (15) 0.64976 (14) 0.83079 (10) 0.0340 (4)
F20 0.32003 (13) 0.70138 (14) 0.74712 (9) 0.0334 (4)
F21 0.06103 (13) 0.80958 (13) 0.68455 (9) 0.0293 (4)
F22 0.18385 (15) 0.68188 (12) 0.66690 (9) 0.0322 (4)
F23 0.15670 (15) 0.93047 (12) 0.58572 (9) 0.0309 (4)
F24 0.30407 (13) 0.81389 (15) 0.58530 (10) 0.0366 (4)
F25 0.06401 (13) 0.82016 (13) 0.52899 (9) 0.0290 (4)
F26 0.22472 (16) 0.73579 (13) 0.50628 (9) 0.0350 (4)
F27 0.08645 (15) 0.98178 (12) 0.43079 (9) 0.0328 (4)
F28 0.25718 (15) 0.92530 (15) 0.42581 (10) 0.0382 (4)
F29 0.09988 (15) 0.81457 (13) 0.37561 (9) 0.0324 (4)
F30 0.26162 (14) 0.81447 (15) 0.34125 (10) 0.0372 (4)
F31 0.02332 (15) 0.99493 (14) 0.28353 (10) 0.0395 (4)
F32 0.17557 (19) 1.02039 (14) 0.26881 (10) 0.0442 (5)
Zn1 0.92431 (2) 0.64810 (2) 0.264318 (16) 0.02023 (8)
Zn2 0.32520 (2) 0.77141 (2) 1.014147 (16) 0.02163 (8)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C1 0.0295 (14) 0.0311 (15) 0.0335 (14) −0.0143 (12) 0.0040 (11) −0.0089 (12)
C2 0.0263 (14) 0.0300 (15) 0.0523 (18) −0.0131 (12) 0.0025 (13) −0.0146 (13)
C3 0.0259 (14) 0.0323 (16) 0.0542 (18) −0.0092 (12) −0.0038 (13) −0.0233 (14)
C4 0.0238 (13) 0.0265 (14) 0.0387 (15) −0.0028 (11) −0.0061 (11) −0.0186 (12)
C5 0.0325 (15) 0.0353 (16) 0.0353 (15) −0.0018 (13) −0.0102 (12) −0.0213 (12)
C6 0.0340 (15) 0.0377 (16) 0.0251 (13) 0.0019 (13) −0.0081 (11) −0.0165 (12)
C7 0.0276 (13) 0.0280 (14) 0.0226 (12) −0.0015 (11) −0.0031 (10) −0.0110 (10)
C8 0.0299 (14) 0.0324 (15) 0.0172 (11) −0.0020 (12) 0.0020 (10) −0.0055 (10)
C9 0.0285 (14) 0.0284 (14) 0.0230 (12) −0.0081 (12) 0.0044 (10) −0.0026 (10)
C10 0.0232 (12) 0.0243 (13) 0.0217 (11) −0.0067 (11) 0.0021 (9) −0.0064 (10)
C11 0.0218 (12) 0.0235 (13) 0.0206 (11) −0.0026 (10) −0.0011 (9) −0.0098 (9)
C12 0.0217 (12) 0.0241 (13) 0.0252 (12) −0.0037 (10) −0.0012 (9) −0.0118 (10)
C13 0.0270 (13) 0.0299 (14) 0.0202 (11) −0.0101 (11) 0.0021 (10) −0.0103 (10)
C14 0.0298 (14) 0.0336 (15) 0.0249 (12) −0.0109 (12) 0.0039 (10) −0.0149 (11)
C15 0.0280 (13) 0.0305 (15) 0.0322 (14) −0.0082 (12) 0.0010 (11) −0.0174 (11)
C16 0.0279 (13) 0.0245 (13) 0.0301 (13) −0.0103 (11) −0.0031 (10) −0.0106 (10)
C17 0.0331 (15) 0.0246 (14) 0.0402 (15) −0.0093 (12) −0.0040 (12) −0.0108 (12)
C18 0.0353 (15) 0.0240 (14) 0.0388 (15) −0.0133 (12) −0.0051 (12) −0.0034 (11)
C19 0.0299 (14) 0.0281 (14) 0.0281 (13) −0.0167 (12) −0.0033 (10) −0.0036 (11)
C20 0.0349 (15) 0.0360 (16) 0.0243 (12) −0.0232 (13) −0.0036 (11) −0.0003 (11)
C21 0.0340 (15) 0.0400 (16) 0.0179 (11) −0.0236 (13) 0.0018 (10) −0.0041 (11)
C22 0.0289 (13) 0.0358 (15) 0.0203 (11) −0.0175 (12) 0.0038 (10) −0.0093 (10)
C23 0.0234 (12) 0.0260 (13) 0.0232 (11) −0.0128 (11) −0.0011 (9) −0.0069 (10)
C24 0.0247 (12) 0.0243 (13) 0.0222 (11) −0.0121 (10) −0.0020 (9) −0.0086 (9)
C25 0.0294 (14) 0.0306 (14) 0.0244 (12) −0.0111 (12) 0.0057 (10) −0.0137 (11)
C26 0.0346 (16) 0.0310 (16) 0.0398 (16) −0.0120 (13) 0.0092 (12) −0.0155 (13)
C27 0.0378 (17) 0.0410 (18) 0.0368 (15) −0.0152 (14) 0.0120 (13) −0.0201 (13)
C28 0.0277 (14) 0.0356 (16) 0.0370 (15) −0.0109 (13) 0.0056 (12) −0.0115 (12)
C30 0.0340 (16) 0.0308 (15) 0.0275 (13) −0.0113 (13) −0.0009 (11) −0.0084 (11)
C31 0.0258 (13) 0.0282 (14) 0.0229 (12) −0.0108 (11) 0.0001 (10) −0.0065 (10)
C32 0.0272 (13) 0.0272 (14) 0.0209 (11) −0.0101 (11) −0.0020 (10) −0.0024 (10)
C33 0.0275 (13) 0.0266 (14) 0.0239 (12) −0.0109 (11) −0.0024 (10) −0.0060 (10)
C34 0.0277 (13) 0.0253 (13) 0.0209 (11) −0.0098 (11) −0.0031 (10) −0.0076 (10)
C35 0.0343 (15) 0.0380 (16) 0.0196 (12) −0.0122 (13) 0.0013 (11) −0.0017 (11)
C36 0.0377 (17) 0.0399 (18) 0.0251 (13) −0.0105 (14) 0.0075 (12) 0.0023 (12)
C37 0.0306 (15) 0.0283 (15) 0.0371 (15) −0.0085 (12) 0.0094 (12) 0.0028 (12)
C38 0.0242 (13) 0.0213 (13) 0.0385 (15) −0.0069 (11) 0.0044 (11) −0.0017 (11)
C39 0.0233 (14) 0.0251 (14) 0.0541 (19) −0.0097 (12) 0.0037 (12) −0.0069 (13)
C40 0.0233 (13) 0.0268 (15) 0.0562 (19) −0.0102 (12) −0.0033 (13) −0.0131 (13)
C41 0.0233 (13) 0.0216 (13) 0.0404 (15) −0.0058 (11) −0.0047 (11) −0.0095 (11)
C42 0.0319 (15) 0.0292 (15) 0.0408 (16) −0.0083 (12) −0.0106 (12) −0.0143 (12)
C43 0.0374 (16) 0.0322 (15) 0.0269 (13) −0.0109 (13) −0.0081 (11) −0.0099 (11)
C44 0.0312 (14) 0.0291 (14) 0.0220 (12) −0.0116 (12) −0.0028 (10) −0.0060 (10)
C45 0.0205 (12) 0.0199 (12) 0.0306 (13) −0.0069 (10) −0.0017 (10) −0.0053 (10)
C46 0.0215 (12) 0.0205 (12) 0.0291 (13) −0.0063 (10) 0.0019 (10) −0.0024 (10)
C47 0.0352 (14) 0.0317 (14) 0.0140 (10) −0.0151 (12) 0.0014 (10) −0.0080 (10)
C48 0.0316 (14) 0.0295 (14) 0.0170 (11) −0.0145 (12) 0.0008 (10) −0.0083 (10)
C49 0.0286 (13) 0.0276 (13) 0.0174 (11) −0.0122 (11) 0.0010 (9) −0.0085 (9)
C50 0.0290 (13) 0.0262 (13) 0.0157 (10) −0.0130 (11) 0.0007 (9) −0.0080 (9)
C51 0.0280 (13) 0.0299 (14) 0.0164 (11) −0.0134 (11) 0.0008 (9) −0.0078 (10)
C52 0.0283 (13) 0.0259 (13) 0.0160 (10) −0.0113 (11) 0.0021 (9) −0.0084 (9)
C53 0.0296 (13) 0.0263 (13) 0.0171 (11) −0.0144 (11) 0.0017 (9) −0.0073 (9)
C54 0.0261 (12) 0.0248 (13) 0.0160 (10) −0.0116 (11) 0.0011 (9) −0.0073 (9)
C55 0.0372 (15) 0.0228 (13) 0.0174 (11) −0.0137 (11) 0.0009 (10) −0.0063 (9)
C56 0.0283 (13) 0.0238 (12) 0.0138 (10) −0.0102 (11) 0.0004 (9) −0.0061 (9)
N1 0.0211 (10) 0.0265 (12) 0.0259 (10) −0.0088 (9) 0.0012 (8) −0.0091 (9)
N2 0.0213 (10) 0.0237 (11) 0.0177 (9) −0.0068 (9) 0.0009 (8) −0.0067 (8)
N3 0.0221 (10) 0.0249 (11) 0.0202 (10) −0.0098 (9) 0.0010 (8) −0.0082 (8)
N4 0.0254 (11) 0.0270 (11) 0.0178 (9) −0.0139 (9) 0.0015 (8) −0.0056 (8)
N5 0.0274 (11) 0.0291 (12) 0.0202 (10) −0.0112 (10) 0.0033 (8) −0.0035 (9)
N6 0.0256 (11) 0.0236 (11) 0.0208 (10) −0.0098 (9) −0.0030 (8) −0.0062 (8)
O1 0.0264 (9) 0.0233 (9) 0.0209 (8) −0.0112 (8) 0.0018 (7) −0.0076 (7)
O2 0.0320 (10) 0.0304 (11) 0.0264 (9) −0.0109 (9) 0.0097 (8) −0.0124 (8)
O3 0.0458 (13) 0.0345 (12) 0.0411 (12) −0.0179 (11) 0.0169 (10) −0.0149 (10)
O4 0.0286 (10) 0.0305 (11) 0.0245 (9) −0.0065 (9) −0.0010 (7) −0.0063 (8)
O5 0.0304 (10) 0.0359 (11) 0.0228 (9) −0.0095 (9) 0.0020 (8) −0.0071 (8)
O6 0.0264 (9) 0.0261 (10) 0.0182 (8) −0.0114 (8) 0.0036 (7) −0.0074 (7)
O7 0.0332 (10) 0.0261 (10) 0.0196 (8) −0.0114 (8) 0.0024 (7) −0.0077 (7)
O8 0.0323 (10) 0.0282 (10) 0.0196 (9) −0.0101 (9) −0.0020 (7) −0.0068 (7)
O9 0.0366 (11) 0.0290 (10) 0.0156 (8) −0.0138 (9) −0.0021 (7) −0.0073 (7)
O10 0.0551 (14) 0.0317 (11) 0.0237 (9) −0.0189 (10) −0.0093 (9) −0.0057 (8)
O11 0.0340 (10) 0.0273 (10) 0.0171 (8) −0.0166 (8) 0.0034 (7) −0.0085 (7)
O12 0.0462 (12) 0.0301 (10) 0.0171 (8) −0.0217 (10) 0.0053 (8) −0.0056 (7)
O13 0.0464 (13) 0.0276 (11) 0.0221 (9) −0.0096 (10) −0.0052 (9) −0.0056 (8)
O14 0.0310 (18) 0.042 (2) 0.042 (2) −0.0169 (15) 0.0105 (14) −0.0263 (16)
F2 0.0543 (13) 0.0726 (16) 0.0839 (16) −0.0447 (12) 0.0374 (12) −0.0618 (14)
F3 0.0319 (9) 0.0499 (11) 0.0463 (10) −0.0197 (9) 0.0060 (8) −0.0248 (9)
F4 0.0620 (13) 0.0349 (10) 0.0261 (8) −0.0101 (9) −0.0012 (8) −0.0066 (7)
F5 0.0439 (11) 0.0534 (13) 0.0437 (11) −0.0091 (10) −0.0086 (9) −0.0124 (9)
F6 0.0568 (13) 0.0447 (12) 0.0471 (11) −0.0248 (10) 0.0211 (10) −0.0193 (9)
F7 0.0680 (15) 0.0402 (12) 0.0777 (16) −0.0299 (11) 0.0433 (13) −0.0325 (11)
F8 0.0382 (11) 0.101 (2) 0.0743 (16) −0.0135 (12) −0.0024 (11) −0.0647 (15)
F9 0.0314 (9) 0.0531 (12) 0.0277 (8) −0.0141 (9) 0.0067 (7) −0.0131 (8)
F10 0.0396 (10) 0.0284 (9) 0.0376 (9) −0.0046 (8) −0.0130 (7) −0.0122 (7)
F11 0.0288 (9) 0.0376 (10) 0.0389 (9) −0.0066 (8) −0.0052 (7) −0.0159 (8)
F12 0.0468 (10) 0.0329 (9) 0.0244 (8) −0.0206 (8) −0.0002 (7) −0.0031 (7)
F13 0.0459 (10) 0.0335 (9) 0.0235 (8) −0.0222 (8) −0.0066 (7) 0.0025 (7)
F14 0.0303 (9) 0.0336 (9) 0.0340 (9) −0.0058 (7) −0.0038 (7) −0.0129 (7)
F15 0.0432 (10) 0.0267 (9) 0.0369 (9) −0.0121 (8) −0.0074 (8) −0.0095 (7)
F16 0.0316 (9) 0.0392 (10) 0.0267 (8) −0.0203 (8) 0.0007 (6) −0.0062 (7)
F17 0.0310 (9) 0.0457 (10) 0.0222 (7) −0.0098 (8) 0.0029 (6) −0.0156 (7)
F18 0.0498 (11) 0.0405 (10) 0.0216 (7) −0.0287 (9) −0.0012 (7) −0.0072 (7)
F19 0.0492 (10) 0.0350 (9) 0.0188 (7) −0.0256 (8) −0.0023 (7) −0.0070 (6)
F20 0.0286 (8) 0.0472 (11) 0.0207 (7) −0.0068 (8) 0.0001 (6) −0.0108 (7)
F21 0.0261 (8) 0.0446 (10) 0.0200 (7) −0.0153 (7) 0.0020 (6) −0.0101 (7)
F22 0.0549 (11) 0.0262 (8) 0.0196 (7) −0.0159 (8) −0.0032 (7) −0.0078 (6)
F23 0.0518 (11) 0.0271 (8) 0.0207 (7) −0.0184 (8) −0.0025 (7) −0.0091 (6)
F24 0.0262 (8) 0.0632 (13) 0.0212 (7) −0.0192 (9) 0.0008 (6) −0.0093 (8)
F25 0.0340 (9) 0.0434 (10) 0.0189 (7) −0.0245 (8) 0.0023 (6) −0.0101 (6)
F26 0.0524 (11) 0.0278 (9) 0.0194 (7) −0.0021 (8) −0.0029 (7) −0.0100 (6)
F27 0.0490 (11) 0.0258 (8) 0.0218 (7) −0.0058 (8) −0.0026 (7) −0.0101 (6)
F28 0.0472 (11) 0.0623 (12) 0.0211 (7) −0.0407 (10) 0.0035 (7) −0.0109 (8)
F29 0.0558 (11) 0.0370 (9) 0.0169 (7) −0.0323 (9) 0.0033 (7) −0.0080 (6)
F30 0.0341 (9) 0.0486 (11) 0.0222 (8) 0.0018 (8) −0.0019 (7) −0.0164 (7)
F31 0.0409 (10) 0.0439 (11) 0.0261 (8) 0.0054 (9) −0.0065 (7) −0.0172 (8)
F32 0.0888 (16) 0.0385 (10) 0.0215 (8) −0.0438 (11) 0.0037 (9) −0.0078 (7)
Zn1 0.02239 (15) 0.02375 (15) 0.01586 (13) −0.00966 (12) 0.00286 (10) −0.00658 (11)
Zn2 0.02592 (15) 0.02748 (16) 0.01473 (13) −0.01326 (13) 0.00132 (10) −0.00631 (11)
Geometric parameters (Å, º)
C1—N1 1.331 (4) C35—N5 1.326 (3)
C1—C2 1.401 (4) C35—C36 1.407 (4)
C1—H1 0.93 C35—H35 0.93
C2—C3 1.368 (5) C36—C37 1.358 (5)
C2—H2 0.93 C36—H36 0.93
C3—C4 1.407 (4) C37—C38 1.411 (4)
C3—H3 0.93 C37—H37 0.93
C4—C12 1.409 (4) C38—C46 1.415 (4)
C4—C5 1.430 (4) C38—C39 1.427 (5)
C5—C6 1.345 (5) C39—C40 1.349 (5)
C5—H5 0.93 C39—H39 0.93
C6—H6 0.93 C40—H40 0.93
C7—C11 1.401 (4) C41—C42 1.403 (4)
C7—C8 1.412 (4) C41—C45 1.411 (4)
C8—C9 1.368 (4) C42—C43 1.374 (4)
C8—H8 0.93 C42—H42 0.93
C9—C10 1.405 (4) C43—C44 1.395 (4)
C9—H9 0.93 C43—H43 0.93
C10—N2 1.330 (3) C44—N6 1.328 (3)
C10—H10 0.93 C44—H44 0.93
C11—N2 1.360 (3) C45—N6 1.357 (3)
C11—C12 1.440 (4) C45—C46 1.440 (4)
C12—N1 1.358 (3) C46—N5 1.346 (4)
C13—N3 1.331 (3) C47—O10 1.226 (4)
C13—C14 1.395 (4) C47—O9 1.256 (3)
C13—H13 0.93 C47—C48 1.562 (4)
C14—C15 1.373 (4) C48—F18 1.346 (3)
C14—H14 0.93 C48—F17 1.357 (3)
C15—C16 1.409 (4) C48—C49 1.548 (3)
C15—H15 0.93 C49—F19 1.342 (3)
C16—C24 1.403 (4) C49—F20 1.349 (3)
C16—C17 1.435 (4) C49—C50 1.557 (3)
C17—C18 1.346 (4) C50—F21 1.338 (3)
C17—H17 0.93 C50—F22 1.349 (3)
C18—C19 1.434 (4) C50—C51 1.558 (3)
C18—H18 0.93 C51—F23 1.339 (3)
C19—C20 1.404 (4) C51—F24 1.342 (3)
C19—C23 1.407 (4) C51—C52 1.553 (3)
C20—C21 1.363 (5) C52—F26 1.338 (3)
C20—H20 0.93 C52—F25 1.346 (3)
C21—C22 1.405 (4) C52—C53 1.553 (3)
C21—H21 0.93 C53—F27 1.338 (3)
C22—N4 1.326 (3) C53—F28 1.345 (3)
C22—H22 0.93 C53—C54 1.559 (3)
C23—N4 1.354 (4) C54—F29 1.340 (3)
C23—C24 1.441 (4) C54—F30 1.345 (3)
C24—N3 1.357 (3) C54—C55 1.541 (4)
C25—O3 1.227 (3) C55—F31 1.343 (3)
C25—O2 1.243 (4) C55—F32 1.359 (3)
C25—C26 1.549 (4) C55—C56 1.557 (3)
C26—F2 1.329 (4) C56—O12 1.237 (3)
C26—F1 1.389 (4) C56—O11 1.246 (3)
C26—C27 1.522 (4) N1—Zn1 2.197 (2)
C27—F3 1.335 (4) N2—Zn1 2.126 (2)
C27—F4 1.369 (4) N3—Zn1 2.206 (2)
C27—C28 1.569 (4) N4—Zn1 2.138 (2)
C28—F6 1.333 (4) N5—Zn2 2.136 (2)
C28—F5 1.367 (4) N6—Zn2 2.168 (2)
C29—F8 1.343 (4) O1—H1A 0.824 (18)
C29—F7 1.347 (4) O1—H1B 0.833 (18)
C29—C29i 1.566 (6) O2—Zn1 2.040 (2)
C30—F10 1.346 (3) O6—Zn2 2.0587 (18)
C30—F9 1.351 (3) O6—H6A 0.823 (18)
C30—C30ii 1.539 (6) O6—H6B 0.818 (18)
C30—C31 1.564 (4) O7—Zn2 2.144 (2)
C31—F11 1.340 (3) O7—H7A 0.840 (19)
C31—F12 1.343 (3) O7—H7B 0.826 (18)
C31—C32 1.541 (4) O8—Zn2 2.113 (2)
C32—F14 1.347 (3) O8—H8A 0.826 (19)
C32—F13 1.346 (3) O8—H8B 0.820 (19)
C32—C33 1.549 (4) O9—Zn2 2.0980 (18)
C33—F15 1.350 (3) O13—H13A 0.822 (19)
C33—F16 1.362 (3) O13—H13B 0.819 (19)
C33—C34 1.561 (4) O14—H14A 0.83 (2)
C34—O4 1.237 (3) O14—H14B 0.82 (2)
C34—O5 1.239 (3)
N1—C1—C2 123.1 (3) C38—C39—H39 119.5
N1—C1—H1 118.4 C39—C40—C41 121.4 (3)
C2—C1—H1 118.4 C39—C40—H40 119.3
C3—C2—C1 119.2 (3) C41—C40—H40 119.3
C3—C2—H2 120.4 C42—C41—C45 117.0 (3)
C1—C2—H2 120.4 C42—C41—C40 123.7 (3)
C2—C3—C4 119.5 (3) C45—C41—C40 119.2 (3)
C2—C3—H3 120.2 C43—C42—C41 119.6 (3)
C4—C3—H3 120.2 C43—C42—H42 120.2
C3—C4—C12 117.5 (3) C41—C42—H42 120.2
C3—C4—C5 123.7 (3) C42—C43—C44 119.5 (3)
C12—C4—C5 118.9 (3) C42—C43—H43 120.3
C6—C5—C4 120.9 (3) C44—C43—H43 120.3
C6—C5—H5 119.5 N6—C44—C43 122.6 (3)
C4—C5—H5 119.5 N6—C44—H44 118.7
C5—C6—C7 121.7 (3) C43—C44—H44 118.7
C5—C6—H6 119.1 N6—C45—C41 122.9 (3)
C7—C6—H6 119.1 N6—C45—C46 117.5 (2)
C11—C7—C8 117.3 (3) C41—C45—C46 119.6 (2)
C11—C7—C6 118.9 (3) N5—C46—C38 122.8 (3)
C8—C7—C6 123.7 (3) N5—C46—C45 118.1 (2)
C9—C8—C7 120.1 (2) C38—C46—C45 119.1 (3)
C9—C8—H8 120.0 O10—C47—O9 130.2 (3)
C7—C8—H8 120.0 O10—C47—C48 117.0 (2)
C8—C9—C10 118.8 (3) O9—C47—C48 112.9 (2)
C8—C9—H9 120.6 F18—C48—F17 107.7 (2)
C10—C9—H9 120.6 F18—C48—C49 108.4 (2)
N2—C10—C9 122.5 (3) F17—C48—C49 107.6 (2)
C9—C10—H10 118.8 F17—C48—C47 108.9 (2)
N2—C11—C7 122.5 (2) C49—C48—C47 112.9 (2)
N2—C11—C12 118.0 (2) F19—C49—F20 108.4 (2)
C7—C11—C12 119.5 (2) F19—C49—C48 109.1 (2)
N1—C12—C4 122.8 (3) F20—C49—C48 107.6 (2)
N1—C12—C11 117.4 (2) F19—C49—C50 108.3 (2)
C4—C12—C11 119.8 (2) F20—C49—C50 108.8 (2)
N3—C13—C14 123.2 (3) C48—C49—C50 114.4 (2)
N3—C13—H13 118.4 F21—C50—F22 108.3 (2)
C14—C13—H13 118.4 F21—C50—C49 108.9 (2)
C15—C14—C13 118.9 (3) F22—C50—C49 108.4 (2)
C15—C14—H14 120.6 F21—C50—C51 108.5 (2)
C13—C14—H14 120.6 F22—C50—C51 108.2 (2)
C14—C15—C16 119.6 (3) C49—C50—C51 114.3 (2)
C14—C15—H15 120.2 F23—C51—F24 109.2 (2)
C16—C15—H15 120.2 F23—C51—C52 108.6 (2)
C24—C16—C15 117.4 (3) F24—C51—C52 108.7 (2)
C24—C16—C17 119.4 (3) F23—C51—C50 108.8 (2)
C15—C16—C17 123.2 (3) F24—C51—C50 108.6 (2)
C18—C17—C16 120.8 (3) C52—C51—C50 113.0 (2)
C18—C17—H17 119.6 F26—C52—F25 108.6 (2)
C16—C17—H17 119.6 F26—C52—C53 108.8 (2)
C17—C18—C19 121.4 (3) F25—C52—C53 107.6 (2)
C17—C18—H18 119.3 F26—C52—C51 108.5 (2)
C19—C18—H18 119.3 F25—C52—C51 107.76 (19)
C20—C19—C23 116.9 (3) C53—C52—C51 115.4 (2)
C20—C19—C18 124.0 (3) F27—C53—F28 108.4 (2)
C23—C19—C18 119.2 (3) F27—C53—C52 108.5 (2)
C21—C20—C19 120.1 (3) F28—C53—C52 108.8 (2)
C21—C20—H20 119.9 F27—C53—C54 109.4 (2)
C19—C20—H20 119.9 F28—C53—C54 108.9 (2)
C20—C21—C22 119.4 (3) C52—C53—C54 112.7 (2)
C20—C21—H21 120.3 F29—C54—F30 108.5 (2)
C22—C21—H21 120.3 F29—C54—C55 108.4 (2)
N4—C22—C21 122.0 (3) F30—C54—C55 108.2 (2)
N4—C22—H22 119.0 F29—C54—C53 107.96 (19)
C21—C22—H22 119.0 F30—C54—C53 107.8 (2)
N4—C23—C19 122.7 (2) C55—C54—C53 115.8 (2)
N4—C23—C24 117.8 (2) F31—C55—F32 107.7 (2)
C19—C23—C24 119.5 (3) F31—C55—C54 108.9 (2)
N3—C24—C16 122.7 (2) F32—C55—C54 106.9 (2)
N3—C24—C23 117.5 (2) F31—C55—C56 109.1 (2)
C16—C24—C23 119.7 (2) F32—C55—C56 110.9 (2)
O3—C25—O2 128.4 (3) C54—C55—C56 113.2 (2)
O3—C25—C26 114.4 (3) O12—C56—O11 129.3 (2)
O2—C25—C26 117.2 (2) O12—C56—C55 115.4 (2)
F2—C26—F1 108.4 (3) O11—C56—C55 115.4 (2)
F1—C26—C27 104.3 (3) C1—N1—Zn1 130.6 (2)
F2—C26—C25 109.6 (3) C12—N1—Zn1 111.09 (17)
F1—C26—C25 107.5 (2) C10—N2—C11 118.8 (2)
C27—C26—C25 117.2 (3) C10—N2—Zn1 127.32 (18)
F3—C27—F4 107.6 (3) C11—N2—Zn1 112.58 (17)
F3—C27—C26 110.9 (3) C13—N3—C24 118.1 (2)
F4—C27—C26 105.7 (3) C13—N3—Zn1 128.79 (19)
F3—C27—C28 104.1 (3) C24—N3—Zn1 112.85 (16)
F4—C27—C28 107.8 (2) C22—N4—C23 118.9 (2)
C26—C27—C28 120.2 (3) C22—N4—Zn1 126.0 (2)
F6—C28—F5 106.1 (3) C23—N4—Zn1 115.05 (16)
F6—C28—C29 110.7 (3) C35—N5—C46 118.7 (2)
F5—C28—C29 106.5 (3) C35—N5—Zn2 127.7 (2)
F6—C28—C27 106.0 (3) C46—N5—Zn2 112.97 (17)
F5—C28—C27 107.5 (3) C44—N6—C45 118.4 (2)
C29—C28—C27 119.4 (3) C44—N6—Zn2 129.24 (19)
F8—C29—F7 109.8 (3) C45—N6—Zn2 111.99 (17)
F8—C29—C28 107.9 (3) Zn1—O1—H1A 121 (3)
F7—C29—C28 107.2 (3) Zn1—O1—H1B 114 (3)
F8—C29—C29i 108.7 (4) H1A—O1—H1B 113 (4)
F7—C29—C29i 108.4 (4) C25—O2—Zn1 119.72 (17)
C28—C29—C29i 114.8 (3) Zn2—O6—H6A 113 (3)
F10—C30—F9 109.3 (2) Zn2—O6—H6B 118 (3)
F10—C30—C30ii 108.3 (3) H6A—O6—H6B 105 (4)
F9—C30—C30ii 107.6 (3) Zn2—O7—H7A 111 (3)
F10—C30—C31 105.1 (2) Zn2—O7—H7B 120 (3)
F9—C30—C31 108.6 (2) H7A—O7—H7B 104 (4)
C30ii—C30—C31 117.8 (3) Zn2—O8—H8A 111 (3)
F11—C31—F12 107.9 (2) Zn2—O8—H8B 120 (3)
F11—C31—C32 109.2 (2) H8A—O8—H8B 109 (4)
F12—C31—C32 107.9 (2) C47—O9—Zn2 127.12 (17)
F11—C31—C30 104.4 (2) H13A—O13—H13B 106 (4)
F12—C31—C30 108.1 (2) H14A—O14—H14B 102 (6)
C32—C31—C30 118.9 (2) O2—Zn1—N2 103.70 (8)
F14—C32—F13 108.9 (2) O2—Zn1—N4 92.33 (9)
F14—C32—C31 108.2 (2) N2—Zn1—N4 160.24 (9)
F13—C32—C31 107.9 (2) O2—Zn1—O1 91.47 (8)
F14—C32—C33 108.8 (2) N2—Zn1—O1 97.71 (8)
F13—C32—C33 107.9 (2) N4—Zn1—O1 93.28 (8)
C31—C32—C33 114.9 (2) O2—Zn1—N1 94.92 (9)
F15—C33—F16 106.7 (2) N2—Zn1—N1 77.40 (8)
F15—C33—C32 106.1 (2) N4—Zn1—N1 89.95 (8)
F16—C33—C32 106.3 (2) O1—Zn1—N1 172.71 (8)
F15—C33—C34 110.2 (2) O2—Zn1—N3 167.92 (8)
F16—C33—C34 110.7 (2) N2—Zn1—N3 88.03 (8)
C32—C33—C34 116.4 (2) N4—Zn1—N3 76.81 (8)
O4—C34—O5 129.9 (3) O1—Zn1—N3 84.00 (8)
O5—C34—C33 114.9 (2) O6—Zn2—O9 95.59 (8)
N5—C35—C36 122.2 (3) O6—Zn2—O8 87.90 (8)
N5—C35—H35 118.9 O9—Zn2—O8 91.13 (8)
C36—C35—H35 118.9 O6—Zn2—N5 171.75 (9)
C37—C36—C35 119.7 (3) O9—Zn2—N5 90.90 (8)
C37—C36—H36 120.2 O8—Zn2—N5 86.88 (9)
C35—C36—H36 120.2 O6—Zn2—O7 88.76 (8)
C36—C37—C38 119.6 (3) O9—Zn2—O7 90.83 (8)
C36—C37—H37 120.2 O8—Zn2—O7 176.28 (7)
C38—C37—H37 120.2 N5—Zn2—O7 96.25 (9)
C37—C38—C46 117.0 (3) O6—Zn2—N6 95.67 (8)
C37—C38—C39 123.4 (3) O9—Zn2—N6 168.64 (8)
C46—C38—C39 119.6 (3) O8—Zn2—N6 87.75 (8)
C40—C39—C38 120.9 (3) N5—Zn2—N6 77.75 (9)
C40—C39—H39 119.5 O7—Zn2—N6 90.95 (8)
N1—C1—C2—C3 2.5 (5) C48—C49—C50—F22 −75.8 (3)
C1—C2—C3—C4 −1.9 (5) F19—C49—C50—C51 41.4 (3)
C2—C3—C4—C12 −0.4 (4) F20—C49—C50—C51 −76.2 (3)
C2—C3—C4—C5 179.9 (3) C48—C49—C50—C51 163.3 (2)
C3—C4—C5—C6 178.4 (3) F21—C50—C51—F23 47.1 (3)
C12—C4—C5—C6 −1.2 (4) F22—C50—C51—F23 164.4 (2)
C4—C5—C6—C7 −2.9 (5) C49—C50—C51—F23 −74.7 (3)
C5—C6—C7—C11 3.9 (4) F21—C50—C51—F24 165.9 (2)
C5—C6—C7—C8 −173.6 (3) F22—C50—C51—F24 −76.9 (3)
C11—C7—C8—C9 −0.9 (4) C49—C50—C51—F24 44.1 (3)
C6—C7—C8—C9 176.5 (3) F21—C50—C51—C52 −73.5 (3)
C7—C8—C9—C10 1.7 (4) F22—C50—C51—C52 43.8 (3)
C8—C9—C10—N2 −0.4 (4) C49—C50—C51—C52 164.7 (2)
C8—C7—C11—N2 −1.1 (4) F23—C51—C52—F26 165.5 (2)
C6—C7—C11—N2 −178.7 (2) F24—C51—C52—F26 46.8 (3)
C8—C7—C11—C12 176.8 (2) C50—C51—C52—F26 −73.8 (3)
C6—C7—C11—C12 −0.8 (4) F23—C51—C52—F25 −77.1 (3)
C3—C4—C12—N1 2.5 (4) F24—C51—C52—F25 164.3 (2)
C5—C4—C12—N1 −177.8 (3) C50—C51—C52—F25 43.7 (3)
C3—C4—C12—C11 −175.4 (3) F23—C51—C52—C53 43.2 (3)
C5—C4—C12—C11 4.3 (4) F24—C51—C52—C53 −75.4 (3)
N2—C11—C12—N1 −3.3 (4) C50—C51—C52—C53 164.0 (2)
C7—C11—C12—N1 178.7 (2) F26—C52—C53—F27 162.4 (2)
N2—C11—C12—C4 174.8 (2) F25—C52—C53—F27 44.9 (3)
C7—C11—C12—C4 −3.2 (4) C51—C52—C53—F27 −75.5 (3)
N3—C13—C14—C15 1.3 (4) F26—C52—C53—F28 −79.9 (3)
C13—C14—C15—C16 −0.2 (4) F25—C52—C53—F28 162.6 (2)
C14—C15—C16—C24 −1.6 (4) C51—C52—C53—F28 42.3 (3)
C14—C15—C16—C17 −179.0 (3) F26—C52—C53—C54 41.1 (3)
C24—C16—C17—C18 −2.4 (4) F25—C52—C53—C54 −76.4 (3)
C15—C16—C17—C18 174.9 (3) C51—C52—C53—C54 163.2 (2)
C17—C18—C19—C20 −176.5 (3) F28—C53—C54—F29 157.4 (2)
C17—C18—C19—C23 2.7 (4) C52—C53—C54—F29 36.6 (3)
C23—C19—C20—C21 1.8 (4) F27—C53—C54—F30 158.8 (2)
C18—C19—C20—C21 −179.0 (3) F28—C53—C54—F30 40.4 (3)
C19—C20—C21—C22 −1.3 (4) C52—C53—C54—F30 −80.4 (3)
C20—C21—C22—N4 −0.6 (4) F27—C53—C54—C55 37.5 (3)
C20—C19—C23—N4 −0.5 (4) F28—C53—C54—C55 −80.9 (3)
C18—C19—C23—N4 −179.8 (2) C52—C53—C54—C55 158.3 (2)
C20—C19—C23—C24 177.3 (2) F29—C54—C55—F31 48.7 (3)
C18—C19—C23—C24 −2.0 (4) F30—C54—C55—F31 166.2 (2)
C15—C16—C24—N3 2.5 (4) C53—C54—C55—F31 −72.7 (3)
C17—C16—C24—N3 179.9 (2) F29—C54—C55—F32 164.8 (2)
C15—C16—C24—C23 −174.4 (2) F30—C54—C55—F32 −77.8 (3)
C17—C16—C24—C23 3.0 (4) C53—C54—C55—F32 43.3 (3)
N4—C23—C24—N3 0.0 (3) F29—C54—C55—C56 −72.8 (3)
C19—C23—C24—N3 −177.9 (2) F30—C54—C55—C56 44.7 (3)
N4—C23—C24—C16 177.1 (2) C53—C54—C55—C56 165.8 (2)
C19—C23—C24—C16 −0.9 (4) F31—C55—C56—O12 105.3 (3)
O3—C25—C26—F2 −55.7 (4) F32—C55—C56—O12 −13.1 (4)
O2—C25—C26—F2 122.0 (3) C54—C55—C56—O12 −133.3 (3)
O3—C25—C26—F1 61.9 (4) F31—C55—C56—O11 −73.9 (3)
O2—C25—C26—F1 −120.3 (3) F32—C55—C56—O11 167.6 (2)
O3—C25—C26—C27 178.9 (3) C54—C55—C56—O11 47.4 (3)
O2—C25—C26—C27 −3.3 (4) C2—C1—N1—C12 −0.4 (4)
F2—C26—C27—F3 −175.3 (3) C2—C1—N1—Zn1 −171.8 (2)
F1—C26—C27—F3 68.9 (3) C4—C12—N1—C1 −2.1 (4)
C25—C26—C27—F3 −49.8 (4) C11—C12—N1—C1 175.9 (3)
F2—C26—C27—F4 −58.9 (3) C4—C12—N1—Zn1 170.9 (2)
F1—C26—C27—F4 −174.7 (2) C11—C12—N1—Zn1 −11.1 (3)
C25—C26—C27—F4 66.6 (3) C9—C10—N2—C11 −1.7 (4)
F2—C26—C27—C28 63.2 (4) C9—C10—N2—Zn1 164.3 (2)
F1—C26—C27—C28 −52.7 (4) C7—C11—N2—C10 2.4 (4)
C25—C26—C27—C28 −171.4 (3) C12—C11—N2—C10 −175.6 (2)
F3—C27—C28—F6 61.9 (3) C7—C11—N2—Zn1 −165.5 (2)
F4—C27—C28—F6 −52.2 (3) C12—C11—N2—Zn1 16.5 (3)
C26—C27—C28—F6 −173.3 (3) C14—C13—N3—C24 −0.5 (4)
F3—C27—C28—F5 −51.2 (3) C14—C13—N3—Zn1 172.9 (2)
F4—C27—C28—F5 −165.3 (2) C16—C24—N3—C13 −1.4 (4)
C26—C27—C28—F5 73.6 (4) C23—C24—N3—C13 175.5 (2)
F3—C27—C28—C29 −172.5 (3) C16—C24—N3—Zn1 −175.9 (2)
F4—C27—C28—C29 73.4 (4) C23—C24—N3—Zn1 1.0 (3)
C26—C27—C28—C29 −47.6 (4) C21—C22—N4—C23 1.9 (4)
F6—C28—C29—F8 76.6 (3) C21—C22—N4—Zn1 −175.95 (19)
F5—C28—C29—F8 −168.5 (2) C19—C23—N4—C22 −1.3 (4)
C27—C28—C29—F8 −46.7 (4) C24—C23—N4—C22 −179.1 (2)
F6—C28—C29—F7 −165.2 (3) C19—C23—N4—Zn1 176.8 (2)
F5—C28—C29—F7 −50.3 (3) C24—C23—N4—Zn1 −1.1 (3)
F6—C28—C29—C29i −44.8 (5) C36—C35—N5—Zn2 170.7 (2)
F5—C28—C29—C29i 70.1 (4) C38—C46—N5—C35 2.1 (4)
C27—C28—C29—C29i −168.1 (3) C45—C46—N5—C35 −177.0 (3)
F10—C30—C31—F11 65.8 (3) C38—C46—N5—Zn2 −169.9 (2)
F9—C30—C31—F11 −51.0 (3) C45—C46—N5—Zn2 11.0 (3)
C30ii—C30—C31—F11 −173.6 (3) C43—C44—N6—C45 −0.2 (4)
F10—C30—C31—F12 −48.9 (3) C43—C44—N6—Zn2 −172.2 (2)
F9—C30—C31—F12 −165.7 (2) C41—C45—N6—C44 −1.6 (4)
C30ii—C30—C31—F12 71.7 (4) C46—C45—N6—C44 178.6 (3)
F10—C30—C31—C32 −172.3 (2) C41—C45—N6—Zn2 171.7 (2)
F9—C30—C31—C32 70.9 (3) C46—C45—N6—Zn2 −8.1 (3)
C30ii—C30—C31—C32 −51.6 (4) O3—C25—O2—Zn1 −14.9 (4)
F11—C31—C32—F14 −162.7 (2) C26—C25—O2—Zn1 167.7 (2)
F12—C31—C32—F14 −45.7 (3) O10—C47—O9—Zn2 −7.1 (4)
C30—C31—C32—F14 77.8 (3) C48—C47—O9—Zn2 173.13 (16)
F11—C31—C32—F13 79.6 (3) C25—O2—Zn1—N2 −38.8 (2)
F12—C31—C32—F13 −163.4 (2) C25—O2—Zn1—N4 152.8 (2)
C30—C31—C32—F13 −39.9 (3) C25—O2—Zn1—O1 59.5 (2)
F11—C31—C32—C33 −40.8 (3) C25—O2—Zn1—N1 −117.0 (2)
F12—C31—C32—C33 76.2 (3) C25—O2—Zn1—N3 127.2 (4)
C30—C31—C32—C33 −160.3 (2) C10—N2—Zn1—O2 84.7 (2)
F14—C32—C33—F15 −49.3 (3) C11—N2—Zn1—O2 −108.61 (18)
F13—C32—C33—F15 68.7 (3) C10—N2—Zn1—N4 −132.0 (3)
C31—C32—C33—F15 −170.9 (2) C11—N2—Zn1—N4 34.8 (3)
F14—C32—C33—F16 −162.6 (2) C10—N2—Zn1—O1 −8.8 (2)
F13—C32—C33—F16 −44.6 (3) C11—N2—Zn1—O1 157.97 (17)
C31—C32—C33—F16 75.8 (3) C10—N2—Zn1—N1 176.7 (2)
F14—C32—C33—C34 73.6 (3) C11—N2—Zn1—N1 −16.55 (17)
F13—C32—C33—C34 −168.3 (2) C10—N2—Zn1—N3 −92.4 (2)
C31—C32—C33—C34 −47.9 (3) C11—N2—Zn1—N3 74.30 (18)
F15—C33—C34—O4 −135.3 (2) C22—N4—Zn1—O2 4.4 (2)
F16—C33—C34—O4 −17.5 (3) C23—N4—Zn1—O2 −173.46 (18)
C32—C33—C34—O4 103.9 (3) C22—N4—Zn1—N2 −140.1 (3)
F15—C33—C34—O5 45.3 (3) C23—N4—Zn1—N2 42.0 (3)
F16—C33—C34—O5 163.1 (2) C22—N4—Zn1—O1 96.0 (2)
C32—C33—C34—O5 −75.4 (3) C23—N4—Zn1—O1 −81.85 (18)
N5—C35—C36—C37 −1.8 (5) C22—N4—Zn1—N1 −90.5 (2)
C35—C36—C37—C38 1.5 (5) C23—N4—Zn1—N1 91.61 (18)
C36—C37—C38—C46 0.3 (4) C22—N4—Zn1—N3 179.1 (2)
C36—C37—C38—C39 179.2 (3) C23—N4—Zn1—N3 1.20 (17)
C37—C38—C39—C40 −175.8 (3) C1—N1—Zn1—O2 −70.4 (3)
C46—C38—C39—C40 3.0 (4) C12—N1—Zn1—O2 117.75 (18)
C38—C39—C40—C41 −1.0 (5) C1—N1—Zn1—N2 −173.4 (3)
C39—C40—C41—C42 178.6 (3) C12—N1—Zn1—N2 14.79 (18)
C39—C40—C41—C45 −2.0 (4) C1—N1—Zn1—N4 21.9 (3)
C45—C41—C42—C43 −1.6 (4) C12—N1—Zn1—N4 −149.91 (19)
C40—C41—C42—C43 177.9 (3) C1—N1—Zn1—N3 98.7 (3)
C42—C43—C44—N6 1.0 (5) C13—N3—Zn1—O2 −148.6 (4)
C42—C41—C45—N6 2.5 (4) C24—N3—Zn1—O2 25.2 (5)
C40—C41—C45—N6 −177.0 (3) C13—N3—Zn1—N2 17.8 (2)
C42—C41—C45—C46 −177.7 (3) C24—N3—Zn1—N2 −168.42 (18)
C40—C41—C45—C46 2.8 (4) C13—N3—Zn1—N4 −175.0 (2)
C37—C38—C46—N5 −2.2 (4) C24—N3—Zn1—N4 −1.18 (17)
C39—C38—C46—N5 178.8 (3) C13—N3—Zn1—O1 −80.1 (2)
C37—C38—C46—C45 176.8 (3) C24—N3—Zn1—O1 93.62 (17)
C39—C38—C46—C45 −2.1 (4) C13—N3—Zn1—N1 95.2 (2)
N6—C45—C46—N5 −1.8 (4) C24—N3—Zn1—N1 −91.04 (18)
C41—C45—C46—N5 178.3 (3) C47—O9—Zn2—O6 −68.2 (2)
N6—C45—C46—C38 179.1 (2) C47—O9—Zn2—O8 −156.2 (2)
C41—C45—C46—C38 −0.8 (4) C47—O9—Zn2—N5 116.9 (2)
O10—C47—C48—F18 12.5 (3) C47—O9—Zn2—O7 20.6 (2)
O9—C47—C48—F18 −167.7 (2) C47—O9—Zn2—N6 119.6 (4)
O10—C47—C48—F17 130.9 (3) C35—N5—Zn2—O9 −3.1 (3)
O9—C47—C48—F17 −49.3 (3) C46—N5—Zn2—O9 168.1 (2)
O10—C47—C48—C49 −109.6 (3) C35—N5—Zn2—O8 −94.2 (3)
O9—C47—C48—C49 70.3 (3) C46—N5—Zn2—O8 77.0 (2)
F18—C48—C49—F19 165.9 (2) C35—N5—Zn2—O7 87.8 (3)
F17—C48—C49—F19 49.7 (3) C46—N5—Zn2—O7 −101.0 (2)
C47—C48—C49—F19 −70.5 (3) C35—N5—Zn2—N6 177.5 (3)
F18—C48—C49—F20 −76.7 (3) C46—N5—Zn2—N6 −11.37 (19)
F17—C48—C49—F20 167.2 (2) C44—N6—Zn2—O6 7.8 (3)
C47—C48—C49—F20 46.9 (3) C45—N6—Zn2—O6 −164.61 (18)
F18—C48—C49—C50 44.4 (3) C44—N6—Zn2—O9 180.0 (4)
F17—C48—C49—C50 −71.8 (3) C45—N6—Zn2—O9 7.6 (5)
C47—C48—C49—C50 168.0 (2) C44—N6—Zn2—O8 95.4 (2)
F19—C49—C50—F21 −80.2 (3) C45—N6—Zn2—O8 −76.94 (19)
F20—C49—C50—F21 162.2 (2) C44—N6—Zn2—N5 −177.3 (3)
C48—C49—C50—F21 41.8 (3) C45—N6—Zn2—N5 10.36 (18)
F19—C49—C50—F22 162.2 (2) C44—N6—Zn2—O7 −81.1 (2)
F20—C49—C50—F22 44.6 (3) C45—N6—Zn2—O7 106.54 (19)
Symmetry codes: (i) −x+1, −y+2, −z+1; (ii) −x+1, −y+1, −z+1.
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
O1—H1A···O11iii 0.82 (2) 2.13 (3) 2.833 (3) 143 (3)
O1—H1A···O3 0.82 (2) 2.55 (3) 3.003 (3) 116 (3)
O1—H1A···F29iii 0.82 (2) 2.54 (3) 3.133 (2) 130 (3)
O1—H1B···O4iv 0.83 (2) 2.01 (2) 2.834 (3) 168 (4)
O6—H6A···O4v 0.82 (2) 1.87 (2) 2.661 (3) 162 (4)
O6—H6B···O11vi 0.82 (2) 1.90 (2) 2.706 (3) 168 (4)
O7—H7A···O10 0.84 (2) 1.96 (3) 2.706 (3) 148 (4)
O7—H7B···O5v 0.83 (2) 1.95 (2) 2.776 (3) 176 (4)
O8—H8B···O12vi 0.82 (2) 1.93 (2) 2.724 (3) 163 (4)
O13—H13A···O3i 0.82 (2) 1.99 (2) 2.806 (3) 170 (4)
O13—H13B···O9 0.82 (2) 2.29 (3) 2.991 (3) 143 (4)
O13—H13B···F17 0.82 (2) 2.50 (3) 3.151 (3) 137 (4)
O14—H14A···O7 0.83 (2) 2.12 (3) 2.930 (4) 163 (6)
O14—H14B···O5 0.82 (2) 2.17 (3) 2.955 (4) 159 (6)
C13—H13···Cg1 0.93 2.84 3.613 (3) 142